The Effect of Lupinus albus and Calcium Chloride on Growth Performance, Body Composition, Plasma Biochemistry and Meat Quality of Male Pigs Immunized Against Gonadotrophin Releasing Factor
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Allocation and Housing
2.2. Diets and Feeding Regime
2.3. Growth Performance
2.4. Dual-Energy X-ray Absorptiometry Analysis
2.5. Slaughter Procedure
2.6. Blood Analysis
2.7. Objective Meat Quality
2.8. Statistical Analysis
3. Results
3.1. Growth and Carcass Performance
3.2. Body Composition
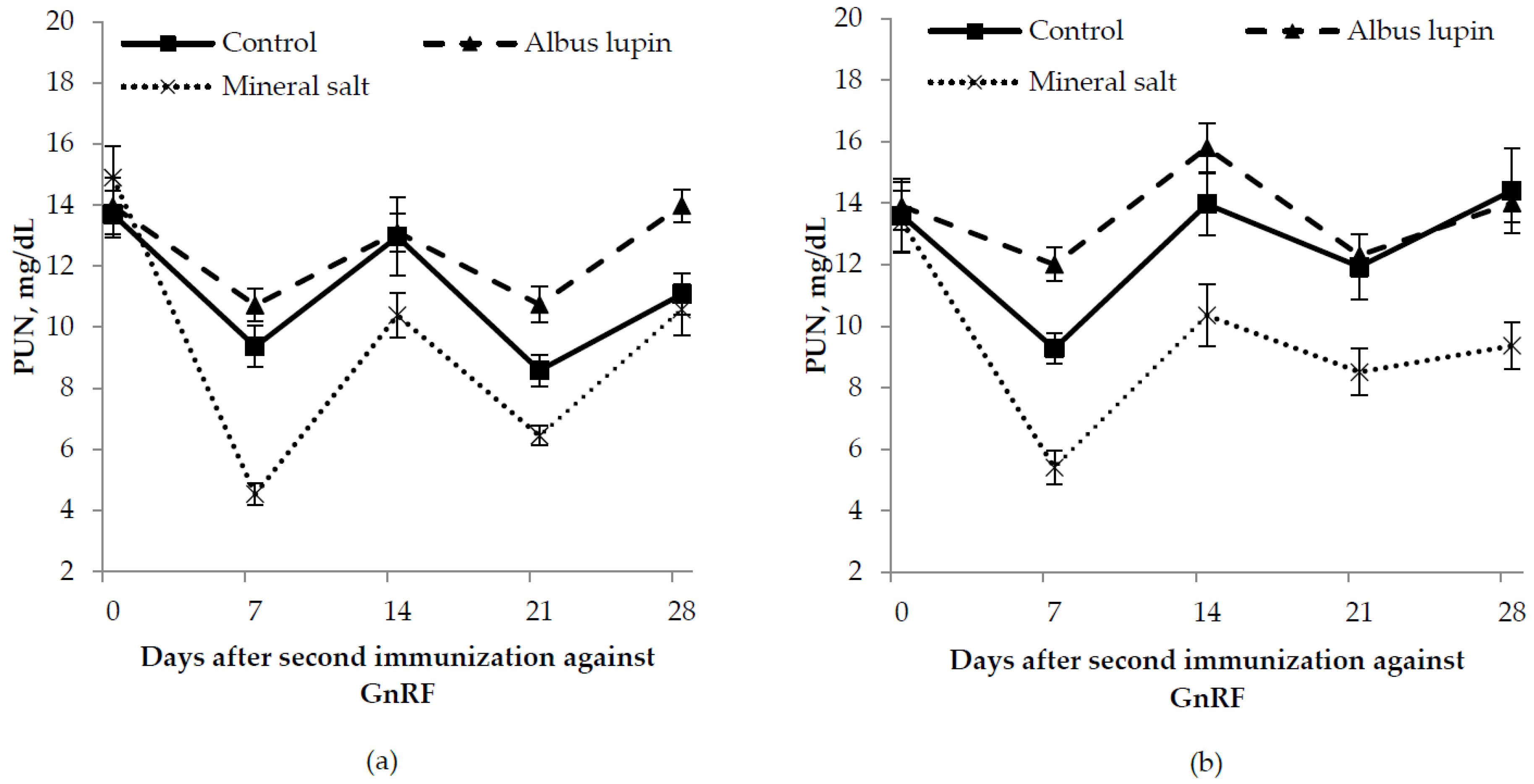
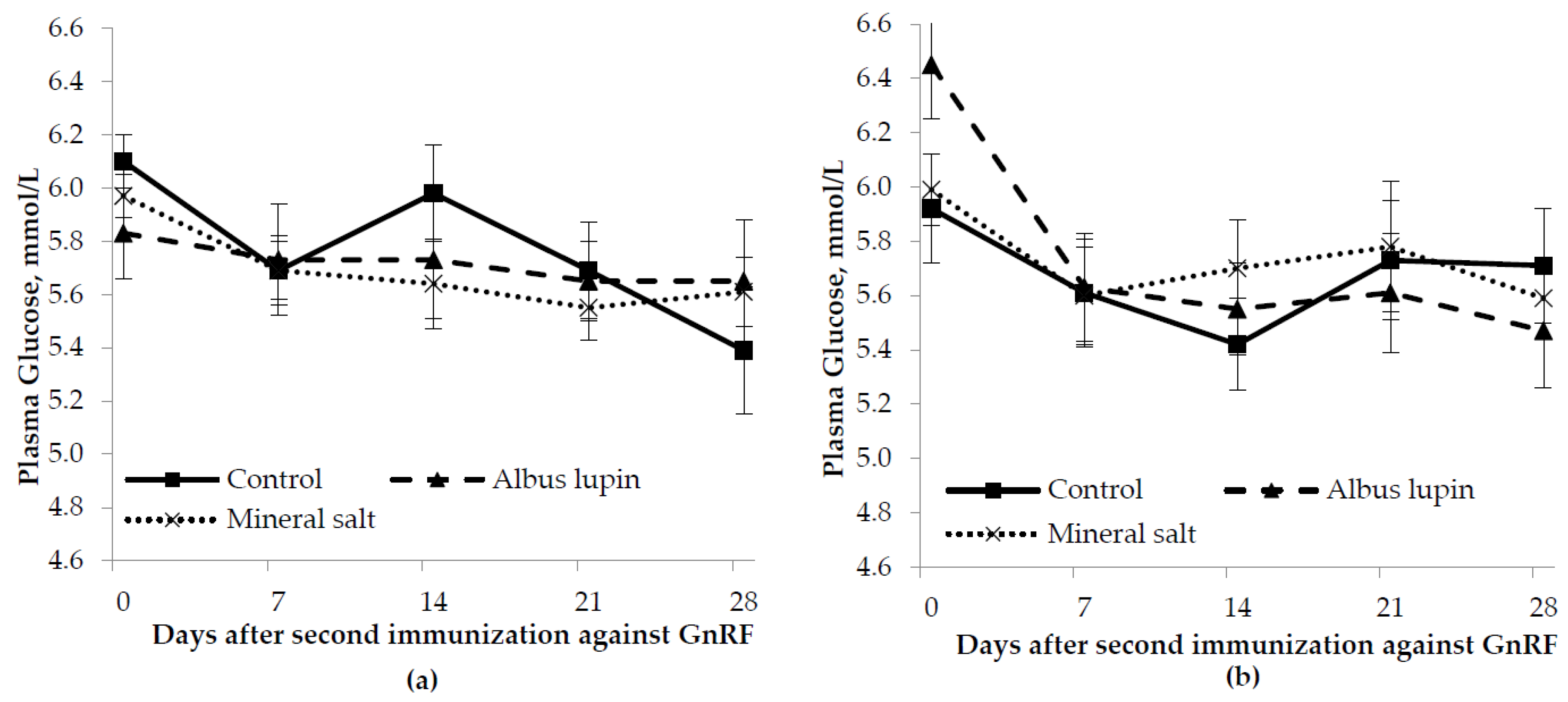
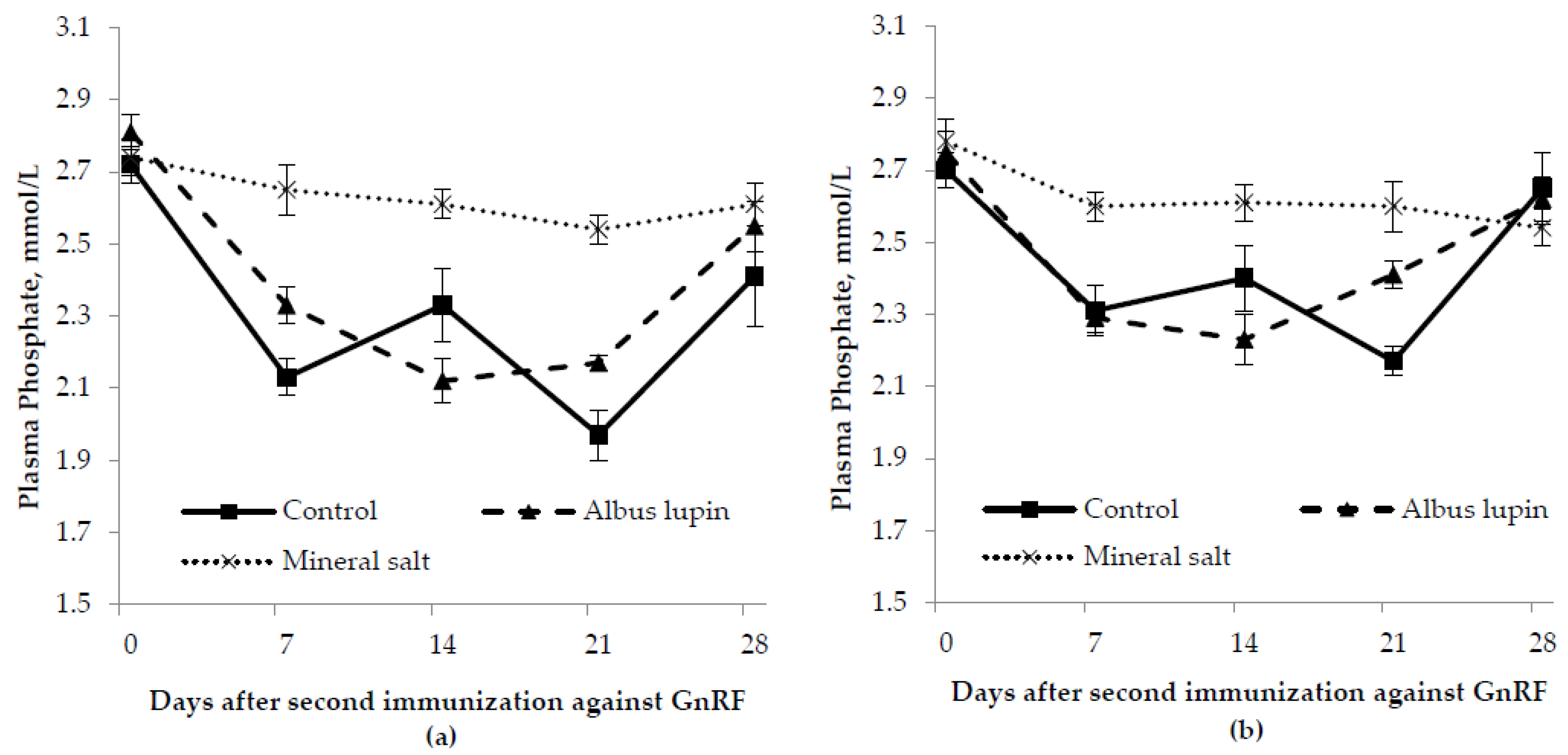
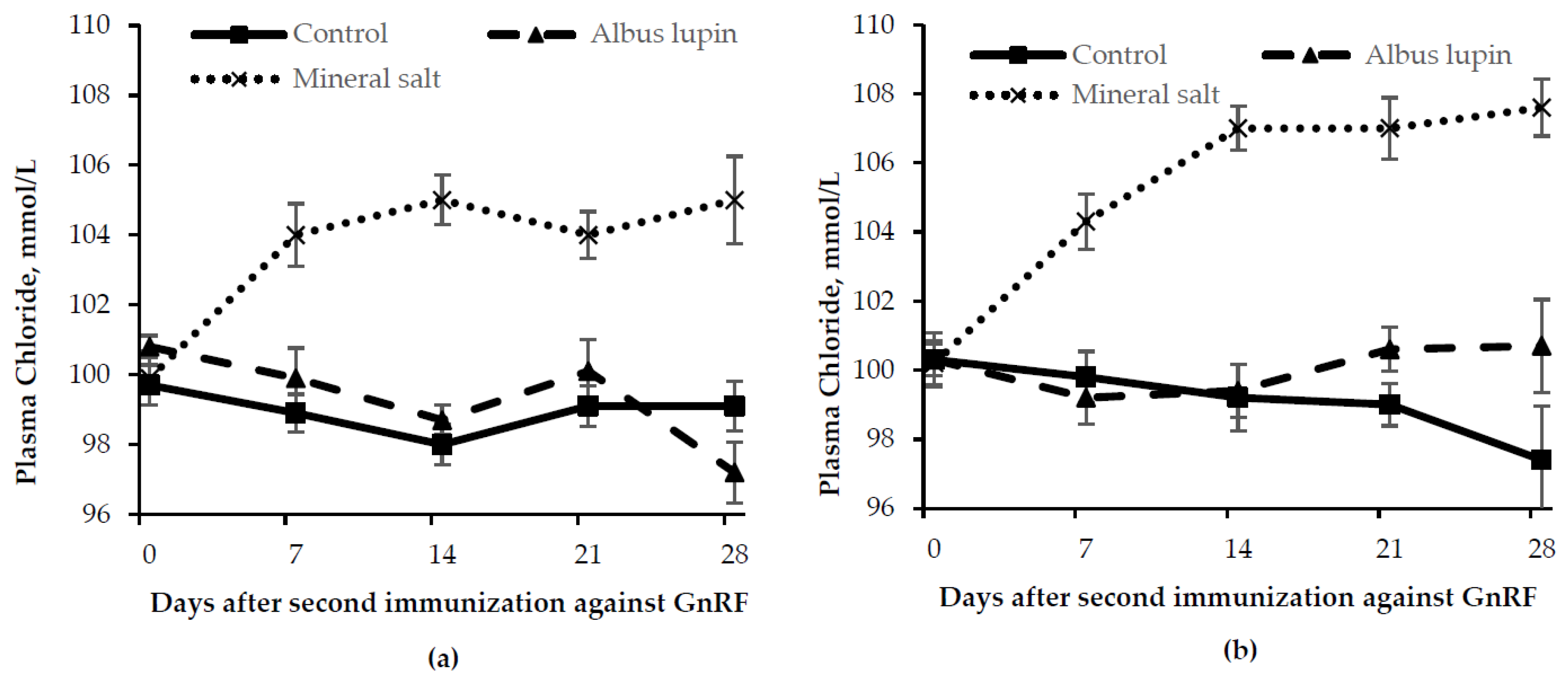
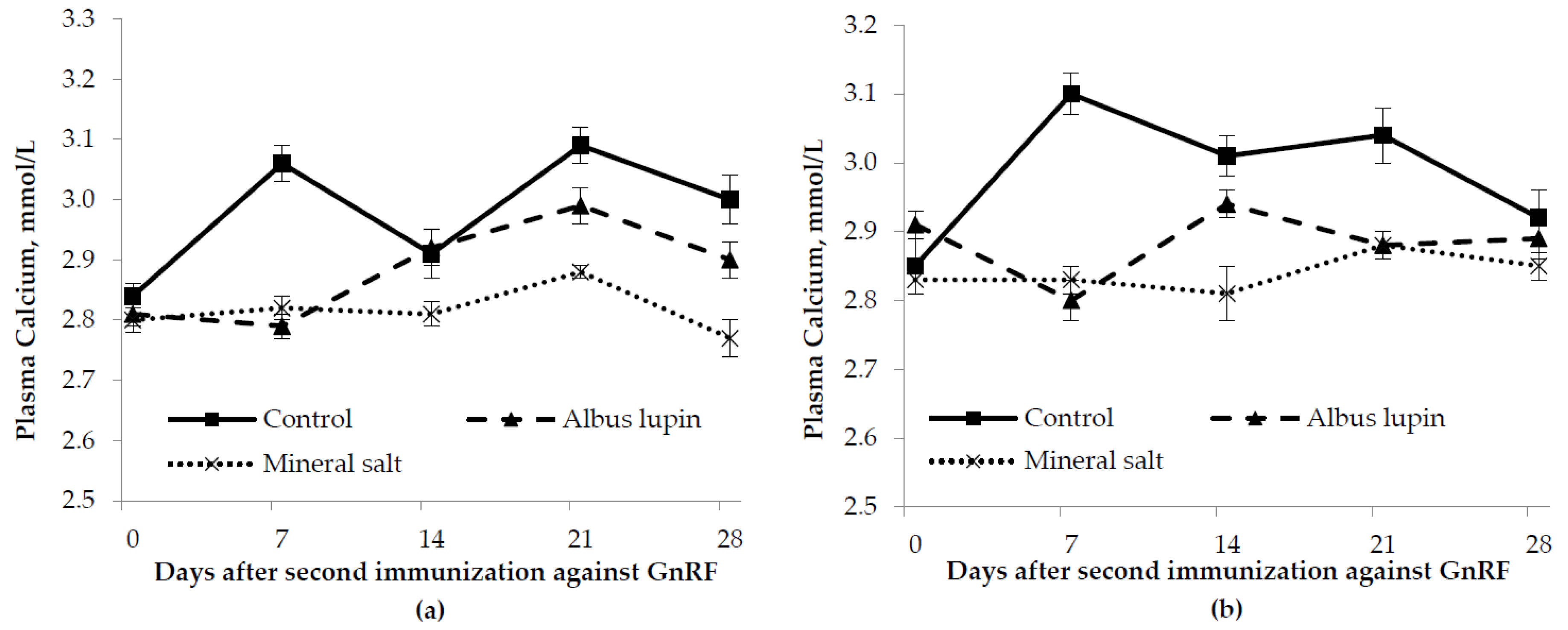
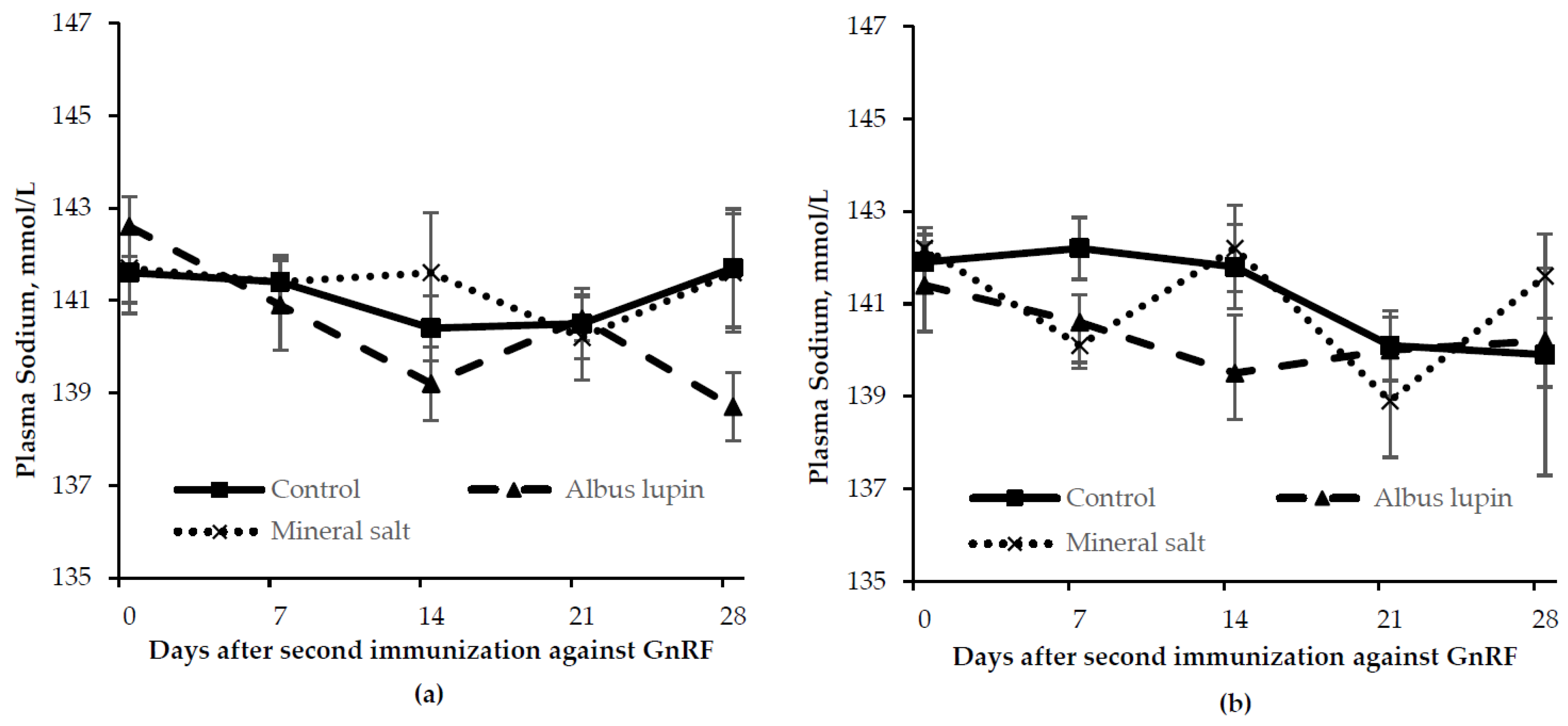
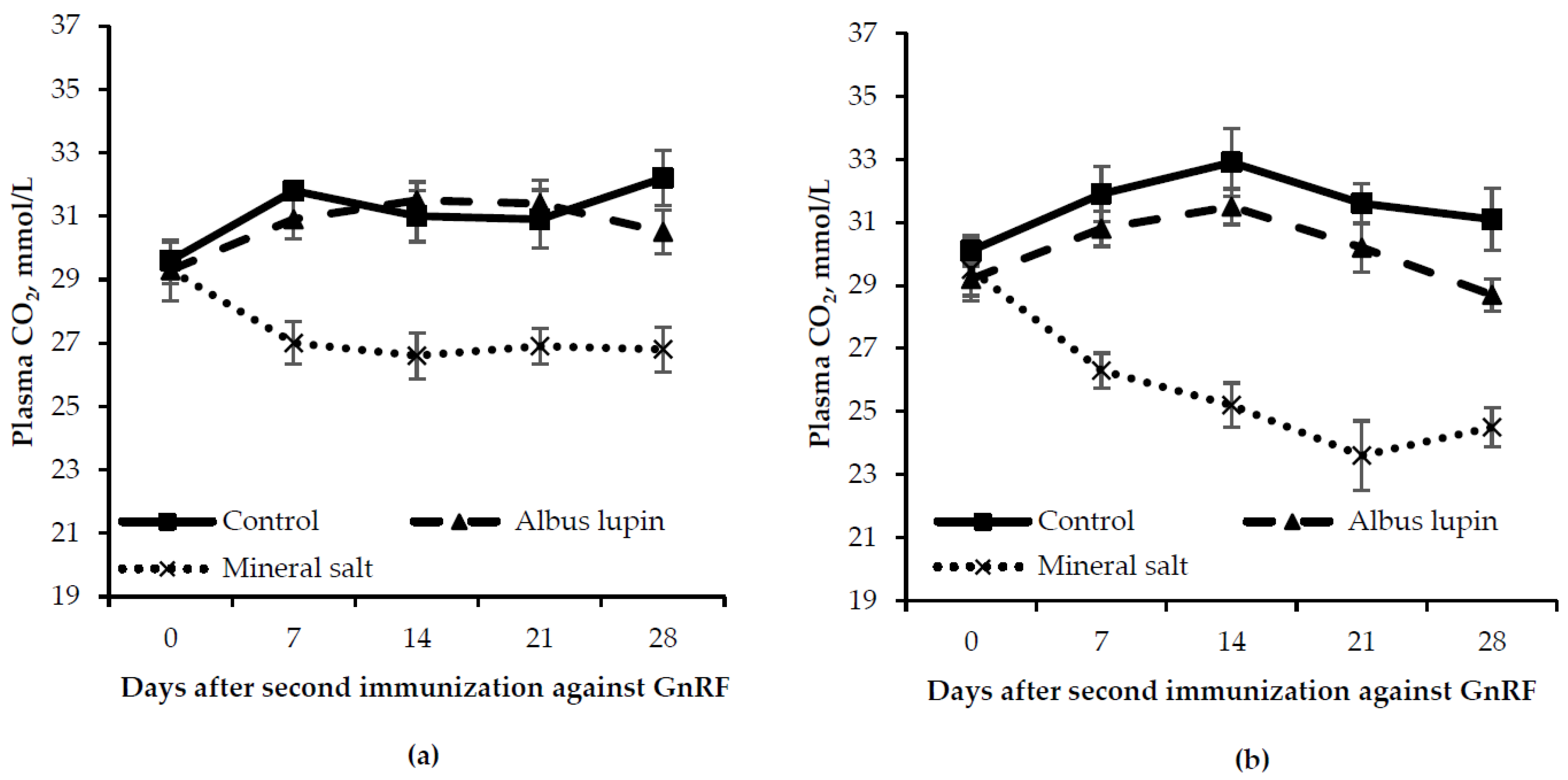
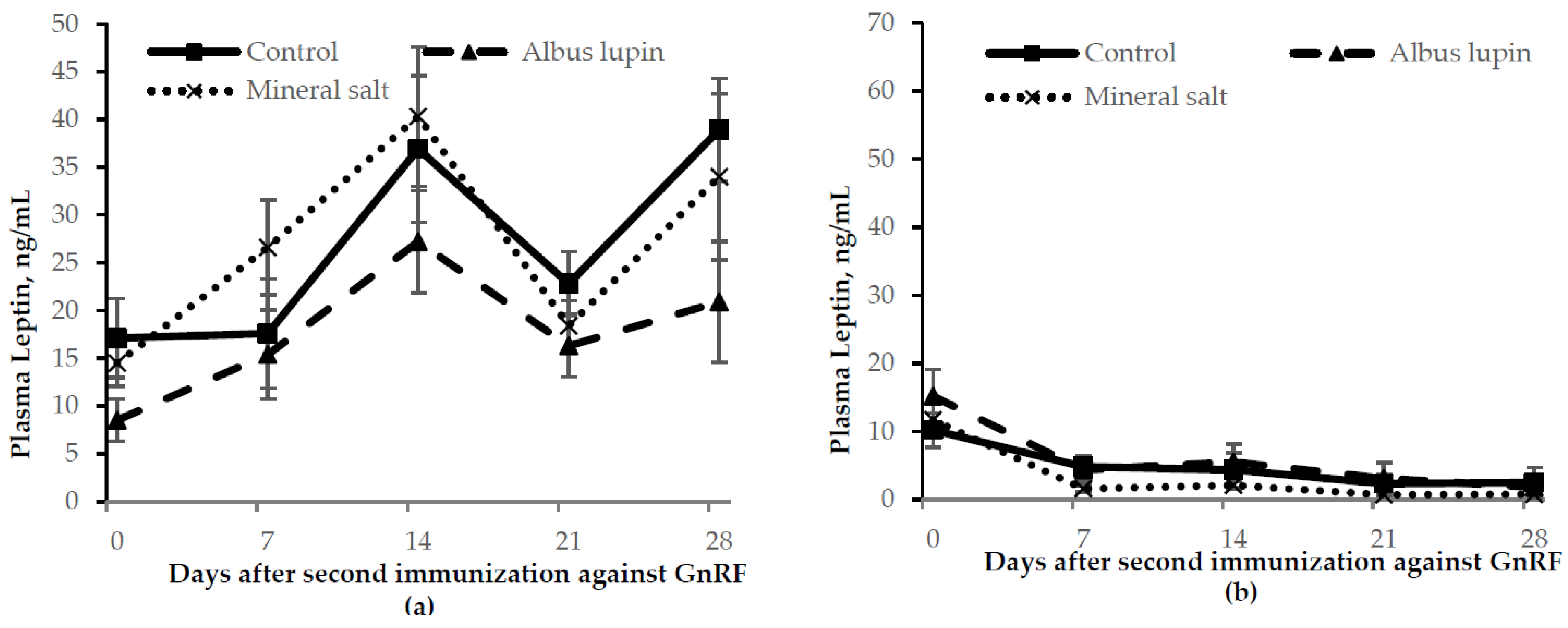
3.3. Physiological Measures
3.4. Objective Meat Quality
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dunshea, F.R. Castration in the swine industry: Physical versus immunological. In Proceedings of the Pfizer International Swine Symposium, Ottawa, ON, Canada, 7–9 October 2009; pp. 26–32.
- Dunshea, F.R.; Calantoni, C.; Howard, K.; McCauley, I.; Jackson, P. Vaccination of boars with a GnRF vaccine (Improvac) eliminates boar taint and increases growth performance. J. Anim. Sci. 2001, 79, 2524–2535. [Google Scholar] [CrossRef] [PubMed]
- Cronin, G.M.; Dunshea, F.R.; Butler, K.R.; McCauley, I.; Barnett, J.L.; Hemsworth, P.H. The effects of immuno- and surgical-castration on the behaviour and consequently growth of group-housed, male finisher pigs. Appl. Anim. Behav. Sci. 2003, 81, 111–126. [Google Scholar] [CrossRef]
- McCauley, I.; Watt, M.; Suster, D.; Kerton, D.J.; Oliver, W.T.; Harrell, R.J.; Dunshea, F.R. A GnRF vaccine (Improvac) and porcine somatotropin (Reporcin) have synergistic effects upon growth performance in both boars and gilts. Aust. J. Agric. Res. 2003, 54, 11–20. [Google Scholar]
- Oliver, W.T.; McCauley, I.; Harell, R.J.; Suster, D.; Kerton, D.J.; Dunshea, F.R. A gonadotropin-releasing factor vaccine (Improvac) and porcine somatotropin have synergistic and additive effects on growth performance in group-housed boars and gilts. J Anim. Sci. 2003, 81, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Lealiifano, A.K.; Pluske, J.R.; Nicholls, R.R.; Dunshea, F.R.; Campbell, R.G.; Hennessy, D.P.; Miller, D.W.; Mullan, B.P. Reducing the length of time between harvest and the secondary GnRF immunization improves growth performance and clears boar taint compounds in male finishing pigs. J. Anim. Sci. 2011, 89, 2782–2792. [Google Scholar] [CrossRef] [PubMed]
- Moore, K. L.; Mullan, B.P.; Kim, J.C.; Dunshea, F.R. Standardized ileal digestible lysine requirements of male pigs immunized against gonadotrophin releasing factor. J. Anim. Sci. 2016, 94, 1982–1992. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.L.; Mullan, B.P.; Kim, J.C.; Payne, H.G.; Dunshea, F.R. Effect of feed restriction and initial body weight on growth performance, body composition and hormones in male pigs immunized against gonadotrophin releasing factor. J. Anim. Sci. 2016, 94, 3. [Google Scholar] [CrossRef]
- Quiniou, N.; Monziols, M.; Colin, F.; Goues, T.; Courboulay, V. Effect of feed restriction on the performance and behaviour of pigs immunologically castrated with Improvac®. Animal 2012, 6, 1420–1426. [Google Scholar] [CrossRef] [PubMed]
- Batorek, N.; Skrlep, M.; Prunier, A.; Louveau, I.; Noblet, J.; Bonneau, M.; Candek-Potokar, M. Effect of feed restriction on hormones, performance, carcass traits, and meat quality in immunocastrated pigs. J. Anim. Sci. 2012, 90, 4593–4603. [Google Scholar] [CrossRef] [PubMed]
- Dunshea, F.R.; Gannon, N.J.; van Barneveld, R.J.; Mullan, B.P.; Campbell, R.G.; King, R.H. Dietary lupins (Lupinus angustifolius and Lupinus albus) can increase digesta retention in the gastrointestinal tract of pigs. Aust. J. Agric. Res. 2001, 52, 593–602. [Google Scholar] [CrossRef]
- Van Nevel, C.; Seynaeve, M.; Van De Voorde, G.; De Smet, S.; Van Driessche, E.; de Wilde, R. Effects of increasing amounts of Lupinus albus seeds without or with whole egg powder in the diet of growing pigs on performance. Anim. Feed Sci. Technol. 2000, 83, 89–101. [Google Scholar] [CrossRef]
- Yen, J.T.; Pond, W.G.; Prior, R.L. Calcium chloride as a regulator of feed intake and weight gain in pigs. J. Anim. Sci. 1981, 52, 778–782. [Google Scholar] [CrossRef]
- Pluske, J.R.; Black, J.L.; Kim, J.C.; Dunshea, F.R. Suppressing the feed intake of finisher pigs: A preliminary study. Anim. Prod. Sci. 2015, 55, 1546. [Google Scholar]
- National Health Medical and Research Council. Australian Code for the Care and Use of Animals for Scientific Purposes, 8th ed.; National Health Medical and Research Council: Canberra, Australia, 2013. [Google Scholar]
- Suster, D.; Leury, B.J.; Kerton, D.J.; Borg, M.R.; Butler, K.L.; Dunshea, F.R. Longitudinal DXA measurements demonstrate lifetime differences in lean and fat tissue deposition between boars and barrows under individual and group-penned systems. Aust. J. Agric. Res. 2006, 57, 1009–1015. [Google Scholar] [CrossRef]
- Rasmussen, A.J.; Andersson, M. New methods for determination of drip loss in pork muscles. In Proceedings of the Meat for the Consumer, 42nd International Congress of Meat Science and Technology, Lillehammer, Norway, 1–6 September 1996; pp. 286–287.
- Bouton, P.E.; Harris, P.V.; Shorthose, W.R. Effect of ultimate pH upon the water holding capacity and tenderness of meat. J. Food Sci. 1971, 36, 435–439. [Google Scholar] [CrossRef]
- Soine, T.O.; Wilson, C.O. Roger’s Inorganic Pharmaceutical Chemistry, 8th ed.; Lea and Febiger: Philadelphia, PA, USA, 1967. [Google Scholar]
- Spurlock, M.E.; Frank, G.R.; Cornelius, S.G.; Ji, S.; Willis, G.M.; Bidwell, C.A. Obese gene expression in porcine adipose tissue is reduced by food deprivation but not by maintenance of submaintenance intake. J. Nutr. 1998, 128, 677–682. [Google Scholar]
- Houseknecht, K.L.; Baile, C.A.; Matteri, R.L.; Spurlock, M.E. The biology of leptin: A review. J. Anim. Sci. 1998, 76, 1405–1420. [Google Scholar] [CrossRef] [PubMed]
- Barb, C.R.; Yan, X.; Azain, M.J.; Kraeling, R.R.; Rampacek, G.B.; Ramsay, T.G. Recombinant leptin reduced feed intake and stimulates growth hormone secretion in swine. Dom. Anim. Endocrinol. 1998, 15, 77–86. [Google Scholar] [CrossRef]
- Amills, M.; Villalba, D.; Tor, M.; Mercade, A.; Gallardo, D.; Cabrera, B.; Jimenez, N.; Noguera, J.L.; Sanchez, A.; Estany, J. Plasma leptin levels in pigs with different leptin and leptin receptor genotypes. J. Anim. Breed. Genet. 2008, 125, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.C.; Mullan, B.P.; Nicholls, R.R.; Pluske, J.R. Effect of Australian sweet lupin (Lupinus angustifolius L.) inclusion levels and enzyme supplementation of the performance, carcass composition and meat quality of grower/finisher pigs. Anim. Prod. Sci. 2011, 51, 37–43. [Google Scholar] [CrossRef]
- Vicenti, A.; Toteda, F.; Di Turi, L.; Cocca, C.; Perrucci, M.; Melodia, L.; Ragni, M. Use of sweet lupin (Lupinus albus L. var. Multialia) in feeding for Podolian young bulls and influence on productive performances and meat quality traits. Meat Sci. 2009, 82, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Batorek, N.; Candek-Potokar, M.; Bonneau, M.; Van Milgen, J. Meta-analysis of the effect of immunocastration on production performance, reproductive organs and boar taint compounds in pigs. Animal 2012, 6, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
| Ingredients g/kg, As-Fed | Control Low 7 | Control High 8 | Albus Lupin Low | Albus Lupin High | Mineral Salts Low | Mineral Salts High |
|---|---|---|---|---|---|---|
| Barley | 400 | 400 | 400 | 400 | 400 | 400 |
| Wheat | 372 | 181 | 170 | 172 | 299 | 190 |
| Mill run, 15% | 50 | 45.7 | 82.3 | 48.5 | 20.0 | 24.6 |
| Lupins, 28% | 100 | 150 | 0 | 0 | 60 | 100 |
| Lupins albus | 0 | 0 | 300 | 300 | 0 | 0 |
| Canola meal, 36% | 0 | 150 | 0 | 0 | 100 | 150 |
| Soybean meal, 48% | 10 | 10 | 0 | 19.5 | 10 | 10 |
| Bloodmeal, 85% | 12.6 | 8.81 | 0 | 20 | 4.15 | 19.1 |
| Tallow | 36.0 | 38.4 | 30.4 | 21.9 | 55 | 55 |
| Limestone | 12.2 | 10.3 | 11.3 | 11.1 | 0 | 0 |
| Salt | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| L-Lysine HCL | 2.13 | 2.12 | 0.22 | 0.42 | 1.98 | 1.96 |
| Methionine | 0.61 | 0.51 | 0.16 | 1.01 | 0 | 0.51 |
| Phytase 1 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Choline chloride, 60% | 1.24 | 0 | 2.05 | 1.93 | 0.47 | 0 |
| Calcium chloride, 77% 2 | 0 | 0 | 0 | 0 | 16.0 | 16.0 |
| Sodium tripolyphosphate 3 | 0 | 0 | 0 | 0 | 3.00 | 3.00 |
| Vitamins and minerals 4 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Nutrient Composition 5 | ||||||
| DE, MJ/kg | 14.0 | 14.0 | 14.0 | 14.0 | 14.0 | 14.0 |
| CP, g/kg | 145 | 188 | 184 | 207 | 150 | 181 |
| Ca, g/kg | 8.00 | 8.00 | 8.00 | 8.00 | 12.2 | 12.5 |
| Total P, g/kg | 5.92 | 6.83 | 6.40 | 6.24 | 10.2 | 10.6 |
| Available P, g/kg | 4.22 | 4.50 | 4.50 | 4.50 | 7.74 | 7.86 |
| Na, g/kg | 1.09 | 1.15 | 0.96 | 1.10 | 6.02 | 6.18 |
| NDF, g/kg | 16.8 | 19.7 | 18.9 | 17.8 | 16.6 | 17.8 |
| ADF, g/kg | 5.30 | 5.63 | 7.00 | 7.10 | 4.34 | 4.74 |
| g SID Lysine/MJ DE 6 | 0.50 | 0.64 | 0.50 | 0.64 | 0.50 | 0.64 |
| Amino acid g/kg, As-Fed | Control Low 1 | Control High 2 | Albus Lupins Low | Albus Lupins High | Mineral Salts Low | Mineral Salts High |
|---|---|---|---|---|---|---|
| Histidine | 3.9 | 4.4 | 3.8 | 4.9 | 3.5 | 5.0 |
| Isoleucine | 5.1 | 6.0 | 6.2 | 6.7 | 5.1 | 5.9 |
| Leucine | 10.3 | 11.5 | 11.2 | 14.0 | 9.6 | 12.9 |
| Lysine | 8.1 | 9.5 | 7.4 | 9.4 | 7.7 | 10.3 |
| Methionine | 2.6 | 2.5 | 1.6 | 2.2 | 1.9 | 1.2 |
| Phenylalanine | 6.5 | 7.1 | 6.8 | 8.3 | 6.1 | 7.8 |
| Threonine | 5.5 | 6.3 | 5.8 | 6.9 | 5.2 | 6.7 |
| Valine | 7.4 | 8.3 | 7.4 | 9.2 | 6.9 | 9.2 |
| Alanine | 6.4 | 7.0 | 6.1 | 7.5 | 5.9 | 7.6 |
| Arginine | 9.4 | 10.8 | 12.9 | 14.8 | 8.2 | 10.8 |
| Aspartic acid | 10.9 | 12.4 | 13.4 | 15.9 | 10.0 | 13.3 |
| Glycine | 7.1 | 7.8 | 7.0 | 7.8 | 6.6 | 7.6 |
| Glutamic acid | 27.8 | 30.4 | 32.1 | 35.0 | 26.5 | 30.9 |
| Proline | 9.6 | 10.2 | 9.5 | 10.2 | 9.5 | 10.2 |
| Serine | 6.7 | 7.5 | 7.7 | 9.0 | 6.1 | 7.9 |
| Tyrosine | 3.4 | 3.8 | 4.2 | 4.8 | 3.0 | 3.6 |
| Entire Male | Immunocastrated Male | SED a | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Mineral Salt | Albus Lupin | Control | Mineral Salt | Albus Lupin | Sex | Diet | Sex × Diet | ||
| Daily gain (kg/day) | ||||||||||
| d 0–14 | 1.00 | 0.984 | 0.648 | 1.07 | 1.00 | 0.673 | 0.037 | 0.11 | <0.001 | 0.67 |
| d 15–28 | 1.09 | 1.06 | 0.930 | 1.23 | 1.25 | 1.050 | 0.036 | <0.001 | <0.001 | 0.38 |
| d 0–28 | 1.05 | 1.02 | 0.789 | 1.15 | 1.13 | 0.861 | 0.026 | <0.001 | <0.001 | 0.63 |
| Feed intake (kg/day) | ||||||||||
| d 0–14 | 2.52 | 2.25 | 1.61 | 2.63 | 2.39 | 1.64 | 0.076 | 0.04 | <0.001 | 0.59 |
| d 15–28 | 3.05 | 2.80 | 2.47 | 3.80 | 3.58 | 3.03 | 0.090 | <0.001 | <0.001 | 0.16 |
| d 0–28 | 2.72 | 2.48 | 1.98 | 3.16 | 2.92 | 2.30 | 0.075 | <0.001 | <0.001 | 0.46 |
| Feed conversion ratio (kg/kg) | ||||||||||
| d 0–14 | 2.52 | 2.29 | 2.51 | 2.48 | 2.39 | 2.45 | 0.094 | 0.98 | 0.05 | 0.45 |
| d 15–28 | 2.81 | 2.64 | 2.67 | 3.10 | 2.86 | 2.90 | 0.091 | <0.001 | 0.007 | 0.82 |
| d 0–28 | 2.60 | 2.43 | 2.52 | 2.75 | 2.59 | 2.67 | 0.058 | <0.001 | 0.001 | 0.98 |
| Carcass characteristics | ||||||||||
| CW c (kg) | 64.7 | 64.3 | 58.8 | 65.6 | 65.2 | 59.8 | 0.820 | 0.06 | <0.001 | 1.00 |
| DP d (%) | 66.8 | 66.9 | 65.7 | 65.9 | 65.6 | 65.1 | 0.428 | <0.001 | 0.01 | 0.40 |
| P2 backfat (mm) b | 9.34 | 8.83 | 7.49 | 10.8 | 10.7 | 8.71 | 0.508 | <0.001 | 0.01 | 0.43 |
| Entire Male | Immunocastrated Male | SED a | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Mineral Salt | Albus Lupin | Control | Mineral Salt | Albus Lupin | Sex | Diet | Sex × Diet | ||
| BMC b (g/day) | ||||||||||
| d −1–13 | 22.0 | 20.6 | 13.4 | 23.3 | 23.1 | 16.9 | 1.94 | 0.03 | <0.001 | 0.73 |
| d 14–27 | 18.1 | 23.9 | 20.5 | 16.5 | 20.5 | 17.1 | 2.00 | 0.02 | 0.003 | 0.76 |
| d −1–27 | 20.2 | 22.3 | 16.9 | 19.6 | 22.7 | 17.0 | 1.39 | 0.98 | <0.001 | 0.88 |
| Lean (g/day) | ||||||||||
| d −1–13 | 736 | 709 | 499 | 783 | 694 | 525 | 43.2 | 0.446 | <0.001 | 0.59 |
| d 14–27 | 706 | 765 | 752 | 737 | 762 | 626 | 52.5 | 0.29 | 0.14 | 0.10 |
| d −1–27 | 725 | 737 | 625 | 758 | 728 | 576 | 34.1 | 0.67 | <0.001 | 0.24 |
| Fat (g/day) | ||||||||||
| d −1–13 | 189 | 196 | 57.6 | 202 | 173 | 97.9 | 30.9 | 0.57 | <0.001 | 0.35 |
| d 14–27 | 238 | 294 | 166 | 465 | 487 | 369 | 38.5 | <0.001 | <0.001 | 0.81 |
| d −1–27 | 222 | 245 | 112 | 321 | 330 | 234 | 23.2 | <0.001 | <0.001 | 0.53 |
| g Fat/kg Metabolic liveweight | ||||||||||
| d −1–13 | 423 | 399 | 160 | 405 | 350 | 261 | 70.6 | 0.78 | <0.001 | 0.29 |
| d 14–27 | 445 | 551 | 367 | 770 | 788 | 712 | 66.0 | <0.001 | 0.03 | 0.47 |
| d −1–27 | 266 | 281 | 170 | 344 | 353 | 304 | 25.6 | <0.001 | <0.001 | 0.18 |
| Entire Male | Immunocastrated Male | SED a | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Mineral Salt | Albus Lupin | Control | Mineral Salt | Albus Lupin | Sex | Diet | Sex × Diet | ||
| pH 45 min | 6.18 | 6.24 | 6.24 | 6.02 | 6.26 | 6.20 | 0.082 | 0.23 | 0.03 | 0.32 |
| pH 24 h | 5.62 | 5.54 | 5.62 | 5.58 | 5.67 | 5.52 | 0.043 | <0.001 | 0.81 | 0.22 |
| L | 49.4 | 48.7 | 48.5 | 50.8 | 50.4 | 51.1 | 1.10 | 0.003 | 0.77 | 0.69 |
| a | 5.82 | 5.56 | 5.08 | 6.01 | 5.31 | 6.04 | 0.377 | 0.17 | 0.17 | 0.08 |
| b | 4.15 | 3.91 | 3.51 | 4.54 | 4.16 | 4.64 | 0.377 | 0.01 | 0.46 | 0.21 |
| Drip loss (%) | 4.75 | 3.79 | 3.57 | 6.12 | 5.04 | 6.18 | 0.725 | <0.001 | 0.14 | 0.34 |
| Cook loss (%) b | 24.5 | 23.6 | 23.6 | 21.9 | 23.1 | 25.8 | 1.14 | 0.73 | 0.35 | 0.12 |
| Shear force (N) b | 47.0 | 42.6 | 45.1 | 41.3 | 43.4 | 44.7 | 2.71 | 0.41 | 0.78 | 0.43 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moore, K.; Mullan, B.; Kim, J.C.; Dunshea, F. The Effect of Lupinus albus and Calcium Chloride on Growth Performance, Body Composition, Plasma Biochemistry and Meat Quality of Male Pigs Immunized Against Gonadotrophin Releasing Factor. Animals 2016, 6, 78. https://doi.org/10.3390/ani6120078
Moore K, Mullan B, Kim JC, Dunshea F. The Effect of Lupinus albus and Calcium Chloride on Growth Performance, Body Composition, Plasma Biochemistry and Meat Quality of Male Pigs Immunized Against Gonadotrophin Releasing Factor. Animals. 2016; 6(12):78. https://doi.org/10.3390/ani6120078
Chicago/Turabian StyleMoore, Karen, Bruce Mullan, Jae Cheol Kim, and Frank Dunshea. 2016. "The Effect of Lupinus albus and Calcium Chloride on Growth Performance, Body Composition, Plasma Biochemistry and Meat Quality of Male Pigs Immunized Against Gonadotrophin Releasing Factor" Animals 6, no. 12: 78. https://doi.org/10.3390/ani6120078
APA StyleMoore, K., Mullan, B., Kim, J. C., & Dunshea, F. (2016). The Effect of Lupinus albus and Calcium Chloride on Growth Performance, Body Composition, Plasma Biochemistry and Meat Quality of Male Pigs Immunized Against Gonadotrophin Releasing Factor. Animals, 6(12), 78. https://doi.org/10.3390/ani6120078






