The Choice of Diet Affects the Oral Health of the Domestic Cat
Abstract
:Simple Summary
Abstract
1. Introduction
2. Experimental Section
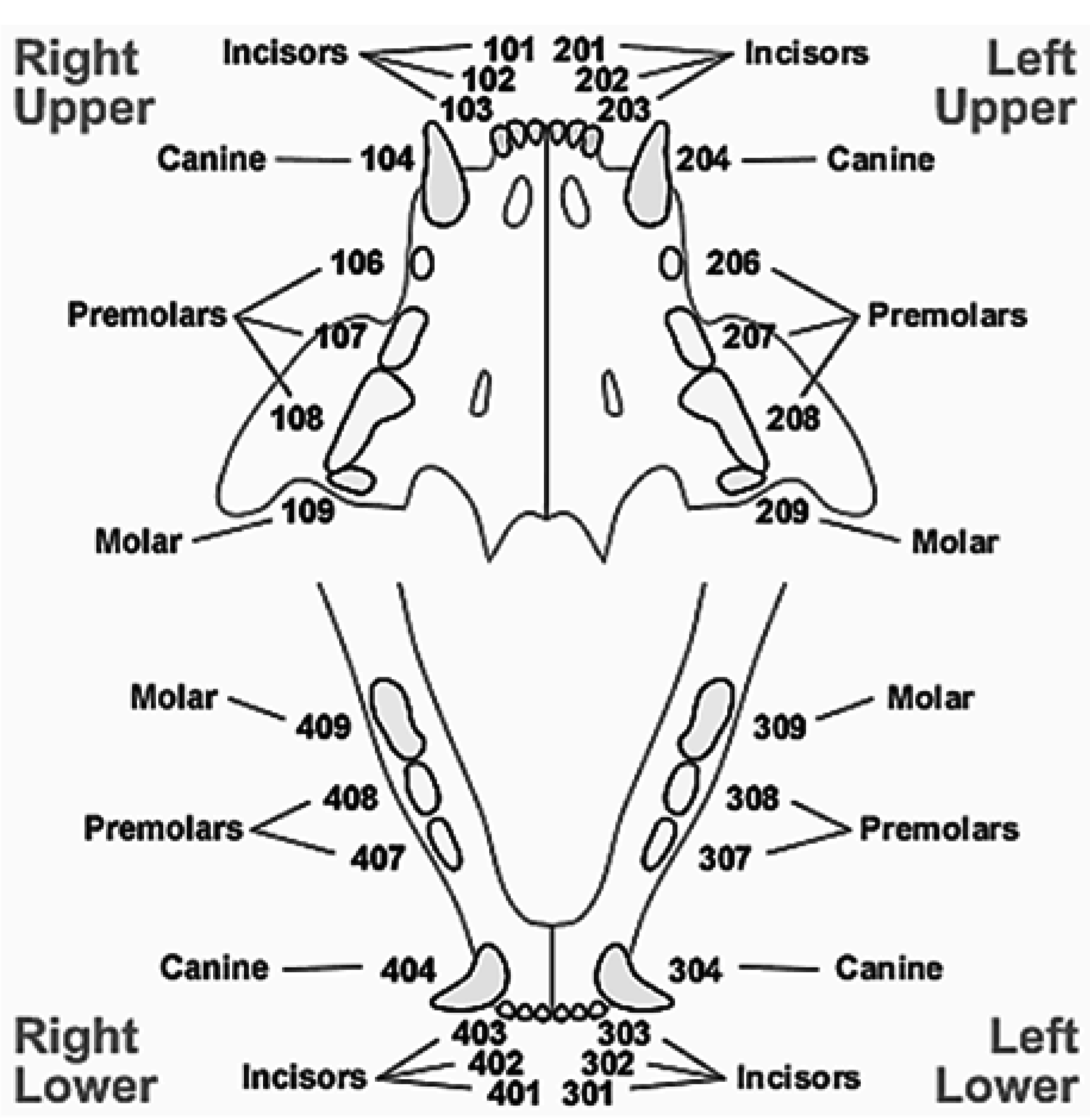
3. Results and Discussion
3.1. Results
| Variable | Parameter Estimation (β) | SE | |
|---|---|---|---|
| Threshold | THS6 | −3.391 | 1.408 |
| THS5 | −2.492 | 1.334 | |
| THS4 | −1.166 | 1.287 | |
| THS3 | 0.154 | 1.263 | |
| THS2 | 1.680 | 1.233 | |
| THS1 | 2.432 | 1.280 | |
| Teeth | Incisors | 1.948 | 2.184 |
| Canines | 0.767 | 1.777 | |
| Premolars | −2.993 | 1.927 | |
| Molars | 0 | ||
| Diet | Dry | −0.051 | 1.477 |
| Wet | −1.826 | 1.451 | |
| Dry + Wet | 1.686 | 0.618 | |
| Homemade | 0 | ||
| Incisors × Young | −0.121 | 1.7749 | |
| Incisors × Adult | 0.699 | 1.1050 | |
| Canines × Young | 0.150 | 1.3088 | |
| Canines × Adult | 0.091 | 0.9347 | |
| Premolars × Young | 2.402 | 1.3880 | |
| Premolars × Adult | 2.263 | 0.9023 | |
| Molars × Young | −0.477 | 1.5227 | |
| Molars × Adult | −2.035 | 1.2142 | |
| Young × Dry | 2.138 | 2.080 | |
| Young × Wet | 1.298 | 2.176 | |
| Young × Dry + Wet | −3.038 | 1.063 | |
| Adult × Dry | 3.010 | 1.511 | |
| Adult × Wet | 2.107 | 1.463 | |
| Incisors × Dry | 1.220 | 2.345 | |
| Incisors × Wet | 2.914 | 2.402 | |
| Incisors × Dry + Wet | −2.241 | 1.308 | |
| Canines × Dry | −0.175 | 1.833 | |
| Canines × Wet | 0.434 | 2.071 | |
| Canines × Dry + Wet | −1.898 | 0.938 | |
| Premolars × Dry | 2.553 | 2.061 | |
| Premolars × Wet | 2.125 | 2.045 | |
| Premolars × Dry + Wet | −0.864 | 0.625 | |
| Incisors × Young × Dry | −1.440 | 2.945 | |
| Incisors × Young × Wet | −1.593 | 2.963 | |
| Incisors × Young × Dry + Wet | 5.461 | 2.191 | |
| Incisors × Adult × Dry | −2.942 | 2.489 | |
| Incisors × Adult × Wet | −5.459 | 2.543 | |
| Canines × Young × Dry | 0.729 | 2.089 | |
| Canines × Young × Wet | −0.255 | 2.481 | |
| Canines × Young × Dry + Wet | 0.505 | 1.227 | |
| Canines × Adult × Dry | −0.755 | 1.916 | |
| Canines × Adult × Wet | −2.343 | 2.136 | |
| Premolars × Young × Dry | −1.768 | 2.581 | |
| Premolars × Young × Wet | −1.226 | 2.628 | |
| Premolars × Young × Dry + Wet | 0.978 | 1.387 | |
| Premolars ×Adult × Dry | −3.023 | 2.075 | |
| Premolars × Adult × Wet | −3.016 | 2.060 | |
| Variables | Probabilities for the Different THS Scores | |||||||
|---|---|---|---|---|---|---|---|---|
| Teeth | Age | Diet | 6 | 5 | 4 | 3 | 2 | 1 |
| incisors | adult | dry | 0.001 | 0.002 | 0.006 | 0.023 | 0.099 | 0.190 |
| incisors | young | dry | 0.001 | 0.002 | 0.008 | 0.028 | 0.118 | 0.221 |
| incisors | young | dry + wet | 0.001 | 0.002 | 0.008 | 0.028 | 0.118 | 0.221 |
| canines | young | dry | 0.001 | 0.002 | 0.009 | 0.032 | 0.133 | 0.245 |
| incisors | old | dry | 0.001 | 0.004 | 0.014 | 0.049 | 0.192 | 0.335 |
| incisors | old | wet | 0.002 | 0.004 | 0.015 | 0.053 | 0.205 | 0.353 |
| canines | adult | dry | 0.002 | 0.005 | 0.017 | 0.061 | 0.230 | 0.388 |
| incisors | adult | homemade | 0.002 | 0.006 | 0.022 | 0.076 | 0.275 | 0.446 |
| incisors | young | wet | 0.002 | 0.006 | 0.022 | 0.078 | 0.281 | 0.453 |
| premolars | young | dry | 0.003 | 0.008 | 0.031 | 0.106 | 0.354 | 0.538 |
| incisors | adult | dry + wet | 0.004 | 0.010 | 0.037 | 0.126 | 0.398 | 0.584 |
| incisors | young | homemade | 0.005 | 0.013 | 0.048 | 0.158 | 0.463 | 0.647 |
| premolars | adult | dry | 0.006 | 0.014 | 0.051 | 0.167 | 0.480 | 0.662 |
| molars | old | dry + wet | 0.006 | 0.015 | 0.055 | 0.178 | 0.498 | 0.678 |
| molars | young | dry | 0.007 | 0.016 | 0.059 | 0.189 | 0.517 | 0.695 |
| incisors | old | dry + wet | 0.008 | 0.020 | 0.072 | 0.225 | 0.571 | 0.739 |
| molars | adult | dry | 0.013 | 0.032 | 0.110 | 0.316 | 0.680 | 0.819 |
| canines | young | homemade | 0.013 | 0.032 | 0.111 | 0.318 | 0.682 | 0.820 |
| canines | adult | homemade | 0.014 | 0.034 | 0.117 | 0.331 | 0.695 | 0.828 |
| canines | adult | dry + wet | 0.017 | 0.042 | 0.140 | 0.379 | 0.738 | 0.856 |
| canines | young | wet | 0.019 | 0.045 | 0.150 | 0.398 | 0.753 | 0.866 |
| canines | old | dry + wet | 0.019 | 0.045 | 0.152 | 0.401 | 0.755 | 0.867 |
| canines | old | dry | 0.019 | 0.046 | 0.154 | 0.404 | 0.757 | 0.869 |
| incisors | adult | wet | 0.022 | 0.053 | 0.175 | 0.443 | 0.785 | 0.886 |
| premolars | adult | dry + wet | 0.030 | 0.070 | 0.221 | 0.515 | 0.830 | 0.912 |
| molars | old | dry | 0.034 | 0.080 | 0.247 | 0.551 | 0.850 | 0.923 |
| premolars | young | wet | 0.040 | 0.094 | 0.280 | 0.592 | 0.870 | 0.934 |
| molars | adult | dry + wet | 0.046 | 0.105 | 0.306 | 0.623 | 0.884 | 0.942 |
| molars | young | homemade | 0.051 | 0.118 | 0.334 | 0.653 | 0.896 | 0.948 |
| premolars | old | dry | 0.052 | 0.119 | 0.337 | 0.656 | 0.898 | 0.949 |
| premolars | young | homemade | 0.057 | 0.130 | 0.360 | 0.678 | 0.906 | 0.954 |
| canines | old | wet | 0.059 | 0.134 | 0.368 | 0.685 | 0.909 | 0.955 |
| premolars | adult | homemade | 0.065 | 0.147 | 0.393 | 0.708 | 0.918 | 0.959 |
| canines | adult | wet | 0.068 | 0.152 | 0.402 | 0.716 | 0.921 | 0.961 |
| molars | young | wet | 0.084 | 0.184 | 0.460 | 0.761 | 0.936 | 0.969 |
| premolars | adult | wet | 0.114 | 0.24 | 0.543 | 0.817 | 0.953 | 0.978 |
| molars | adult | wet | 0.163 | 0.323 | 0.643 | 0.871 | 0.969 | 0.985 |
| molars | old | wet | 0.173 | 0.339 | 0.659 | 0.879 | 0.971 | 0.986 |
| canines | young | dry + wet | 0.173 | 0.340 | 0.660 | 0.879 | 0.971 | 0.986 |
| premolars | young | dry + wet | 0.173 | 0.340 | 0.660 | 0.879 | 0.971 | 0.986 |
| molars | young | dry + wet | 0.173 | 0.340 | 0.660 | 0.879 | 0.971 | 0.986 |
| molars | adult | homemade | 0.205 | 0.388 | 0.705 | 0.899 | 0.976 | 0.989 |
| premolars | old | dry + wet | 0.228 | 0.420 | 0.732 | 0.911 | 0.979 | 0.990 |
| premolars | old | wet | 0.332 | 0.550 | 0.822 | 0.945 | 0.988 | 0.994 |
3.2. Discussion
4. Conclusions
Acknowledgements
Conflicts of Interest
References
- Ingham, K.; Gorrel, C.; Blackburn, J.; Farnsworth, W. The effect of toothbrushing on periodontal disease in cats. J. Nutr. 2012, 132, 1740S–1741S. [Google Scholar]
- Verhaert, L.; van Wetter, C. Survey of oral diseases in cats in Flanders. Vlaams Diergeneeskd. Tijdschr. 2004, 73, 331–341. [Google Scholar]
- Southerden, P. Review of feline oral disease: 1 Periodontitis and chronic gingivostomatitis. Practice 2010, 32, 2–7. [Google Scholar] [CrossRef]
- Watson, A. Diet and periodontal disease in dogs and cats. Aust. Vet. J. 1994, 71, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Niemiec, B. Periodontal Disease. Top. Companion Anim. Med. 2008, 23, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.; Cameron, A. Relationship between diet, dental calculus and periodontal disease in domestic and feral cats in Australia. Aust. Vet. J. 1998, 76, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Mealey, B. Periodontal disease and diabetes. J. Am. Dent. Assoc. 2006, 137, 26S–31S. [Google Scholar] [CrossRef] [PubMed]
- Ramfjord, S. The periodontal disease index (PDI). J. Periodontol. 1967, 38, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Loe, H.; Silness, J. Periodontal disease in pregnancy. Acta Odontol. Scand. 1963, 21, 533–551. [Google Scholar] [CrossRef] [PubMed]
- Gawor, J.; Reiter, A.; Jodkowska, K.; Kurski, G.; Wojtacki, M.; Kurek, A. Influence of diet in oral health in cats and dogs. J. Nutr. 2006, 136, 2021S–2023S. [Google Scholar] [PubMed]
- Floyd, M.R. The modified Triadan system: Nomenclature for veterinary dentistry. J. Vet. Dent. 1991, 8, 18–19. [Google Scholar] [PubMed]
- Crossley, D. Veterinary Dentistry Basics; eMedia Unit. Royal Veterinary College: London, UK, 2002. Available online: http://www.rvc.ac.uk/review/Dentistry/Basics/intro.html (accessed on 11 February 2015).
- Vrieling, H.; Theyse, L.; van Winkelhoff, A.; Dijkshoorn, N.; Logan, E.; Picavet, P. Effectiveness of feeding large kibbles with mechanical cleaning properties in cats with gingivitis. Tijdschr. Diergeneeskd. 2005, 130, 136–140. [Google Scholar] [PubMed]
- Buckley, C.; Colyer, A.; Skrzywanek, M.; Jodkowska, K.; Kurski, G.; Gawor, J.; Ceregrzyn, M. The impact of home-prepared diets and home oral hygiene on oral health in cats and dogs. Br. J. Nutr. 2011, 106, S124–S127. [Google Scholar] [CrossRef] [PubMed]
- Gorrel, C.; Inskeep, G.; Inskeep, T. Benefits of a “dental hygiene chew” on the periodontal health of cats. J. Vet. Dent. 1998, 15, 135–138. [Google Scholar] [PubMed]
- Harley, R.; Gruffydd-Jones, T.; Day, M. Salivary and serum immunoglobulin levels in cats with chronic gingivostomatitis. Vet. Rec. 2003, 152, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Kornya, M.; Little, S.; Scherk, M.; Sears, W.; Bienzle, D. Association between oral health status and retrovirus test results in cats. J. Am. Vet. Med. Assoc. 2014, 245, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.; Hawthorne, A.; Colyer, A.; Stevenson, A. Effect of dietary water intake on urinary output, specific gravity and relative supersaturation for calcium oxalate and struvite in the cat. Br. J. Nutr. 2011, 106, S128–S130. [Google Scholar] [CrossRef] [PubMed]
- Thrall, B.; Miller, L. Water turnover in cats fed dry rations. Feline Pract. 1976, 6, 10–17. [Google Scholar]
- Zoran, D. The carnivore connection to nutrition in cats. J. Am. Vet. Med. Assoc.n 2002, 221, 1559–1567. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mata, F. The Choice of Diet Affects the Oral Health of the Domestic Cat. Animals 2015, 5, 101-109. https://doi.org/10.3390/ani5010101
Mata F. The Choice of Diet Affects the Oral Health of the Domestic Cat. Animals. 2015; 5(1):101-109. https://doi.org/10.3390/ani5010101
Chicago/Turabian StyleMata, Fernando. 2015. "The Choice of Diet Affects the Oral Health of the Domestic Cat" Animals 5, no. 1: 101-109. https://doi.org/10.3390/ani5010101
APA StyleMata, F. (2015). The Choice of Diet Affects the Oral Health of the Domestic Cat. Animals, 5(1), 101-109. https://doi.org/10.3390/ani5010101





