1. Introduction
Animal genetic resources are of strategic importance for ensuring national food security, promoting the sustainable development of the livestock industry, and safeguarding agricultural biodiversity [
1]. As a pivotal genetic resource for global animal protein production, broiler breeders see their economic value and conversion efficiency directly influenced by the stability of their production performance, making their conservation and efficient utilization is a central challenge in modern animal husbandry [
2]. In commercial production, however, egg production and egg quality decline as age [
3], a process often exacerbated by the compromised antioxidant capacity of the birds due to accumulated reactive oxygen species (ROS) and free radicals [
4]. Therefore, extending the productive lifespan of high-performing individuals represents a key strategy in animal production for maximizing their genetic potential and economic value, ultimately promoting the efficient utilization of elite genetic resources [
5,
6,
7].
Induced molting is a pivotal management technique employed to rejuvenate aging broiler breeders, enabling a rapid recovery of physical condition and reproductive function to initiate a second highly productive laying cycle [
8]. Nevertheless, this process itself induces significant physiological stress and can compromise intestinal health, creating a critical need for effective post-molting strategies. The post-molting period is thus a decisive stage for the determining the success of recovery in terms of subsequent egg production, fertilization rate, hatchability and other aspects [
9]. Given the physiological challenges of this period, nutritional interventions that can mitigate stress and support recovery are of great interest.
Probiotics, recognized as safe and sustainable feed additives, have consequently garnered significant research interest and now widely applied in livestock and poultry production. Probiotics contribute to poultry health by modulating the gut microbiota, protecting gastrointestinal health, enhancing immunity, and consequently promoting growth performance [
10]. Specific strains relevant to this study have demonstrated considerable benefits. For example,
Bacillus subtilis can improve the production performance of broiler breeders, enhance egg quality and reproductive performance, strengthen immune function, and maintain the stability of intestinal microbiota [
11]. Dietary supplementation with
Bacillus licheniformis exerts beneficial effects on laying hens, including improved egg quality, enhanced intestinal health, and reinforced antioxidant and immune functions [
12].
Clostridium butyricum plays a crucial role in broiler production. Its primary metabolite, butyric acid, helps maintain intestinal morphological integrity, improve growth performance, and enhance antioxidant capacity and immunity [
13].
Enterococcus faecalis is beneficial for optimizing the cecal microbiota structure of broiler breeders and improving serum biochemical indices [
14]. Compound probiotic preparations are processed from a combination of multiple probiotic strains, which can integrate the probiotic functions of each strain and synergistically promote animal growth and production performance [
15]. Indeed, recent studies have indicated that compound probiotic can improve the production performance and immunity of broiler breeders [
16]. However, while the general benefits of probiotics are established, their efficacy in mitigating the specific stresses of the post-molting period in aging broiler breeders, particularly concerning systemic and reproductive tract antioxidant capacity, remains less systematically investigated. Therefore, this study aimed to investigate the effects of a compound probiotic preparation—composed of
Bacillus subtilis,
Bacillus licheniformis,
Clostridium butyricum, and
Enterococcus faecalis—supplemented via drinking water on the production performance, antioxidant status, and key health parameters of broiler breeders during the post-molting period. The findings aim to provide a scientific basis for the application of this probiotic strategy, thereby evaluating its potential not only to enhance immediate production metrics but also to support the conservation of valuable genetic resources by extending the productive lifespan of high-performing flocks. The findings offer a practical strategy to enhance production performance and support genetic resource conservation by extending the productive lifespan of elite flocks.
4. Discussion
Currently, substantial research has been conducted on the effects of probiotics on animal production performance. The compound probiotic preparation utilized in this study consisted of
Bacillus subtilis,
Bacillus licheniformis,
Clostridium butyricum, and
Enterococcus faecalis, which function synergistically. In contrast to previous studies that predominantly focused on single-strain probiotics or dietary supplementation methods [
21,
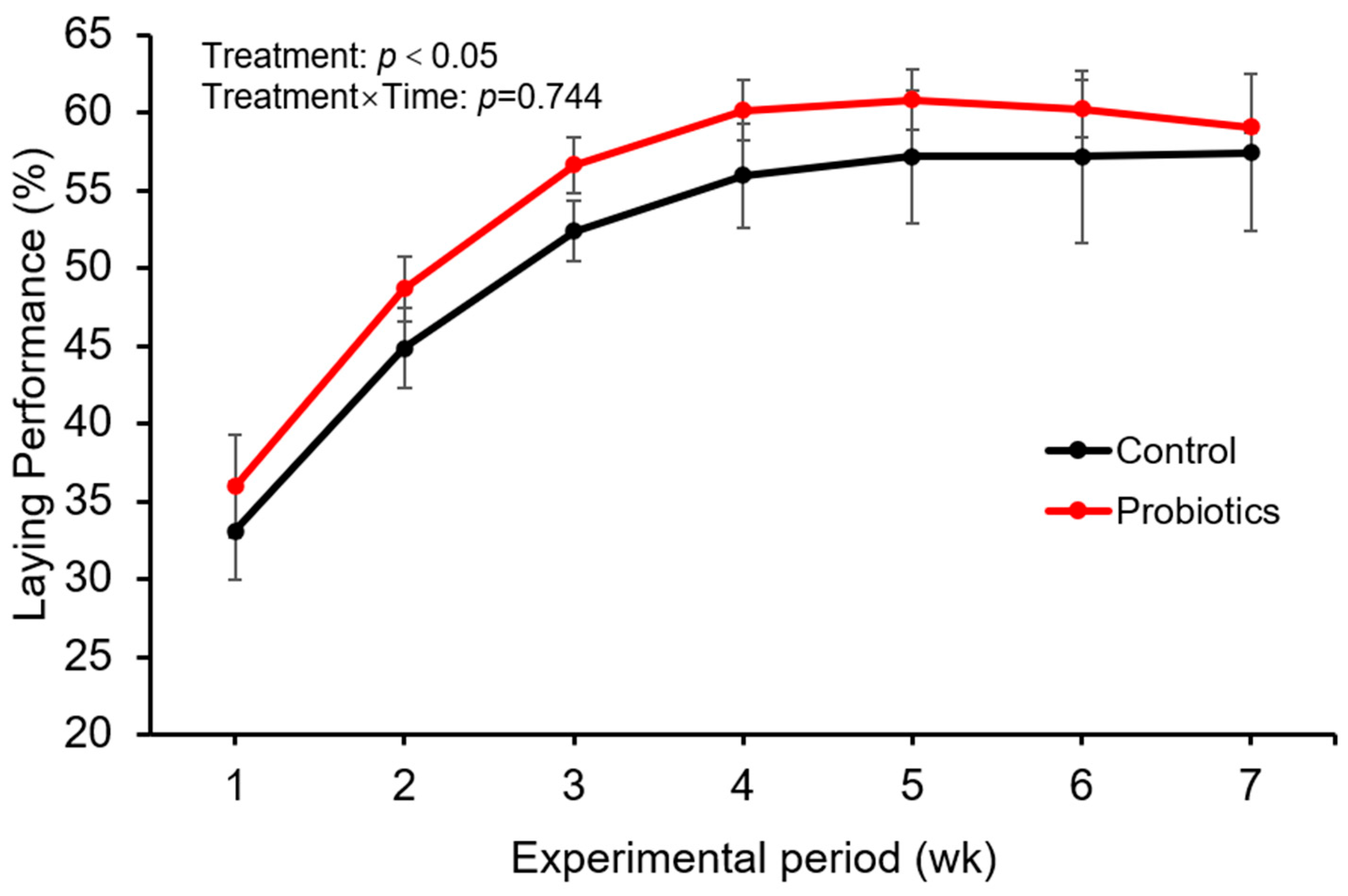
22], the present study innovatively evaluated a multi-strain probiotic complex administered via drinking water. During the critical stress period of post-molting, this intervention demonstrated considerable potential for enhancing the overall performance of broiler breeders, providing a practical and effective nutritional strategy. The results indicated that supplementing drinking water with the compound probiotic preparation significantly improved the laying rate of broiler breeders during the post-molting period, thereby enhancing production performance. These findings are consistent with earlier reports by Salem et al. [
23] and Zhao et al. [
24] The underlying mechanisms may involve, but are not limited to, enhanced nutrient digestion and improved gut health. For instance,
Bacillus subtilis and
Bacillus licheniformis can produce a variety of digestive enzymes that promote nutrient digestion and absorption [
25,
26]. Meanwhile, the inclusion of
Clostridium butyricum and
Enterococcus faecalis synergistically enhanced this effect by complementing the spectrum of digestive enzymes [
27]. Additionally, the compound probiotic preparation may improve intestinal development and maintain gut health through multiple pathways, ultimately enhancing nutrient utilization and production performance.
Optimal reproductive performance during the post-molting period is crucial for economic returns in broiler breeders. This study revealed that supplementation with the compound probiotic preparation significantly increased the fertilization rate and hatching rate of broiler breeders, and significantly improved the intestinal index of offspring chicks. These results are consistent with those reported by Lebedev et al. [
28] and Qu et al. [
29] The improvement in fertilization rate may be attributed to probiotic-induced regulation of reproductive hormone secretion and an enhanced follicular development microenvironment [
30]. The significant improvement in hatchability observed in this study largely reflects the direct result of the enhanced fertility rate. This is a key finding as it directly correlates with economic returns. However, it is also acknowledged that hatchability is a composite metric, jointly influenced by both fertilization success and the survival rate of embryos in fertilized eggs. Therefore, hatchability holds indispensable practical value as a key performance indicator for assessing overall production efficiency. Furthermore, the probiotic may have improved male fertility. The anti-inflammatory and antioxidant effects conferred to the hens could have been transferred to the roosters via direct contact during natural mating or via the environment, potentially improving semen quality, sperm motility, and viability by reducing oxidative damage to sperm cells. The enhancement likely involved improved female reproductive efficiency. The significantly improved antioxidant capacity in the oviduct (increased SOD and T-AOC, decreased MDA) would create a more favorable microenvironment for sperm storage, survival, and function within the hen’s sperm storage tubules (SSTs). This could prolong sperm viability and increase the likelihood of successful fertilization. Therefore, the probiotic’s effect on fertility is likely mediated through a combination of improved sperm quality and an optimized oviductal environment for fertilization.
A notable finding of this study was the significant reduction in serum TP and GLB levels in the probiotic-supplemented group. While serum albumin concentration remained unchanged, the decrease in GLB, may indicate an alleviation of systemic inflammatory status [
31]. This potential mitigation of inflammation could be attributed to the anti-inflammatory properties of probiotic-derived metabolites, such as butyrate [
32].
Age-related structural changes in hepatocytes can exacerbate oxidative stress. In aging laying hens, the level of ROS increases with age, while the capacity of the endogenous antioxidant system to neutralize free radicals and peroxides declines [
33]. This study revealed that supplementing drinking water with the compound probiotic preparation significantly improved systemic and tissue antioxidant status, as evidenced by increased T-AOC and T-SOD activities in serum, liver, ovary, and oviduct, with a concomitant trend toward reduction in MDA levels, and elevated serum IgM levels in post-molting broiler breeders. These observations are consistent with the findings of Bai et al. [
34], Zhao et al. [
35], and Zhang et al. [
36] The collective improvement in antioxidant parameters across serum, liver, and reproductive organs indicates that probiotic supplementation effectively alleviated oxidative stress during the post-molting period. T-AOC serves as a comprehensive biomarker reflecting the non-specific and integrated antioxidant defense status in animals [
37]. The observed enhancement in T-AOC indicates that the probiotic supplementation conferred a systemic, broad-spectrum antioxidant benefit. This effect may be mediated through indirect mechanisms involving the modulation of gut microbiota homeostasis by the compound probiotic preparation, leading to reduced production of endogenous oxidants. Concurrently, specific probiotic strains contributed via distinct pathways:
Bacillus subtilis directly eliminated free radicals through the production of antioxidant enzymes such as superoxide dismutase and catalase [
38], while
Bacillus licheniformis upregulated the Nrf2 signaling pathway to enhance the expression of antioxidant enzymes [
39,
40]. Moreover, butyrate, produced by
Clostridium butyricum, likely played a pivotal role by promoting the transcription of antioxidant-related genes via epigenetic regulation and activating the Nrf2/ARE signaling pathway, thereby upregulating downstream antioxidant enzymes [
41,
42,
43]. The significant increase in serum IgM levels suggests that the probiotics may enhance antioxidant status indirectly through immunomodulation. It is well established that appropriate immune activation can strengthen the body’s antioxidant defense capacity, whereas excessive immune responses may aggravate oxidative stress [
44]. The moderate elevation in IgM observed in this study likely reflects a beneficial immunomodulatory effect induced by the probiotic supplementation. Although the present study confirmed the antioxidant benefits of the compound probiotic, the precise underlying pathways remain to be fully elucidated. Future studies should employ integrated transcriptomic and metabolomic approaches (e.g., RNA-seq and LC-MS/GC-MS, respectively) to clarify the molecular mechanisms by which these probiotics regulate the antioxidant system, with particular emphasis on the synergistic interactions among the constituent bacterial strains.
The cecum serves as the primary site for microbial fermentation in poultry and plays a crucial role in SCFA production [
45]. SCFAs function as key mediators of host-microbiota crosstalk, providing energy to the host and promoting overall health [
46]. This study found that supplementation with the compound probiotic preparation during the post-molting period significantly increased the concentrations of SCFAs such as butyrate and isobutyrate in the cecum of broiler breeders. This result is in agreement with reports by Xu et al. [
47] and Śliżewska et al. [
48] Butyrate, an essential microbial metabolite [
49], is critical for maintaining intestinal barrier integrity and functions as an important signaling molecule with anti-inflammatory and epigenetic regulatory properties. The notable increase in cecal butyrate levels is likely due to probiotic-induced enhancement in the abundance and metabolic activity of butyrate-producing bacteria within the cecal microbiota [
50]. Previous studies have demonstrated that
Clostridium butyricum directly synthesizes butyrate and promotes the proliferation of other butyrate-producing bacteria via cross-feeding mechanisms [
51,
52].
Enterococcus faecalis produces lactate, a metabolic precursor that fuels the growth and butyrate-synthesizing activity of butyrate-producing bacteria through cross-feeding [
53,
54]. Furthermore,
Bacillus subtilis and
Bacillus licheniformis improve the intestinal environment, thereby facilitating the colonization and metabolic function of
Clostridium butyricum [
55,
56]. Therefore, the efficacy of the composite probiotic arises not only from the additive effects of individual strains but also from bacterial interactions that collectively activate the endogenous butyrate-producing metabolic network. Consistent with Zeng et al. [
57] but contrasting with Zhang et al. [
58], probiotic supplementation did not significantly alter the concentrations of other cecal SCFAs, such as acetate and propionate. These discrepancies may be attributed to differences in animal physiological stage, probiotic strain specificity, and dosage. This suggests that the compound probiotics modulate microbial metabolism in a highly specific manner, and the selective stimulation of butyrate production underlies their unique efficacy.
However, a potential limitation of this study is the lack of quantitative assessment of the dynamic survival rate of probiotics within the drinking system. The significant physiological improvements observed provide strong indirect evidence that viable probiotics were successfully delivered and functionally active. Furthermore, the probiotic product used in this study exhibited high stability (100% solubility and stable viable counts ≥ 1010 CFU/g), and the inclusion of Bacillusstrains in spore form ensured strong environmental tolerance. Combined with strict water quality control and fresh preparation practices, these factors collectively supported the effective delivery of probiotics. Future studies should incorporate regular monitoring of viable bacterial counts at the endpoint of drinking lines to more precisely quantify the actual dosage ingested by the birds.