Effects of Dietary Vitamin C Supplementation on Vitamin C Synthesis, Transport, and Egg Deposition in Breeding Geese
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds and Experimental Design
2.2. Sample Collection and Preparations
2.3. Measurement of Vitamin C Content in Serum and Produced Eggs
2.4. Quantitative Real-Time PCR
2.5. Statistical Analysis
3. Results
3.1. The Concentration of Vitamin C in Breeding Eggs and Serum
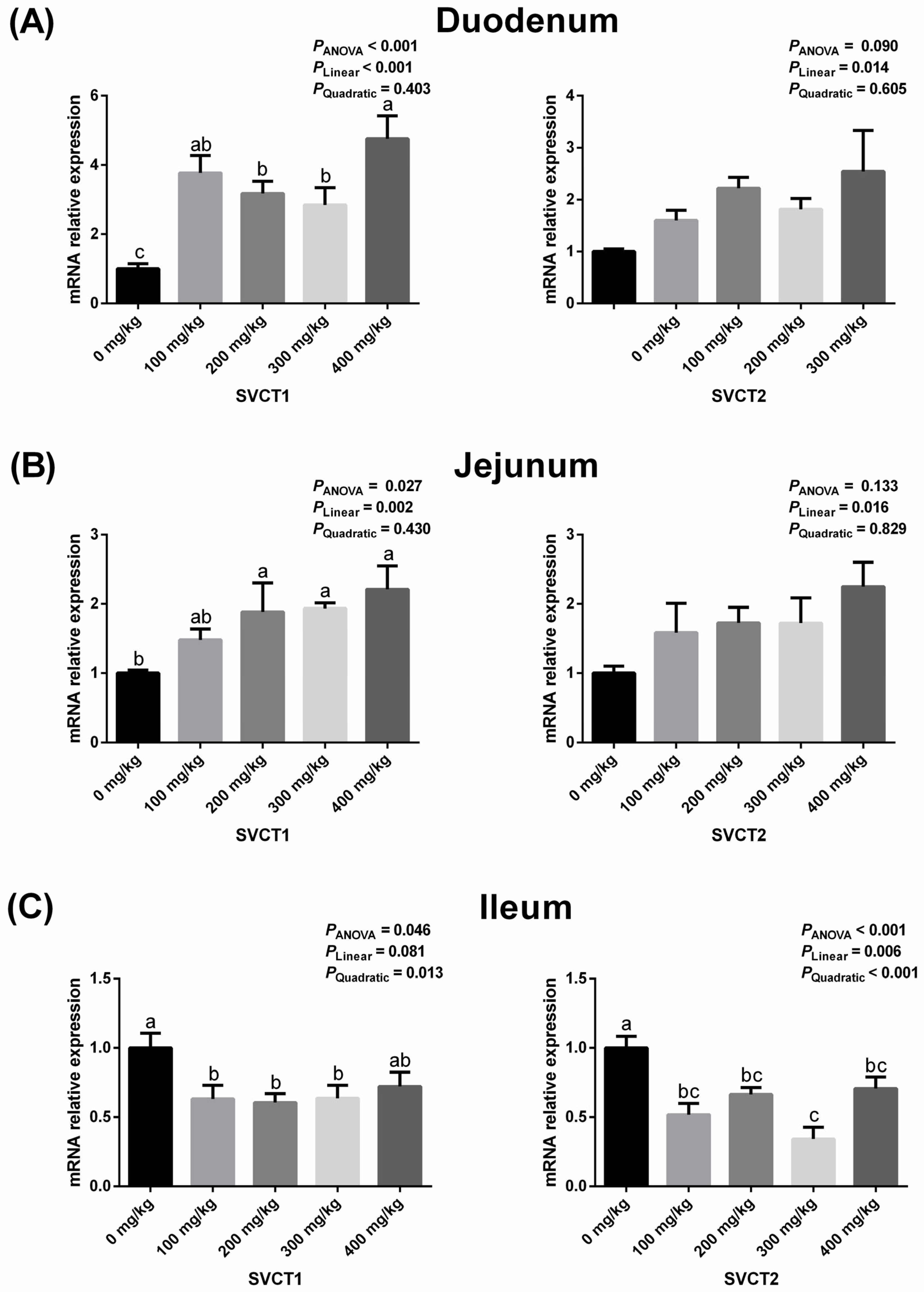
3.2. Intestinal Expressions of SVCT1 and SVCT2 Related to Vitamin C Absorption
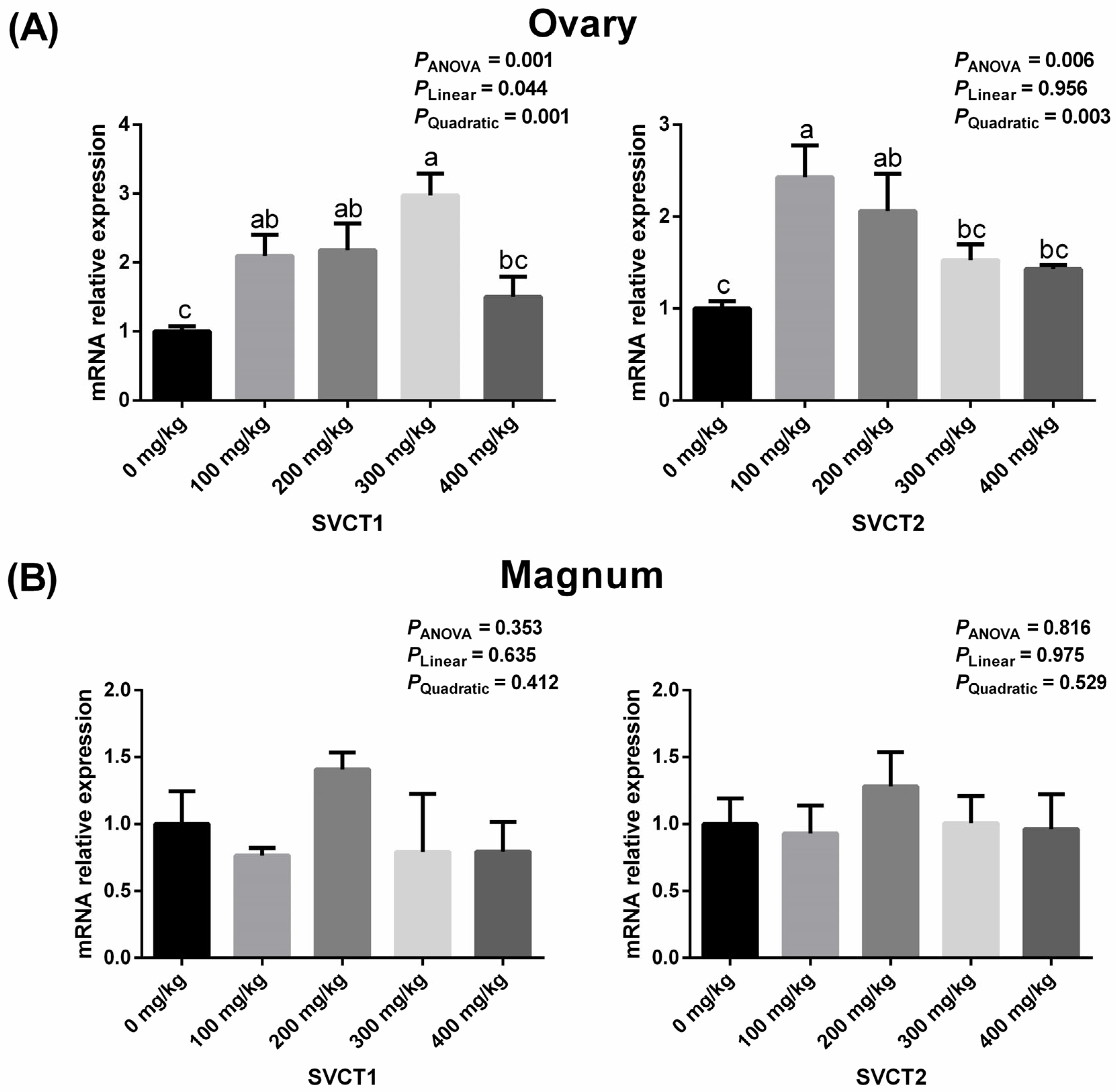
3.3. Ovarian and Magnum Expressions of SVCT1 and SVCT2 Related to Vitamin C Transport
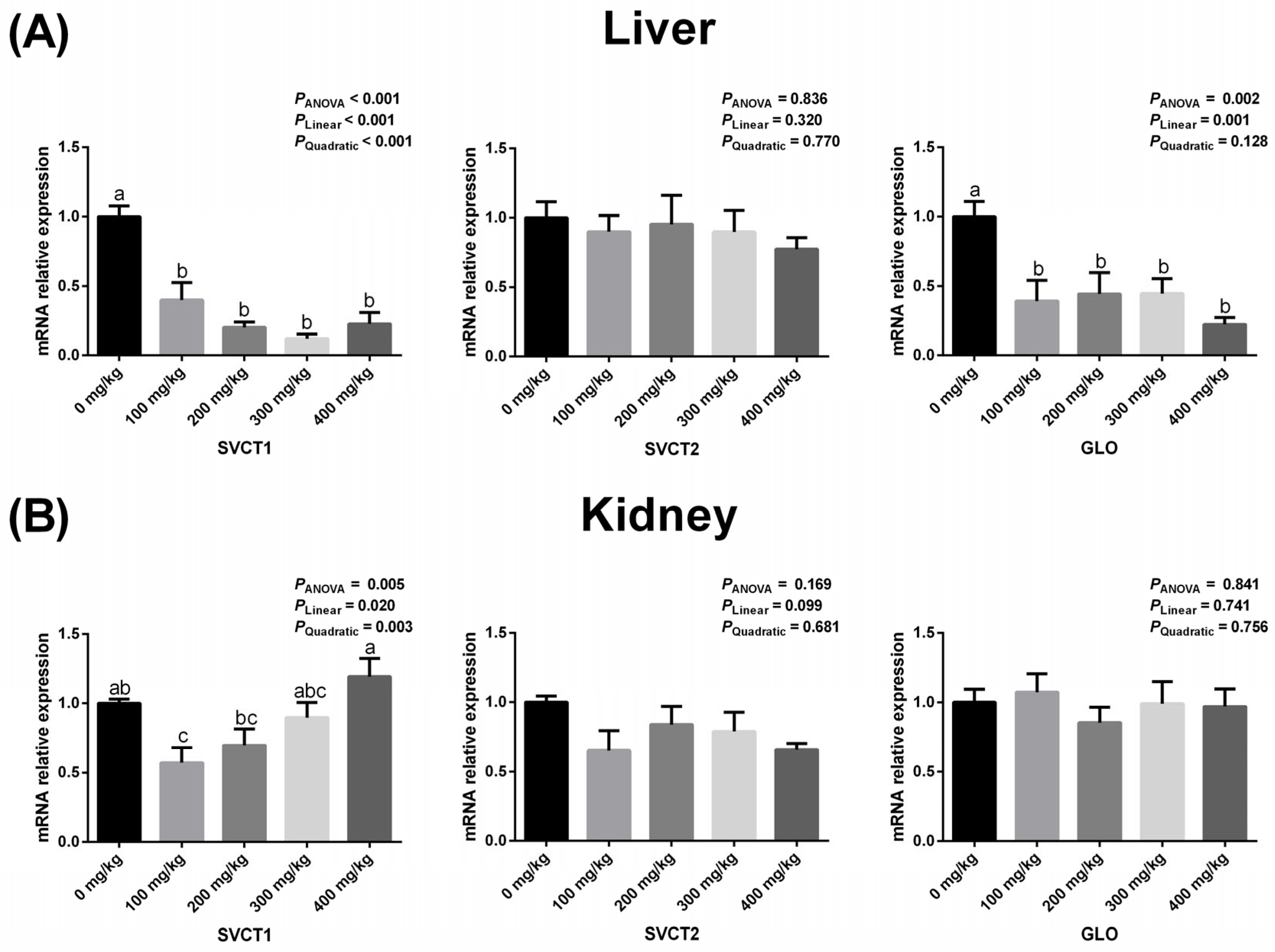
3.4. Hepatic and Renal Expressions of GLO, SVCT1, and SVCT2 Related to Vitamin C Synthesis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GLO | L-gulonolactone oxidase |
| SEM | Standard error of the mean. |
| SVCT1 | Sodium-dependent vitamin C transporter 1 |
| SVCT2 | Sodium-dependent vitamin C transporter 2 |
References
- Monacelli, F.; Acquarone, E.; Giannotti, C.; Borghi, R.; Nencioni, A. Vitamin C, aging and Alzheimer’s disease. Nutrients 2017, 9, 670. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Wang, J.; Yang, Z.; Wang, J.; Wang, Z.Y.; Yang, H.M. Effects of vitamin C on reproductive performance, egg quality and immune function of breeding geese. Chin. J. Anim. Nutr. 2025, 37, 4556–4563. [Google Scholar]
- Kuo, S.M.; Tan, C.H.; Dragan, M.; Wilson, J.X. Endotoxin increases ascorbate recycling and concentration in mouse liver. J. Nutr. 2005, 135, 2411–2416. [Google Scholar] [CrossRef]
- Corpe, C.P.; Tu, H.; Eck, P.; Wang, J.; Faulhaber-Walter, R.; Schnermann, J.; Margolis, S.; Padayatty, S.; Sun, H.; Wang, Y.; et al. Vitamin C transporter Slc23a1 links renal reabsorption, vitamin C tissue accumulation, and perinatal survival in mice. J. Clin. Invest. 2010, 120, 1069–1083. [Google Scholar] [CrossRef]
- Linster, C.L.; Van Schaftingen, E. Vitamin C: Biosynthesis, recycling and degradation in mammals. FEBS J. 2007, 274, 1–22. [Google Scholar] [CrossRef]
- Hooper, C.L.; Maurice, D.V.; Lightsey, S.F.; Toler, J.E. Factors affecting ascorbic acid (AsA) biosynthesis in chickens. II. Effect of dietary AsA and strain of chicken. J. Anim. Physiol. Anim. Nutr. 2002, 86, 326–332. [Google Scholar] [CrossRef]
- Maurice, D.V.; Lightsey, S.F.; Abudabos, A.; Toler, J.E. Factors affecting ascorbic acid biosynthesis in chickens: III. Effect of dietary fluoride on L-gulonolactone oxidase activity and tissue ascorbic acid (AsA) concentration. J. Anim. Physiol. Anim. Nutr. 2002, 86, 383–388. [Google Scholar] [CrossRef]
- Gan, L.; Fan, H.; Nie, W.; Guo, Y. Ascorbic acid synthesis and transportation capacity in old laying hens and the effects of dietary supplementation with ascorbic acid. J. Anim. Sci. Biotechnol. 2018, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Guo, W.; Zhao, J.; Qin, K.; Yan, J.; Huang, X.; Ren, Z.; Yang, X.; Liu, Y.; Yang, X. Alterations on vitamin C synthesis and transportation and egg deposition induced by dietary vitamin C supplementation in Hy-Line Brown layer model. Anim. Nut. 2021, 7, 973–980. [Google Scholar] [CrossRef]
- Ahmed, W.; Ahmad, S.; Ahsan, U.; Kamran, Z. Response of laying hens to vitamin C supplementation through drinking water under sub-tropical conditions. Avian Biol. Res. 2008, 1, 59–63. [Google Scholar] [CrossRef]
- Kucuk, O.; Sahin, N.; Sahin, K.; Gursu, M.F.; Gulcu, F.; Ozcelik, M.; Issi, M. Egg production, egg quality, and lipid peroxidation status in laying hens maintained at a low ambient temperature (6 °C) and fed a vitamin C and vitamin E-supplemented diet. Veterinární Medicína 2003, 48, 33–40. [Google Scholar] [CrossRef]
- Panda, A.K.; Ramarao, S.V.; Raju, M.V.L.N.; Chatterjee, R.N. Effect of dietary supplementation with vitamins E and C on production performance, immune responses and antioxidant status of White Leghorn layers under tropical summer conditions. Br. Poult. Sci. 2008, 49, 592–599. [Google Scholar] [CrossRef]
- Ajakaiye, J.J.; Pérez, B.A.; Mollineda, T.A. Effects of high temperature on production in layer chickens supplemented with vitamins C and E. Rev. MVZ Córdoba 2011, 16, 2283–2291. [Google Scholar]
- Al-Khalaifah, H.; Kamel, N.N.; Gabr, S.; Gouda, A. The Synergistic Effect of vitamin C supplementation and early feed withdrawal on heat stress mitigation in broiler chickens. Animals 2025, 15, 2996. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Tveden-Nyborg, P. The pharmacokinetics of vitamin C. Nutrients 2019, 11, 2412. [Google Scholar] [CrossRef] [PubMed]
- Savini, I.; Rossi, A.; Pierro, C.; Avigliano, L.; Catani, M.V. SVCT1 and SVCT2: Key proteins for vitamin C uptake. Amino Acids 2008, 34, 347–355. [Google Scholar] [CrossRef]
- Eck, P.; Kwon, O.; Chen, S.; Mian, O.; Levine, M. The human sodium-dependent ascorbic acid transporters SLC23A1 and SLC23A2 do not mediate ascorbic acid release in the proximal renal epithelial cell. Physiol. Rep. 2013, 1, e00136. [Google Scholar] [CrossRef]
- Yang, H.M.; Wang, Y.; Wang, Z.Y.; Wang, X.X. Seasonal and photoperiodic regulation of reproductive hormones and related genes in Yangzhou geese. Poult. Sci. 2017, 96, 486–490. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Feng, Y.; Mei, H.; Dai, Z.; Guo, B.; He, B.; Zhu, H. Transcriptomic insights into photoperiodic regulation of goose reproduction: Galanin emerges as a potential player. Poult. Sci. 2025, 104, 105585. [Google Scholar] [CrossRef] [PubMed]
- Ndunguru, S.F.; Reda, G.K.; Csernus, B.; Knop, R.; Gulyás, G.; Szabó, C.; Czeglédi, L.; Lendvai, Á.Z. Embryonic methionine triggers post-natal developmental programming in Japanese quail. J. Comp. Physiol. B 2024, 194, 179–189. [Google Scholar] [CrossRef]
- Wang, G.; Deng, H.; Wang, T.; Zheng, X. Nutritional supplementation of breeding hens may promote embryonic development through the growth hormone-insulin like growth factor axis. Poult. Sci. 2024, 103, 103945. [Google Scholar] [CrossRef]
- van Der Wagt, I.; de Jong, I.C.; Mitchell, M.A.; Molenaar, R.; van Den Brand, H. A review on yolk sac utilization in poultry. Poult. Sci. 2020, 99, 2162–2175. [Google Scholar] [CrossRef]
- Ravindran, V.; Abdollahi, M.R. Nutrition and digestive physiology of the broiler chick: State of the art and outlook. Animals 2021, 11, 2795. [Google Scholar] [CrossRef]
- Estienne, A.; Brossaud, A.; Reverchon, M.; Rame, C.; Froment, P.; Dupont, J. Adipokines expression and effects in oocyte maturation, fertilization and early embryo development: Lessons from mammals and birds. Int. J. Mol. Sci. 2020, 21, 3581. [Google Scholar] [CrossRef]
- Ma, C.; Wu, H. Ovulation and egg formation in the white Roman geese. Acta Vet. Zootech. Sin. 1996, 27, 32–49. [Google Scholar]
- Zhu, Y.F.; Li, S.Z.; Sun, Q.Z.; Yang, X.J. Effect of in ovo feeding of vitamin C on antioxidation and immune function of broiler chickens. Animals 2019, 13, 1927–1933. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.F.; Li, S.Z.; Duan, Y.L.; Ren, Z.Z.; Yang, X.; Yang, X.J. Effects of in ovo feeding of vitamin C on post-hatch performance, immune status and DNA methylation-related gene expression in broiler chickens. Br. J. Nutr. 2020, 124, 903–911. [Google Scholar] [CrossRef]
- Zhu, Y.F.; Zhao, J.F.; Wang, C.X.; Zhang, F.; Huang, X.H.; Ren, Z.Z.; Yang, X.; Liu, Y.L.; Yang, X.J. Exploring the effectiveness of in ovo feeding of vitamin C based on the embryonic vitamin C synthesis and absorption in broiler chickens. J. Animal Sci. Biotechnol. 2021, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (NRC). Nutrient Requirements of Poultry, 9th ed.; The National Academies Press: Washington, DC, USA, 1994. [Google Scholar]
- Fu, Z.; Zhong, T.; Wan, X.; Xu, L.; Yang, H.; Han, H.; Wang, Z. Effects of dietary vitamin E supplementation on reproductive performance, egg characteristics, antioxidant capacity, and immune status in breeding geese during the late laying period. Antioxidants 2022, 11, 2070. [Google Scholar] [CrossRef]
- Yu, J.; Yang, H.M.; Wang, J.; Chen, S.; Huang, Z.X.; Wang, J.; Wang, Z.Y. Effects of gossypol acetate on growth, serum biochemical parameters, and intestinal health of goslings. Poult. Sci. 2024, 103, 104025. [Google Scholar] [CrossRef]
- Fenech, M.; Amaya, I.; Valpuesta, V.; Botella, M.A. Vitamin C content in fruits: Biosynthesis and regulation. Front. Plant Sci. 2019, 9, 2006. [Google Scholar] [CrossRef] [PubMed]
- Furuita, H.; Ishida, T.; Suzuki, T.; Unuma, T.; Kurokawa, T.; Sugita, T.; Yamamoto, T. Vitamin content and quality of eggs produced by broodstock injected with vitamins C and E during artificial maturation in Japanese eel Anguilla japonica. Aquaculture 2009, 289, 334–339. [Google Scholar] [CrossRef]
- Amano, A.; Aigaki, T.; Maruyama, N.; Ishigami, A. Ascorbic acid depletion enhances expression of the sodium-dependent vitamin C transporters, SVCT1 and SVCT2, and uptake of ascorbic acid in livers of SMP30/GNL knockout mice. Arch. Biochem. Biophys. 2010, 496, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Gregoraszczuk, E.L.; Zajda, K.; Tekla, J.; Respekta, N.; Zdybał, P.; Such, A. Vitamin C supplementation had no side effect in non-cancer, but had anticancer properties in ovarian cancer cells. Int. J. Vitam. Nutr. Res. 2021, 91, 293–303. [Google Scholar] [CrossRef] [PubMed]
| Item | Content |
|---|---|
| Ingredient (%) | |
| Corn | 64 |
| Soybean meal | 22 |
| Rice husk | 6 |
| Wheat bran | 0.5 |
| Salt | 0.3 |
| Limestone | 5.1 |
| Calcium hydrogen phosphate | 0.9 |
| DL-Methionine | 0.2 |
| Premix 1 | 1 |
| Total | 100 |
| Nutrient levels 2 (%) | |
| Metabolizable energy (MJ/kg) | 11.08 |
| Crude protein | 14.74 |
| Crude fiber | 4.88 |
| Calcium | 2.17 |
| Total phosphorus | 0.49 |
| Methionine | 0.43 |
| Lysine | 0.74 |
| Gene Name | Primer Sequence (5′–3′) | Product Size (bp) | Accession Number |
|---|---|---|---|
| SVCT1 | F: GTCCATCGTCCTCATCGTCC | 133 | XM_048064718.1 |
| R: GCCAGGATGATTGGGAACA | |||
| SVCT2 | F: CCAGGTTGTCATGTGCTCCT | 124 | XM_048053964.1 |
| R: GGCTGGAAGAAGTGGATCC | |||
| GLO | F: CAGCGTCATCTACCAGGACC | 150 | XM_048079397.1 |
| R: AGAAGCGGTTGATCCAGCA | |||
| β-actin | F: GCACCCAGCACGATGAAAAT | 150 | XM_013174886.1 |
| R: GACAATGGAGGGTCCGGATT |
| Item | 0 mg/kg | 100 mg/kg | 200 mg/kg | 300 mg/kg | 400 mg/kg | SEM | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|
| ANOVA | Linear | Quadratic | |||||||
| Yolk | 11.83 b | 14.14 ab | 17.43 ab | 17.66 a | 17.46 ab | 0.719 | 0.019 | 0.0024 | 0.129 |
| Albumen | 12.56 | 13.48 | 14.13 | 17.12 | 15.60 | 0.802 | 0.421 | 0.099 | 0.708 |
| Serum | 1.38 c | 1.41 c | 2.02 b | 3.03 a | 1.93 b | 0.113 | <0.001 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Hu, Y.; Xu, R.; Zhou, Y.; Li, N.; Yang, H.; Wang, J.; Zhao, H.; Yu, J. Effects of Dietary Vitamin C Supplementation on Vitamin C Synthesis, Transport, and Egg Deposition in Breeding Geese. Animals 2026, 16, 148. https://doi.org/10.3390/ani16010148
Hu Y, Xu R, Zhou Y, Li N, Yang H, Wang J, Zhao H, Yu J. Effects of Dietary Vitamin C Supplementation on Vitamin C Synthesis, Transport, and Egg Deposition in Breeding Geese. Animals. 2026; 16(1):148. https://doi.org/10.3390/ani16010148
Chicago/Turabian StyleHu, Yanglei, Rong Xu, Yating Zhou, Ning Li, Haiming Yang, Jian Wang, Hongchang Zhao, and Jun Yu. 2026. "Effects of Dietary Vitamin C Supplementation on Vitamin C Synthesis, Transport, and Egg Deposition in Breeding Geese" Animals 16, no. 1: 148. https://doi.org/10.3390/ani16010148
APA StyleHu, Y., Xu, R., Zhou, Y., Li, N., Yang, H., Wang, J., Zhao, H., & Yu, J. (2026). Effects of Dietary Vitamin C Supplementation on Vitamin C Synthesis, Transport, and Egg Deposition in Breeding Geese. Animals, 16(1), 148. https://doi.org/10.3390/ani16010148





