Home-Cage Training for Non-Human Primates: An Opportunity to Reduce Stress and Study Natural Behavior in Neurophysiology Experiments
Simple Summary
Abstract
1. Balancing Animal Welfare and Scientific Validity: Managing Stress in Laboratory-Housed NHPs
2. The Evolution of Home-Cage-Based Methods for NHP Training
2.1. Historical and Technological Milestones in Non-Invasive Cognitive Testing Approaches for NHPs
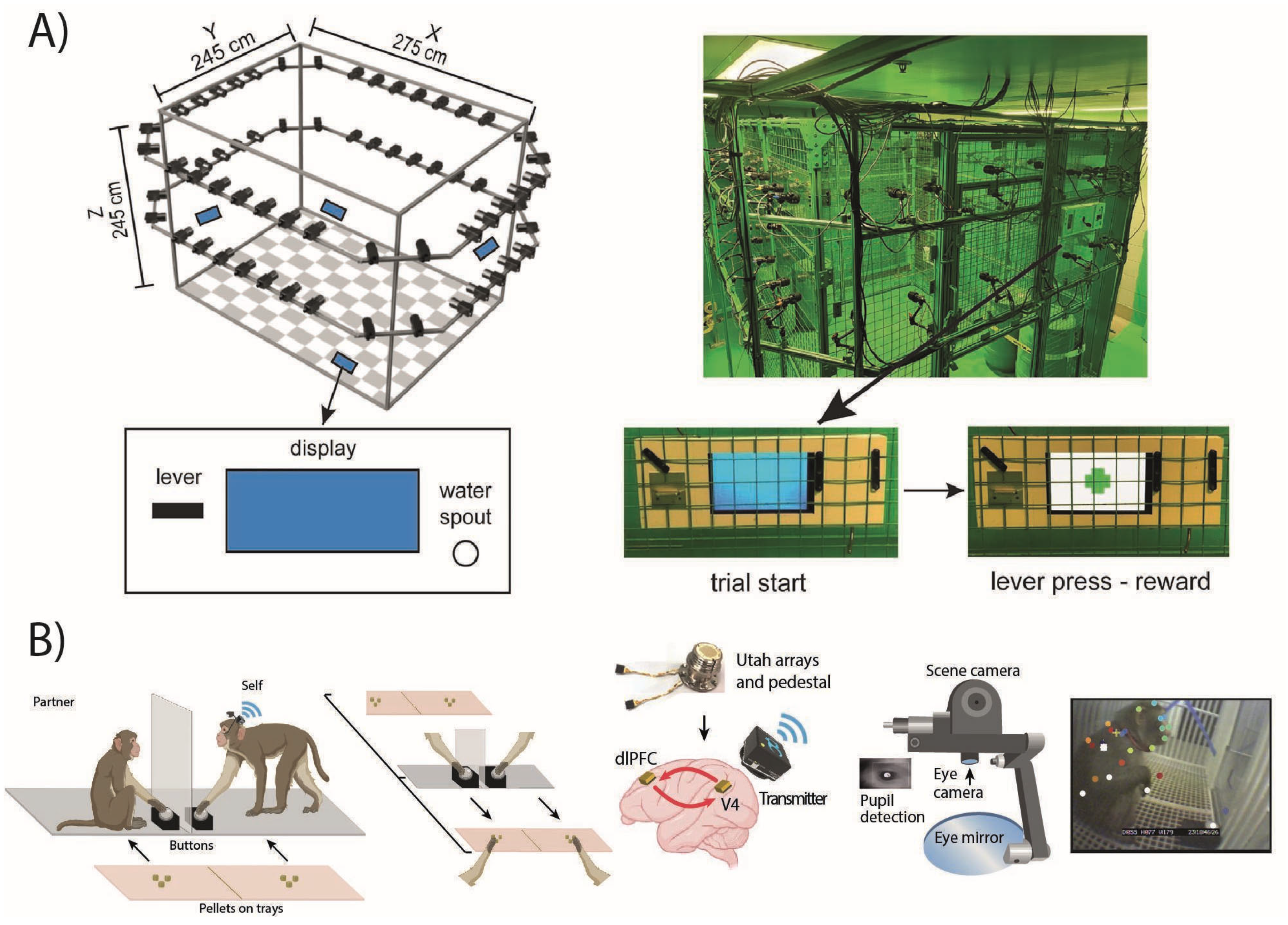
2.2. Home-Cage Systems: Key Aspects of the Current Proposed Approaches and Progressive Advancement Toward Automated Cognitive Testing
| Method Article | Task Devices | Reward System | Primate Species | Automated Step Training | Session Automatization | Primate Recognition | Required Component List |
|---|---|---|---|---|---|---|---|
| [87] Butler & Kennerley, 2019. | Android tablet | Liquid reward | Rhesus macaques | No | No | Face recognition | Yes |
| [81] Calapai et al., 2017. | Touchscreen | Liquid reward | Rhesus macaques | Yes | Yes | Manual post-session identification | No |
| [89] Curry et al., 2017. | Touchscreen | Liquid reward | Rhesus macaques | Yes | No | Separation from conspecifics | Yes |
| [93] Evans et al., 2008. | Monitor + Joystick | Solid reward | Tufted capuchin monkeys | Yes | No | Separation from conspecifics | Yes |
| [92] Fagot & Paleressompoulle, 2009. | Touchscreen | Solid reward | Guinea baboons | No | Yes | RFID | No |
| [88] Fizet et al., 2017. | Touchscreen | Solid reward | Rhesus macaques | Yes | Yes | RFID | No |
| [95] Sacchetti et al., 2022. | Touchscreen | Liquid reward | Rhesus macaques | Yes | No | Separation from conspecifics | No |
| [84] Womelsdorf et al., 2021. | Touchscreen | Solid and liquid reward | Rhesus macaques | Yes | No | Separation from conspecifics | Yes |
| [98] Scott et al., 2024. | Touchscreen | Liquid reward | Common marmosets | Yes | Yes | Separation from conspecifics | Yes |
| [91] Martin et al., 2022 | Touch-screen | Solid reward | Multiple macaque’s species | Yes | Yes | None | Yes |

3. Advancing Neurophysiology with Wireless Recording: Insights into Naturalistic Behavior
3.1. Wireless Recording Technologies in Behavioral Neurophysiology
3.2. From Restraint to Free-Moving Paradigms: Neural Correlates Across Behavioral Domains in NHPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique, Special ed., [Nachdr. der Ausg.] London 1959; Universities Federation for Animal Welfare: Potters Bar, UK, 1992; ISBN 978-0-900767-78-4. [Google Scholar]
- Selye, H. Stress without Distress. In Psychopathology of Human Adaptation; Serban, G., Ed.; Springer US: Boston, MA, USA, 1976; pp. 137–146. ISBN 978-1-4684-2240-5. [Google Scholar]
- Yerkes, R.M.; Dodson, J.D. The Relation of Strength of Stimulus to Rapidity of Habit-formation. J. Comp. Neurol. Psychol. 1908, 18, 459–482. [Google Scholar] [CrossRef]
- Diamond, D.M. Cognitive, Endocrine and Mechanistic Perspectives on Non-Linear Relationships between Arousal and Brain Function. Nonlinearity Biol. Toxicol. Med. 2005, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Luksys, G.; Sandi, C. Neural Mechanisms and Computations Underlying Stress Effects on Learning and Memory. Curr. Opin. Neurobiol. 2011, 21, 502–508. [Google Scholar] [CrossRef]
- Sandi, C. Stress and Cognition. WIRES Cogn. Sci. 2013, 4, 245–261. [Google Scholar] [CrossRef]
- Arnsten, A.F.T. Stress Signalling Pathways That Impair Prefrontal Cortex Structure and Function. Nat. Rev. Neurosci. 2009, 10, 410–422. [Google Scholar] [CrossRef]
- Clark, K.L.; Noudoost, B. The Role of Prefrontal Catecholamines in Attention and Working Memory. Front. Neural Circuits 2014, 8, 33. [Google Scholar] [CrossRef]
- Cools, R.; Arnsten, A.F.T. Neuromodulation of Prefrontal Cortex Cognitive Function in Primates: The Powerful Roles of Monoamines and Acetylcholine. Neuropsychopharmacology 2022, 47, 309–328. [Google Scholar] [CrossRef]
- Bouras, N.N.; Mack, N.R.; Gao, W.-J. Prefrontal Modulation of Anxiety through a Lens of Noradrenergic Signaling. Front. Syst. Neurosci. 2023, 17, 1173326. [Google Scholar] [CrossRef] [PubMed]
- Finlay, J.M.; Zigmond, M.J.; Abercrombie, E.D. Increased Dopamine and Norepinephrine Release in Medial Prefrontal Cortex Induced by Acute and Chronic Stress: Effects of Diazepam. Neuroscience 1995, 64, 619–628. [Google Scholar] [CrossRef]
- Goldstein, L.E.; Rasmusson, A.M.; Bunney, B.S.; Roth, R.H. Role of the Amygdala in the Coordination of Behavioral, Neuroendocrine, and Prefrontal Cortical Monoamine Responses to Psychological Stress in the Rat. J. Neurosci. 1996, 16, 4787–4798. [Google Scholar] [CrossRef]
- Arnsten, A.F.T. Stress Impairs Prefrontal Cortical Function in Rats and Monkeys: Role of Dopamine D1 and Norepinephrine α-1 Receptor Mechanisms. Prog. Brain Res. 2000, 126, 183–192. [Google Scholar] [CrossRef]
- Arnsten, A.F.T.; Goldman-Rakic, P.S. Noise Stress Impairs Prefrontal Cortical Cognitive Function in Monkeys: Evidence for a Hyperdopaminergic Mechanism. Arch. Gen. Psychiatry 1998, 55, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Li, B. Alpha-2 Adrenergic Modulation of Prefrontal Cortical Neuronal Activity Related to Spatial Working Memory in Monkeys. Neuropsychopharmacology 1999, 21, 601–610. [Google Scholar] [CrossRef]
- Birnbaum, S.G.; Yuan, P.X.; Wang, M.; Vijayraghavan, S.; Bloom, A.K.; Davis, D.J.; Gobeske, K.T.; Sweatt, J.D.; Manji, H.K.; Arnsten, A.F.T. Protein Kinase C Overactivity Impairs Prefrontal Cortical Regulation of Working Memory. Science 2004, 306, 882–884. [Google Scholar] [CrossRef]
- Vijayraghavan, S.; Wang, M.; Birnbaum, S.G.; Williams, G.V.; Arnsten, A.F.T. Inverted-U Dopamine D1 Receptor Actions on Prefrontal Neurons Engaged in Working Memory. Nat. Neurosci. 2007, 10, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Arnsten, A.F.T. Catecholamine Influences on Dorsolateral Prefrontal Cortical Networks. Biol. Psychiatry 2011, 69, e89–e99. [Google Scholar] [CrossRef]
- Datta, D.; Yang, S.-T.; Galvin, V.C.; Solder, J.; Luo, F.; Morozov, Y.M.; Arellano, J.; Duque, A.; Rakic, P.; Arnsten, A.F.T.; et al. Noradrenergic A1-Adrenoceptor Actions in the Primate Dorsolateral Prefrontal Cortex. J. Neurosci. 2019, 39, 2722–2734. [Google Scholar] [CrossRef] [PubMed]
- Shafiei, N.; Gray, M.; Viau, V.; Floresco, S.B. Acute Stress Induces Selective Alterations in Cost/Benefit Decision-Making. Neuropsychopharmacology 2012, 37, 2194–2209. [Google Scholar] [CrossRef]
- Butts, K.A.; Floresco, S.B.; Phillips, A.G. Acute Stress Impairs Set-Shifting but Not Reversal Learning. Behav. Brain Res. 2013, 252, 222–229. [Google Scholar] [CrossRef]
- Park, J.; Moghaddam, B. Impact of Anxiety on Prefrontal Cortex Encoding of Cognitive Flexibility. Neuroscience 2017, 345, 193–202. [Google Scholar] [CrossRef]
- Park, J.; Wood, J.; Bondi, C.; Del Arco, A.; Moghaddam, B. Anxiety Evokes Hypofrontality and Disrupts Rule-Relevant Encoding by Dorsomedial Prefrontal Cortex Neurons. J. Neurosci. 2016, 36, 3322–3335. [Google Scholar] [CrossRef] [PubMed]
- Major, A.J.; Vijayraghavan, S.; Everling, S. Cholinergic Overstimulation Attenuates Rule Selectivity in Macaque Prefrontal Cortex. J. Neurosci. 2018, 38, 1137–1150. [Google Scholar] [CrossRef]
- Bahari, Z.; Meftahi, G.H.; Meftahi, M.A. Dopamine Effects on Stress-Induced Working Memory Deficits. Behav. Pharmacol. 2018, 29, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Lugenbühl, J.F.; Viho, E.M.G.; Binder, E.B.; Daskalakis, N.P. Stress Molecular Signaling in Interaction with Cognition. Biol. Psychiatry 2025, 97, 349–358. [Google Scholar] [CrossRef]
- Page, C.E.; Coutellier, L. Prefrontal Excitatory/Inhibitory Balance in Stress and Emotional Disorders: Evidence for over-Inhibition. Neurosci. Biobehav. Rev. 2019, 105, 39–51. [Google Scholar] [CrossRef]
- Negrón-Oyarzo, I.; Aboitiz, F.; Fuentealba, P. Impaired Functional Connectivity in the Prefrontal Cortex: A Mechanism for Chronic Stress-Induced Neuropsychiatric Disorders. Neural Plast. 2016, 2016, 1–16. [Google Scholar] [CrossRef]
- Buchanan-Smith, H.M. Environmental Enrichment for Primates in Laboratories. Adv. Sci. Res. 2011, 5, 41–56. [Google Scholar] [CrossRef]
- Bethell, E.; Holmes, A.; MacLarnon, A.; Semple, S. Cognitive Bias in a Non-Human Primate: Husbandry Procedures Influence Cognitive Indicators of Psychological Well-Being in Captive Rhesus Macaques. Anim. Welf. 2012, 21, 185–195. [Google Scholar] [CrossRef]
- Bassett, L.; Buchanan-Smith, H.M.; McKinley, J.; Smith, T.E. Effects of Training on Stress-Related Behavior of the Common Marmoset (Callithrix Jacchus) in Relation to Coping With Routine Husbandry Procedures. J. Appl. Anim. Welf. Sci. 2003, 6, 221–233. [Google Scholar] [CrossRef]
- Laule, G.E.; Bloomsmith, M.A.; Schapiro, S.J. The Use of Positive Reinforcement Training Techniques to Enhance the Care, Management, and Welfare of Primates in the Laboratory. J. Appl. Anim. Welf. Sci. 2003, 6, 163–173. [Google Scholar] [CrossRef]
- Coleman, K.; Maier, A. The Use of Positive Reinforcement Training to Reduce Stereotypic Behavior in Rhesus Macaques. Appl. Anim. Behav. Sci. 2010, 124, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Kemp, C.; Thatcher, H.; Farningham, D.; Witham, C.; MacLarnon, A.; Holmes, A.; Semple, S.; Bethell, E.J. A Protocol for Training Group-Housed Rhesus Macaques (Macaca mulatta) to Cooperate with Husbandry and Research Procedures Using Positive Reinforcement. Appl. Anim. Behav. Sci. 2017, 197, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.; Wegener, D. Emphasizing the “Positive” in Positive Reinforcement: Using Nonbinary Rewarding for Training Monkeys on Cognitive Tasks. J. Neurophysiol. 2018, 120, 115–128. [Google Scholar] [CrossRef]
- McMillan, J.L.; Perlman, J.E.; Galvan, A.; Wichmann, T.; Bloomsmith, M.A. Refining the Pole-and-Collar Method of Restraint: Emphasizing the Use of Positive Training Techniques with Rhesus Macaques. J. Am. Assoc. Lab. Anim. Sci. 2014, 53, 61–68. [Google Scholar] [PubMed]
- Bliss-Moreau, E.; Theil, J.H.; Moadab, G. Efficient Cooperative Restraint Training With Rhesus Macaques. J. Appl. Anim. Welf. Sci. 2013, 16, 98–117. [Google Scholar] [CrossRef][Green Version]
- Mason, S.; Premereur, E.; Pelekanos, V.; Emberton, A.; Honess, P.; Mitchell, A.S. Effective Chair Training Methods for Neuroscience Research Involving Rhesus Macaques (Macaca mulatta). J. Neurosci. Methods 2019, 317, 82–93. [Google Scholar] [CrossRef]
- Hadj-Bouziane, F.; Monfardini, E.; Guedj, C.; Gardechaux, G.; Hynaux, C.; Farnè, A.; Meunier, M. The Helmet Head Restraint System: A Viable Solution for Resting State fMRI in Awake Monkeys. NeuroImage 2014, 86, 536–543. [Google Scholar] [CrossRef]
- Drucker, C.B.; Carlson, M.L.; Toda, K.; DeWind, N.K.; Platt, M.L. Non-Invasive Primate Head Restraint Using Thermoplastic Masks. J. Neurosci. Methods 2015, 253, 90–100. [Google Scholar] [CrossRef]
- Amemori, S.; Amemori, K.; Cantor, M.L.; Graybiel, A.M. A Non-Invasive Head-Holding Device for Chronic Neural Recordings in Awake Behaving Monkeys. J. Neurosci. Methods 2015, 240, 154–160. [Google Scholar] [CrossRef]
- Slater, H.; Milne, A.E.; Wilson, B.; Muers, R.S.; Balezeau, F.; Hunter, D.; Thiele, A.; Griffiths, T.D.; Petkov, C.I. Individually Customisable Non-Invasive Head Immobilisation System for Non-Human Primates with an Option for Voluntary Engagement. J. Neurosci. Methods 2016, 269, 46–60. [Google Scholar] [CrossRef]
- Rima, S.; Greilsamer, J.; Haag, M.; Cadena-Valencia, J.; Sansonnens, M.; Francovich, A.; Lanz, F.; Zbinden, A.; Bergadano, A.; Schmid, M.C. A Chinrest-Based Approach to Measure Eye Movements and Experimental Task Engagement in Macaques with Minimal Restraint. J. Neurosci. Methods 2024, 408, 110173. [Google Scholar] [CrossRef] [PubMed]
- Ponce, C.R.; Genecin, M.P.; Perez-Melara, G.; Livingstone, M.S. Automated Chair-Training of Rhesus Macaques. J. Neurosci. Methods 2016, 263, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Ruys, J.; Mendoza, S.; Capitanio, J.; Mason, W. Behavioral and Physiological Adaptation to Repeated Chair Restraint in Rhesus Macaques. Physiol. Behav. 2004, 82, 205–213. [Google Scholar] [CrossRef]
- Florence, G.; Riondet, L.; Malecki, H.; Blanquie, J.; Martin, F.; Viso, M.; Milhaud, C.L. A Restraining System for Rhesus Monkeys Used in Space Research. J. Med. Primatol. 1995, 24, 61–67. [Google Scholar] [CrossRef]
- Henry, K.R.; Bowman, R.E. A Long Term Restraint Device for Primates. Physiol. Behav. 1971, 7, 271–272. [Google Scholar] [CrossRef] [PubMed]
- Spence, K. Analysis of the Formation of Visual Discrimination Habits in Chimpanzee. J. Comp. Psychol. 1937, 23, 77–100. [Google Scholar] [CrossRef]
- Parron, C.; Call, J.; Fagot, J. Behavioural Responses to Photographs by Pictorially Naïve Baboons (Papio anubis), Gorillas (Gorilla gorilla) and Chimpanzees (Pan troglodytes). Behav. Process. 2008, 78, 351–357. [Google Scholar] [CrossRef]
- Pope, S.M.; Fagot, J.; Meguerditchian, A.; Watzek, J.; Lew-Levy, S.; Autrey, M.M.; Hopkins, W.D. Optional-Switch Cognitive Flexibility in Primates: Chimpanzees’ (Pan troglodytes) Intermediate Susceptibility to Cognitive Set. J. Comp. Psychol. 2020, 134, 98–109. [Google Scholar] [CrossRef]
- Fagot, J.; Tomonaga, M. Global and Local Processing in Humans (Homo sapiens) and Chimpanzees (Pan troglodytes): Use of a Visual Search Task with Compound Stimuli. J. Comp. Psychol. 1999, 113, 3–12. [Google Scholar] [CrossRef]
- Fagot, J.; Tomonaga, M. Effects of Element Separation on Perceptual Grouping by Humans (Homo sapiens) and Chimpanzees (Pan troglodytes): Perception of Kanizsa Illusory Figures. Anim. Cogn. 2001, 4, 171–177. [Google Scholar] [CrossRef]
- Harlow, H.F.; Bromer, J.A. A Test-Apparatus for Monkeys. Psychol. Rec. 1938, 2, 434–436. [Google Scholar] [CrossRef]
- Harlow, H.F. The Formation of Learning Sets. Psychol. Rev. 1949, 56, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Schrier, A.M. A Modified Version of the Wisconsin General Test Apparatus. J. Psychol. 1961, 52, 193–200. [Google Scholar] [CrossRef]
- Davenport, J.W.; Chamove, A.S.; Harlow, H.F. The Semiautomatic Wisconsin General Test Apparatus. Behav. Res. Meth. Instru. 1970, 2, 135–138. [Google Scholar] [CrossRef]
- Wright, D.C.; French, G.M.; Pinsker, H.M. A Semiautomated Wisconsin General Test Apparatus. Behav. Res. Meth. Instru. 1971, 3, 189–192. [Google Scholar] [CrossRef]
- Meyer, D.R.; Polidora, V.J.; McConnell, D.G. Effects of Spatial S-R Contiguity and Response Delay upon Discriminative Performances by Monkeys. J. Comp. Physiol. Psychol. 1961, 54, 175–177. [Google Scholar] [CrossRef]
- Polidora, V.J.; Main, W.T. Punched card programming and recording techniques employed in the automation of the wgta. J. Exper. Anal. Behav. 1963, 6, 599–603. [Google Scholar] [CrossRef]
- Schuck, J.R.; Polidora, V.J.; McConnell, D.G.; Meyer, D.R. Response Location as a Factor in Primate Pattern Discrimination. J. Comp. Physiol. Psychol. 1961, 54, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Otteson, M.I.; Sheridan, C.L.; Meyer, D.R. Effects of Stimulus-Response Isolation on Primate Pattern Discrimination Learning. J. Comp. Physiol. Psychol. 1962, 55, 935–938. [Google Scholar] [CrossRef]
- Meyer, D.R.; Treichler, F.R.; Meyer, P.M. Discrete-Trial Training Techniques and Stimulus Variables. In Behavior of Nonhuman Primates; Elsevier: Amsterdam, The Netherlands, 1965; Volume 1, pp. 1–49. ISBN 978-1-4832-2820-4. [Google Scholar]
- Wilde, J.; Vauclair, J.; Fagot, J. Eye Movements in Baboons Performing a Matching-to-Sample Task Presented in a Divided-Field Format. Behav. Brain Res. 1994, 63, 61–70. [Google Scholar] [CrossRef]
- Fagot, J.; Vauclair, J. Video-Task Assessment of Stimulus Novelty Effects on Hemispheric Lateralization in Baboons (Papio papio). J. Comp. Psychol. 1994, 108, 156–163. [Google Scholar] [CrossRef]
- Fagot, J.; Goldstein, J.; Davidoff, J.; Pickering, A. Cross-Species Differences in Color Categorization. Psychon. Bull. Rev. 2006, 13, 275–280. [Google Scholar] [CrossRef]
- Dépy, D.; Fagot, J.; Vauclair, J. Processing of above/below Categorical Spatial Relations by Baboons (Papio papio). Behav. Process. 1999, 48, 1–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fagot, J.; Kruschke, J.K.; Dépy, D.; Vauclair, J. Associative Learning in Baboons (Papio papio) and Humans (Homo sapiens): Species Differences in Learned Attention to Visual Features. Anim. Cogn. 1998, 1, 123–133. [Google Scholar] [CrossRef]
- Falcone, R.; Brunamonti, E.; Genovesio, A. Vicarious Learning from Human Models in Monkeys. PLoS ONE 2012, 7, e40283. [Google Scholar] [CrossRef] [PubMed]
- Meunier, M.; Monfardini, E.; Boussaoud, D. Learning by Observation in Rhesus Monkeys. Neurobiol. Learn. Mem. 2007, 88, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Bevacqua, S.; Cerasti, E.; Falcone, R.; Cervelloni, M.; Brunamonti, E.; Ferraina, S.; Genovesio, A. Macaque Monkeys Can Learn Token Values from Human Models through Vicarious Reward. PLoS ONE 2013, 8, e59961. [Google Scholar] [CrossRef]
- Monfardini, E.; Hadj-Bouziane, F.; Meunier, M. Model-Observer Similarity, Error Modeling and Social Learning in Rhesus Macaques. PLoS ONE 2014, 9, e89825. [Google Scholar] [CrossRef]
- Washburn, D.A.; Hopkins, W.D.; Rumbaugh, D.M. Automation of Learning-Set Testing: The Video-Task Paradigm. Behav. Res. Methods Instrum. Comput. 1989, 21, 281–284. [Google Scholar] [CrossRef]
- Washburn, D.A. PC-Compatible Computer-Generated Stimuli for Video-Task Testing. Behav. Res. Methods Instrum. Comput. 1990, 22, 132–135. [Google Scholar] [CrossRef]
- Richardson, W.K.; Washburn, D.A.; Hopkins, W.D.; Savage-rumbaugh, E.S.; Rumbaugh, D.M. The NASA/LRC Computerized Test System. Behav. Res. Methods Instrum. Comput. 1990, 22, 127–131. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Perdue, B.M.; Beran, M.J.; Washburn, D.A. A Computerized Testing System for Primates: Cognition, Welfare, and the Rumbaughx. Behav. Process. 2018, 156, 37–50. [Google Scholar] [CrossRef]
- Washburn, D.A.; Rumbaugh, D.M. Rhesus Monkey (Macaca mulatta) Complex Learning Skills Reassessed. Int. J. Primatol. 1991, 12, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Rumbaugh, D.M.; Richardson, W.K.; Washburn, D.A.; Savage-Rumbaugh, E.S.; Hopkins, W.D. Rhesus Monkeys (Macaca mulatta), Video Tasks, and Implications for Stimulus-Response Spatial Contiguity. J. Comp. Psychol. 1989, 103, 32–38. [Google Scholar] [CrossRef]
- Hopkins, W.D.; Washburn, D.A. Matching Visual Stimuli on the Basis of Global and Local Features by Chimpanzees (Pan troglodytes) and Rhesus Monkeys (Macaca mulatta). Anim. Cogn. 2002, 5, 27–31. [Google Scholar] [CrossRef]
- Washburn, D.A.; Rumbaugh, D.M. Ordinal Judgments of Numerical Symbols by Macaques (Macaca mulatta). Psychol. Sci. 1991, 2, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Washburn, D.A. Analyzing the Path of Responding in Maze-Solving and Other Tasks. Behav. Res. Methods Instrum. Comput. 1992, 24, 248–252. [Google Scholar] [CrossRef]
- Calapai, A.; Berger, M.; Niessing, M.; Heisig, K.; Brockhausen, R.; Treue, S.; Gail, A. A Cage-Based Training, Cognitive Testing and Enrichment System Optimized for Rhesus Macaques in Neuroscience Research. Behav. Res. 2017, 49, 35–45. [Google Scholar] [CrossRef]
- Calapai, A.; Cabrera-Moreno, J.; Moser, T.; Jeschke, M. Flexible Auditory Training, Psychophysics, and Enrichment of Common Marmosets with an Automated, Touchscreen-Based System. Nat. Commun. 2022, 13, 1648. [Google Scholar] [CrossRef]
- Calapai, A.; Pfefferle, D.; Cassidy, L.C.; Nazari, A.; Yurt, P.; Brockhausen, R.R.; Treue, S. A Touchscreen-Based, Multiple-Choice Approach to Cognitive Enrichment of Captive Rhesus Macaques (Macaca mulatta). Animals 2023, 13, 2702. [Google Scholar] [CrossRef]
- Womelsdorf, T.; Thomas, C.; Neumann, A.; Watson, M.R.; Banaie Boroujeni, K.; Hassani, S.A.; Parker, J.; Hoffman, K.L. A Kiosk Station for the Assessment of Multiple Cognitive Domains and Cognitive Enrichment of Monkeys. Front. Behav. Neurosci. 2021, 15, 721069. [Google Scholar] [CrossRef] [PubMed]
- Hansmeyer, L.; Yurt, P.; Agha, N.; Trunk, A.; Berger, M.; Calapai, A.; Treue, S.; Gail, A. Home-Enclosure-Based Behavioral and Wireless Neural Recording Setup for Unrestrained Rhesus Macaques. eNeuro 2023, 10, ENEURO.0285-22.2022. [Google Scholar] [CrossRef] [PubMed]
- Jacob, G.; Katti, H.; Cherian, T.; Das, J.; Zhivago, K.; Arun, S. A Naturalistic Environment to Study Visual Cognition in Unrestrained Monkeys. eLife 2021, 10, e63816. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.L.; Kennerley, S.W. Mymou: A Low-Cost, Wireless Touchscreen System for Automated Training of Nonhuman Primates. Behav. Res. 2019, 51, 2559–2572. [Google Scholar] [CrossRef]
- Fizet, J.; Rimele, A.; Pebayle, T.; Cassel, J.-C.; Kelche, C.; Meunier, H. An Autonomous, Automated and Mobile Device to Concurrently Assess Several Cognitive Functions in Group-Living Non-Human Primates. Neurobiol. Learn. Mem. 2017, 145, 45–58. [Google Scholar] [CrossRef]
- Curry, M.D.; Zimmermann, A.; Parsa, M.; Dehaqani, M.-R.A.; L Clark, K.; Noudoost, B. A Cage-Based Training System for Non-Human Primates. AIMS Neurosci. 2017, 4, 102–119. [Google Scholar] [CrossRef]
- Griggs, D.J.; Bloch, J.; Chavan, S.; Coubrough, K.M.; Conley, W.; Morrisroe, K.; Yazdan-Shahmorad, A. Autonomous Cage-Side System for Remote Training of Non-Human Primates. J. Neurosci. Methods 2021, 348, 108969. [Google Scholar] [CrossRef]
- Martin, C.F.; Muramatsu, A.; Matsuzawa, T. Apex and ApeTouch: Development of a Portable Touchscreen System and Software for Primates at Zoos. Animals 2022, 12, 1660. [Google Scholar] [CrossRef]
- Fagot, J.; Paleressompoulle, D. Automatic Testing of Cognitive Performance in Baboons Maintained in Social Groups. Behav. Res. Methods 2009, 41, 396–404. [Google Scholar] [CrossRef]
- Evans, T.A.; Beran, M.J.; Chan, B.; Klein, E.D.; Menzel, C.R. An Efficient Computerized Testing Method for the Capuchin Monkey (Cebus apella): Adaptation of the LRC-CTS to a Socially Housed Nonhuman Primate Species. Behav. Res. Methods 2008, 40, 590–596. [Google Scholar] [CrossRef]
- Tulip, J.; Zimmermann, J.B.; Farningham, D.; Jackson, A. An Automated System for Positive Reinforcement Training of Group-Housed Macaque Monkeys at Breeding and Research Facilities. J. Neurosci. Methods 2017, 285, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, S.; Ceccarelli, F.; Ferrucci, L.; Benozzo, D.; Brunamonti, E.; Nougaret, S.; Genovesio, A. Macaque Monkeys Learn and Perform a Non-Match-to-Goal Task Using an Automated Home Cage Training Procedure. Sci. Rep. 2021, 11, 2700. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Calapai, A.; Stephan, V.; Niessing, M.; Burchardt, L.; Gail, A.; Treue, S. Standardized Automated Training of Rhesus Monkeys for Neuroscience Research in Their Housing Environment. J. Neurophysiol. 2018, 119, 796–807. [Google Scholar] [CrossRef]
- Cabrera-Moreno, J.; Jeanson, L.; Jeschke, M.; Calapai, A. Group-Based, Autonomous, Individualized Training and Testing of Long-Tailed Macaques (Macaca Fascicularis) in Their Home Enclosure to a Visuo-Acoustic Discrimination Task. Front. Psychol. 2022, 13, 1047242. [Google Scholar] [CrossRef]
- Scott, J.T.; Mendivez Vasquez, B.L.; Stewart, B.J.; Panacheril, D.D.; Rajit, D.K.J.; Fan, A.Y.; Bourne, J.A. CalliCog: An Open-Source Cognitive Neuroscience Toolkit for Freely Behaving Nonhuman Primates. bioRxiv 2024. [Google Scholar] [CrossRef]
- Fagot, J.; Wasserman, E.A.; Young, M.E. Discriminating the Relation between Relations: The Role of Entropy in Abstract Conceptualization by Baboons (Papio papio) and Humans (Homo sapiens). J. Exp. Psychol. Anim. Behav. Process. 2001, 27, 316–328. [Google Scholar] [CrossRef]
- Malassis, R.; Rey, A.; Fagot, J. Non-adjacent Dependencies Processing in Human and Non-human Primates. Cogn. Sci. 2018, 42, 1677–1699. [Google Scholar] [CrossRef]
- Shields, W.E.; Smith, J.D.; Guttmannova, K.; Washburn, D.A. Confidence Judgments by Humans and Rhesus Monkeys. J. Gen. Psychol. 2005, 132, 165–186. [Google Scholar] [PubMed] [PubMed Central]
- Smith, J.D.; Shields, W.E.; Schull, J.; Washburn, A. The Uncertain Response in Humans and Animals. Cognition 1997, 62, 75–97. [Google Scholar] [CrossRef]
- Smith, J.D.; Shields, W.E.; Allendoerfer, K.R.; Washburn, D.A. Memory Monitoring by Animals and Humans. J. Exp. Psychol. Gen. 1998, 127, 227–250. [Google Scholar] [CrossRef]
- Washburn, D.A. Stroop-Like Effects for Monkeys and Humans: Processing Speed or Strength of Association? Psychol. Sci. 1994, 5, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Murai, T.; Bailey, L.; Schultz, L.; Mongeau, L.; DeSana, A.; Silva, A.C.; Roberts, A.C.; Sukoff Rizzo, S.J. Improving Preclinical to Clinical Translation of Cognitive Function for Aging-Related Disorders: The Utility of Comprehensive Touchscreen Testing Batteries in Common Marmosets. Cogn. Affect. Behav. Neurosci. 2024, 24, 325–348. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.R.; Traczewski, N.; Dunghana, S.; Boroujeni, K.B.; Neumann, A.; Wen, X.; Womelsdorf, T. A Multi-Task Platform for Profiling Cognitive and Motivational Constructs in Humans and Nonhuman Primates. bioRxiv 2023. [Google Scholar] [CrossRef]
- Fiori, L.; Ramawat, S.; Marc, I.B.; Giuffrida, V.; Ranavolo, A.; Draicchio, F.; Pani, P.; Ferraina, S.; Brunamonti, E. Balancing Postural Control and Motor Inhibition during Gait Initiation. iScience 2025, 28, 111970. [Google Scholar] [CrossRef]
- Giuffrida, V.; Marc, I.B.; Ramawat, S.; Fontana, R.; Fiori, L.; Bardella, G.; Fagioli, S.; Ferraina, S.; Brunamonti, E.; Pani, P. Reward Prospect Affects Strategic Adjustments in Stop Signal Task. Front. Psychol. 2023, 14, 1125066. [Google Scholar] [CrossRef]
- Spinelli, S.; Pennanen, L.; Dettling, A.C.; Feldon, J.; Higgins, G.A.; Pryce, C.R. Performance of the Marmoset Monkey on Computerized Tasks of Attention and Working Memory. Cogn. Brain Res. 2004, 19, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, G.; DePass, M.; Baspinar, E.; Andujar, M.; Ramawat, S.; Pani, P.; Ferraina, S.; Destexhe, A.; Moreno-Bote, R.; Cos, I. Cognitive Mechanisms of Learning in Sequential Decision-Making under Uncertainty: An Experimental and Theoretical Approach. Front. Behav. Neurosci. 2024, 18, 1399394. [Google Scholar] [CrossRef]
- Weed, M.R.; Taffe, M.A.; Polis, I.; Roberts, A.C.; Robbins, T.W.; Koob, G.F.; Bloom, F.E.; Gold, L.H. Performance Norms for a Rhesus Monkey Neuropsychological Testing Battery: Acquisition and Long-Term Performance. Cogn. Brain Res. 1999, 8, 185–201. [Google Scholar] [CrossRef]
- Ramawat, S.; Marc, I.B.; Di Bello, F.; Bardella, G.; Ferraina, S.; Pani, P.; Brunamonti, E. Force Monitoring Reveals Single Trial Dynamics of Motor Control in a Stop Signal Task. Physiol. Rep. 2024, 12, e70127. [Google Scholar] [CrossRef]
- Marc, I.B.; Giuffrida, V.; Ramawat, S.; Fiori, L.; Fontana, R.; Bardella, G.; Fagioli, S.; Ferraina, S.; Pani, P.; Brunamonti, E. Restart Errors Reaction Time of a Two-Step Inhibition Process Account for the Violation of the Race Model’s Independence in Multi-Effector Selective Stop Signal Task. Front. Hum. Neurosci. 2023, 17, 1106298. [Google Scholar] [CrossRef]
- Nagahara, A.H.; Bernot, T.; Tuszynski, M.H. Age-Related Cognitive Deficits in Rhesus Monkeys Mirror Human Deficits on an Automated Test Battery. Neurobiol. Aging 2010, 31, 1020–1031. [Google Scholar] [CrossRef] [PubMed]
- Levaux, M.-N.; Potvin, S.; Sepehry, A.A.; Sablier, J.; Mendrek, A.; Stip, E. Computerized Assessment of Cognition in Schizophrenia: Promises and Pitfalls of CANTAB. Eur. Psychiatry 2007, 22, 104–115. [Google Scholar] [CrossRef]
- Ozonoff, S.; Cook, I.; Coon, H.; Dawson, G.; Joseph, R.M.; Klin, A.; McMahon, W.M.; Minshew, N.; Munson, J.A.; Pennington, B.F.; et al. Performance on Cambridge Neuropsychological Test Automated Battery Subtests Sensitive to Frontal Lobe Function in People with Autistic Disorder: Evidence from the Collaborative Programs of Excellence in Autism Network. J. Autism Dev. Disord. 2004, 34, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.S.; Zϋrcher, N.R.; Bartlett, T.Q.; Nathanielsz, P.W.; Nijland, M.J. CANTAB Delayed Matching to Sample Task Performance in Juvenile Baboons. J. Neurosci. Methods 2011, 196, 258–263. [Google Scholar] [CrossRef]
- Shnitko, T.A.; Allen, D.C.; Gonzales, S.W.; Walter, N.A.R.; Grant, K.A. Ranking Cognitive Flexibility in a Group Setting of Rhesus Monkeys with a Set-Shifting Procedure. Front. Behav. Neurosci. 2017, 11, 55. [Google Scholar] [CrossRef]
- Crofts, H.S.; Muggleton, N.G.; Bowditch, A.P.; Pearce, P.C.; Nutt, D.J.; Scott, E.A.M. Home Cage Presentation of Complex Discrimination Tasks to Marmosets and Rhesus Monkeys. Lab. Anim. 1999, 33, 207–214. [Google Scholar] [CrossRef]
- Zürcher, N.R.; Rodriguez, J.S.; Jenkins, S.L.; Keenan, K.; Bartlett, T.Q.; McDonald, T.J.; Nathanielsz, P.W.; Nijland, M.J. Performance of Juvenile Baboons on Neuropsychological Tests Assessing Associative Learning, Motivation and Attention. J. Neurosci. Methods 2010, 188, 219–225. [Google Scholar] [CrossRef]
- Fagot, J.; Bonté, E. Automated Testing of Cognitive Performance in Monkeys: Use of a Battery of Computerized Test Systems by a Troop of Semi-Free-Ranging Baboons (Papio papio). Behav. Res. Methods 2010, 42, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Gazes, R.P.; Brown, E.K.; Basile, B.M.; Hampton, R.R. Automated Cognitive Testing of Monkeys in Social Groups Yields Results Comparable to Individual Laboratory-Based Testing. Anim. Cogn. 2013, 16, 445–458. [Google Scholar] [CrossRef]
- Fagot, J.; Gullstrand, J.; Kemp, C.; Defilles, C.; Mekaouche, M. Effects of Freely Accessible Computerized Test Systems on the Spontaneous Behaviors and Stress Level of Guinea Baboons (Papio papio). Am. J. Primatol. 2014, 76, 56–64. [Google Scholar] [CrossRef]
- Matsuzawa, T. WISH Cages: Constructing Multiple Habitats for Captive Chimpanzees. Primates 2020, 61, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Yamanashi, Y.; Hayashi, M. Assessing the Effects of Cognitive Experiments on the Welfare of Captive Chimpanzees (Pan troglodytes) by Direct Comparison of Activity Budget between Wild and Captive Chimpanzees. Am. J. Primatol. 2011, 73, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T. Contrafreeloading and the Value of Control over Visual Stimuli in Japanese Macaques (Macaca fuscata). Anim. Cogn. 2011, 14, 427–431. [Google Scholar] [CrossRef]
- Cisek, P.; Green, A.M. Toward a Neuroscience of Natural Behavior. Curr. Opin. Neurobiol. 2024, 86, 102859. [Google Scholar] [CrossRef]
- Schwarz, D.A.; Lebedev, M.A.; Hanson, T.L.; Dimitrov, D.F.; Lehew, G.; Meloy, J.; Rajangam, S.; Subramanian, V.; Ifft, P.J.; Li, Z.; et al. Chronic, Wireless Recordings of Large-Scale Brain Activity in Freely Moving Rhesus Monkeys. Nat. Methods 2014, 11, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Zanos, S.; Richardson, A.G.; Shupe, L.; Miles, F.P.; Fetz, E.E. The Neurochip-2: An Autonomous Head-Fixed Computer for Recording and Stimulating in Freely Behaving Monkeys. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 427–435. [Google Scholar] [CrossRef]
- Mao, D.; Avila, E.; Caziot, B.; Laurens, J.; Dickman, J.D.; Angelaki, D.E. Spatial Modulation of Hippocampal Activity in Freely Moving Macaques. Neuron 2021, 109, 3521–3534.e6. [Google Scholar] [CrossRef]
- Bukhtiyarova, O.; Chauvette, S.; Seigneur, J.; Timofeev, I. Brain States in Freely Behaving Marmosets. Sleep 2022, 45, zsac106. [Google Scholar] [CrossRef]
- Testard, C.; Tremblay, S.; Parodi, F.; DiTullio, R.W.; Acevedo-Ithier, A.; Gardiner, K.L.; Kording, K.; Platt, M.L. Neural Signatures of Natural Behaviour in Socializing Macaques. Nature 2024, 628, 381–390. [Google Scholar] [CrossRef]
- Yin, M.; Borton, D.A.; Komar, J.; Agha, N.; Lu, Y.; Li, H.; Laurens, J.; Lang, Y.; Li, Q.; Bull, C.; et al. Wireless Neurosensor for Full-Spectrum Electrophysiology Recordings during Free Behavior. Neuron 2014, 84, 1170–1182. [Google Scholar] [CrossRef]
- Zhou, A.; Santacruz, S.R.; Johnson, B.C.; Alexandrov, G.; Moin, A.; Burghardt, F.L.; Rabaey, J.M.; Carmena, J.M.; Muller, R. A Wireless and Artefact-Free 128-Channel Neuromodulation Device for Closed-Loop Stimulation and Recording in Non-Human Primates. Nat. Biomed. Eng. 2018, 3, 15–26. [Google Scholar] [CrossRef]
- Berger, M.; Agha, N.S.; Gail, A. Wireless Recording from Unrestrained Monkeys Reveals Motor Goal Encoding beyond Immediate Reach in Frontoparietal Cortex. eLife 2020, 9, e51322. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Li, H.; Bull, C.; Borton, D.A.; Aceros, J.; Larson, L.; Nurmikko, A.V. An Externally Head-Mounted Wireless Neural Recording Device for Laboratory Animal Research and Possible Human Clinical Use. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 3109–3114. [Google Scholar] [CrossRef]
- Su, Y.; Routhu, S.; Moon, K.; Lee, S.; Youm, W.; Ozturk, Y. A Wireless 32-Channel Implantable Bidirectional Brain Machine Interface. Sensors 2016, 16, 1582. [Google Scholar] [CrossRef] [PubMed]
- Won, S.M.; Cai, L.; Gutruf, P.; Rogers, J.A. Wireless and Battery-Free Technologies for Neuroengineering. Nat. Biomed. Eng. 2021, 7, 405–423. [Google Scholar] [CrossRef]
- Lei, Y.; Sun, N.; Wilson, F.A.W.; Wang, X.; Chen, N.; Yang, J.; Peng, Y.; Wang, J.; Tian, S.; Wang, M.; et al. Telemetric Recordings of Single Neuron Activity and Visual Scenes in Monkeys Walking in an Open Field. J. Neurosci. Methods 2004, 135, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.L.; Lei, Y.L.; Kim, B.-H.; Ryou, J.-W.; Ma, Y.-Y.; Wilson, F.A.W. Neurophysiological Recordings in Freely Moving Monkeys. Methods 2006, 38, 202–209. [Google Scholar] [CrossRef]
- Hampson, R.E.; Collins, V.; Deadwyler, S.A. A Wireless Recording System That Utilizes Bluetooth Technology to Transmit Neural Activity in Freely Moving Animals. J. Neurosci. Methods 2009, 182, 195–204. [Google Scholar] [CrossRef]
- Szuts, T.A.; Fadeyev, V.; Kachiguine, S.; Sher, A.; Grivich, M.V.; Agrochão, M.; Hottowy, P.; Dabrowski, W.; Lubenov, E.V.; Siapas, A.G.; et al. A Wireless Multi-Channel Neural Amplifier for Freely Moving Animals. Nat. Neurosci. 2011, 14, 263–269. [Google Scholar] [CrossRef]
- Ji, B.; Liang, Z.; Yuan, X.; Xu, H.; Wang, M.; Yin, E.; Guo, Z.; Wang, L.; Zhou, Y.; Feng, H.; et al. Recent Advances in Wireless Epicortical and Intracortical Neuronal Recording Systems. Sci. China Inf. Sci. 2022, 65, 140401. [Google Scholar] [CrossRef]
- Datta, S.R.; Anderson, D.J.; Branson, K.; Perona, P.; Leifer, A. Computational Neuroethology: A Call to Action. Neuron 2019, 104, 11–24. [Google Scholar] [CrossRef]
- Miller, C.T.; Gire, D.; Hoke, K.; Huk, A.C.; Kelley, D.; Leopold, D.A.; Smear, M.C.; Theunissen, F.; Yartsev, M.; Niell, C.M. Natural Behavior Is the Language of the Brain. Curr. Biol. 2022, 32, R482–R493. [Google Scholar] [CrossRef] [PubMed]
- Grohrock, P.; Häusler, U.; Jürgens, U. Dual-Channel Telemetry System for Recording Vocalization-Correlated Neuronal Activity in Freely Moving Squirrel Monkeys. J. Neurosci. Methods 1997, 76, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Düsterhöft, F.; Häusler, U.; Jürgens, U. On the search for the vocal pattern generator. A single-unit recording study. NeuroReport 2000, 11, 2031–2034. [Google Scholar] [CrossRef] [PubMed]
- Tammer, R.; Ehrenreich, L.; Jürgens, U. Telemetrically Recorded Neuronal Activity in the Inferior Colliculus and Bordering Tegmentum during Vocal Communication in Squirrel Monkeys (Saimiri sciureus). Behav. Brain Res. 2004, 151, 331–336. [Google Scholar] [CrossRef]
- Eliades, S.J.; Wang, X. Chronic Multi-Electrode Neural Recording in Free-Roaming Monkeys. J. Neurosci. Methods 2008, 172, 201–214. [Google Scholar] [CrossRef]
- Eliades, S.J.; Wang, X. Neural Substrates of Vocalization Feedback Monitoring in Primate Auditory Cortex. Nature 2008, 453, 1102–1106. [Google Scholar] [CrossRef]
- Roy, S.; Wang, X. Wireless Multi-Channel Single Unit Recording in Freely Moving and Vocalizing Primates. J. Neurosci. Methods 2012, 203, 28–40. [Google Scholar] [CrossRef]
- Miller, C.T.; Wren Thomas, A. Individual Recognition during Bouts of Antiphonal Calling in Common Marmosets. J. Comp. Physiol. A 2012, 198, 337–346. [Google Scholar] [CrossRef]
- Miller, C.T.; Thomas, A.W.; Nummela, S.U.; De La Mothe, L.A. Responses of Primate Frontal Cortex Neurons during Natural Vocal Communication. J. Neurophysiol. 2015, 114, 1158–1171. [Google Scholar] [CrossRef]
- Roy, S.; Zhao, L.; Wang, X. Distinct Neural Activities in Premotor Cortex during Natural Vocal Behaviors in a New World Primate, the Common Marmoset (Callithrix jacchus). J. Neurosci. 2016, 36, 12168–12179. [Google Scholar] [CrossRef]
- Nummela, S.U.; Jovanovic, V.; De La Mothe, L.; Miller, C.T. Social Context-Dependent Activity in Marmoset Frontal Cortex Populations during Natural Conversations. J. Neurosci. 2017, 37, 7036–7047. [Google Scholar] [CrossRef] [PubMed]
- Ludvig, N.; Botero, J.M.; Tang, H.M.; Gohil, B.; Kral, J.G. Single-Cell Recording from the Brain of Freely Moving Monkeys. J. Neurosci. Methods 2001, 106, 179–187. [Google Scholar] [CrossRef]
- Eliav, T.; Maimon, S.R.; Aljadeff, J.; Tsodyks, M.; Ginosar, G.; Las, L.; Ulanovsky, N. Multiscale Representation of Very Large Environments in the Hippocampus of Flying Bats. Science 2021, 372, eabg4020. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.; Dostrovsky, J. The Hippocampus as a Spatial Map. Preliminary Evidence from Unit Activity in the Freely-Moving Rat. Brain Res. 1971, 34, 171–175. [Google Scholar] [CrossRef]
- Leutgeb, S.; Leutgeb, J.K.; Moser, M.-B.; Moser, E.I. Place Cells, Spatial Maps and the Population Code for Memory. Curr. Opin. Neurobiol. 2005, 15, 738–746. [Google Scholar] [CrossRef]
- Ludvig, N.; Tang, H.M.; Gohil, B.C.; Botero, J.M. Detecting Location-Specific Neuronal Firing Rate Increases in the Hippocampus of Freely-Moving Monkeys. Brain Res. 2004, 1014, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Hazama, Y.; Tamura, R. Effects of Self-Locomotion on the Activity of Place Cells in the Hippocampus of a Freely Behaving Monkey. Neurosci. Lett. 2019, 701, 32–37. [Google Scholar] [CrossRef]
- Courellis, H.S.; Nummela, S.U.; Metke, M.; Diehl, G.W.; Bussell, R.; Cauwenberghs, G.; Miller, C.T. Spatial Encoding in Primate Hippocampus during Free Navigation. PLoS Biol. 2019, 17, e3000546. [Google Scholar] [CrossRef]
- Piza, D.B.; Corrigan, B.W.; Gulli, R.A.; Do Carmo, S.; Cuello, A.C.; Muller, L.; Martinez-Trujillo, J. Primacy of Vision Shapes Behavioral Strategies and Neural Substrates of Spatial Navigation in Marmoset Hippocampus. Nat. Commun. 2024, 15, 4053. [Google Scholar] [CrossRef]
- Maisson, D.J.-N.; Cervera, R.L.; Voloh, B.; Conover, I.; Zambre, M.; Zimmermann, J.; Hayden, B.Y. Widespread Coding of Navigational Variables in Prefrontal Cortex. Curr. Biol. 2023, 33, 3478–3488.e3. [Google Scholar] [CrossRef]
- Shahidi, N.; Franch, M.; Parajuli, A.; Schrater, P.; Wright, A.; Pitkow, X.; Dragoi, V. Population Coding of Strategic Variables during Foraging in Freely Moving Macaques. Nat. Neurosci. 2024, 27, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Lanzarini, F.; Maranesi, M.; Rondoni, E.H.; Albertini, D.; Ferretti, E.; Lanzilotto, M.; Micera, S.; Mazzoni, A.; Bonini, L. Neuroethology of Natural Actions in Freely Moving Monkeys. Science 2025, 387, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T.; Inoue, K.; Browning, A. Activity of Primate Subgenual Cingulate Cortex Neurons Is Related to Sleep. J. Neurophysiol. 2003, 90, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Gabbott, P.L.; Rolls, E.T. Increased Neuronal Firing in Resting and Sleep in Areas of the Macaque Medial Prefrontal Cortex. Eur. J. Neurosci. 2013, 37, 1737–1746. [Google Scholar] [CrossRef]
- Hall, T.M.; de Carvalho, F.; Jackson, A. A Common Structure Underlies Low-Frequency Cortical Dynamics in Movement, Sleep, and Sedation. Neuron 2014, 83, 1185–1199. [Google Scholar] [CrossRef]
- Van Someren, E.J.W.; Van Der Werf, Y.D.; Roelfsema, P.R.; Mansvelder, H.D.; Lopes Da Silva, F.H. Slow Brain Oscillations of Sleep, Resting State, and Vigilance. Prog. Brain Res. 2011, 193, 3–15. [Google Scholar] [CrossRef]
- Hsieh, K.-C.; Robinson, E.L.; Fuller, C.A. Sleep Architecture in Unrestrained Rhesus Monkeys (Macaca Mulatta) Synchronized to 24-Hour Light-Dark Cycles. Sleep 2008, 31, 1239–1250. [Google Scholar]
- Xu, W.; De Carvalho, F.; Jackson, A. Sequential Neural Activity in Primary Motor Cortex during Sleep. J. Neurosci. 2019, 39, 3698–3712. [Google Scholar] [CrossRef]
- Milton, R.; Shahidi, N.; Dragoi, V. Dynamic States of Population Activity in Prefrontal Cortical Networks of Freely-Moving Macaque. Nat. Commun. 2020, 11, 1948. [Google Scholar] [CrossRef]
- Walker, J.D.; Pirschel, F.; Sundiang, M.; Niekrasz, M.; MacLean, J.N.; Hatsopoulos, N.G. Chronic Wireless Neural Population Recordings with Common Marmosets. Cell Rep. 2021, 36, 109379. [Google Scholar] [CrossRef]
- Testard, C.; Tremblay, S.; Platt, M. From the Field to the Lab and Back: Neuroethology of Primate Social Behavior. Curr. Opin. Neurobiol. 2021, 68, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Saito, N.; Iriki, A.; Isoda, M. Representation of Others’ Action by Neurons in Monkey Medial Frontal Cortex. Curr. Biol. 2011, 21, 249–253. [Google Scholar] [CrossRef]
- Yoshida, K.; Saito, N.; Iriki, A.; Isoda, M. Social Error Monitoring in Macaque Frontal Cortex. Nat. Neurosci. 2012, 15, 1307–1312. [Google Scholar] [CrossRef]
- Cirillo, R.; Ferrucci, L.; Marcos, E.; Ferraina, S.; Genovesio, A. Coding of Self and Other’s Future Choices in Dorsal Premotor Cortex during Social Interaction. Cell Rep. 2018, 24, 1679–1686. [Google Scholar] [CrossRef]
- Ferrucci, L.; Nougaret, S.; Ceccarelli, F.; Sacchetti, S.; Fascianelli, V.; Benozzo, D.; Genovesio, A. Social Monitoring of Actions in the Macaque Frontopolar Cortex. Prog. Neurobiol. 2022, 218, 102339. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Nougaret, S.; Falcone, R.; Cirillo, R.; Ceccarelli, F.; Genovesio, A. Dedicated Representation of Others in the Macaque Frontal Cortex: From Action Monitoring and Prediction to Outcome Evaluation. Cereb. Cortex 2022, 32, 891–907. [Google Scholar] [CrossRef]
- Chang, S.W.C.; Gariépy, J.-F.; Platt, M.L. Neuronal Reference Frames for Social Decisions in Primate Frontal Cortex. Nat. Neurosci. 2013, 16, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Noritake, A.; Ninomiya, T.; Isoda, M. Social Reward Monitoring and Valuation in the Macaque Brain. Nat. Neurosci. 2018, 21, 1452–1462. [Google Scholar] [CrossRef]
- Noritake, A.; Ninomiya, T.; Isoda, M. Representation of Distinct Reward Variables for Self and Other in Primate Lateral Hypothalamus. Proc. Natl. Acad. Sci. USA 2020, 117, 5516–5524. [Google Scholar] [CrossRef]
- Haroush, K.; Williams, Z.M. Neuronal Prediction of Opponent’s Behavior during Cooperative Social Interchange in Primates. Cell 2015, 160, 1233–1245. [Google Scholar] [CrossRef]
- Hosokawa, T.; Watanabe, M. Prefrontal Neurons Represent Winning and Losing during Competitive Video Shooting Games between Monkeys. J. Neurosci. 2012, 32, 7662–7671. [Google Scholar] [CrossRef]
- Visco-Comandini, F.; Ferrari-Toniolo, S.; Satta, E.; Papazachariadis, O.; Gupta, R.; Nalbant, L.E.; Battaglia-Mayer, A. Do Non-Human Primates Cooperate? Evidences of Motor Coordination during a Joint Action Task in Macaque Monkeys. Cortex 2015, 70, 115–127. [Google Scholar] [CrossRef]
- Grabenhorst, F.; Báez-Mendoza, R.; Genest, W.; Deco, G.; Schultz, W. Primate Amygdala Neurons Simulate Decision Processes of Social Partners. Cell 2019, 177, 986–998.e15. [Google Scholar] [CrossRef] [PubMed]
- Tsao, D.Y.; Freiwald, W.A.; Tootell, R.B.H.; Livingstone, M.S. A Cortical Region Consisting Entirely of Face-Selective Cells. Science 2006, 311, 670–674. [Google Scholar] [CrossRef]
- Tazumi, T.; Hori, E.; Maior, R.S.; Ono, T.; Nishijo, H. Neural Correlates to Seen Gaze-Direction and Head Orientation in the Macaque Monkey Amygdala. Neuroscience 2010, 169, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Dal Monte, O.; Nair, A.R.; Fagan, N.A.; Chang, S.W.C. Closed-Loop Microstimulations of the Orbitofrontal Cortex during Real-Life Gaze Interaction Enhance Dynamic Social Attention. Neuron 2024, 112, 2631–2644.e6. [Google Scholar] [CrossRef]
- Giamundo, M.; Trapeau, R.; Thoret, E.; Renaud, L.; Nougaret, S.; Brochier, T.G.; Belin, P. A Population of Neurons Selective for Human Voice in the Monkey Brain. Proc. Natl. Acad. Sci. USA 2024, 121, e2405588121. [Google Scholar] [CrossRef] [PubMed]
- Lacal, I.; Babicola, L.; Caminiti, R.; Ferrari-Toniolo, S.; Schito, A.; Nalbant, L.E.; Gupta, R.K.; Battaglia-Mayer, A. Evidence for a We-Representation in Monkeys When Acting Together. Cortex 2022, 149, 123–136. [Google Scholar] [CrossRef]
- Báez-Mendoza, R.; Mastrobattista, E.P.; Wang, A.J.; Williams, Z.M. Social Agent Identity Cells in the Prefrontal Cortex of Interacting Groups of Primates. Science 2021, 374, eabb4149. [Google Scholar] [CrossRef]
- Breveglieri, R.; Vaccari, F.E.; Bosco, A.; Gamberini, M.; Fattori, P.; Galletti, C. Neurons Modulated by Action Execution and Observation in the Macaque Medial Parietal Cortex. Curr. Biol. 2019, 29, 1218–1225.e3. [Google Scholar] [CrossRef]
- Papadourakis, V.; Raos, V. Neurons in the Macaque Dorsal Premotor Cortex Respond to Execution and Observation of Actions. Cereb. Cortex 2019, 29, 4223–4237. [Google Scholar] [CrossRef]
- Falcone, R.; Cirillo, R.; Ceccarelli, F.; Genovesio, A. Neural Representation of Others during Action Observation in Posterior Medial Prefrontal Cortex. Cereb. Cortex 2022, 32, 4512–4523. [Google Scholar] [CrossRef]
- Di Bello, F.; Falcone, R.; Genovesio, A. Simultaneous Oscillatory Encoding of “Hot” and “Cold” Information during Social Interactions in the Monkey Medial Prefrontal Cortex. iScience 2024, 27, 109559. [Google Scholar] [CrossRef]
- Cisek, P.; Kalaska, J.F. Neural Correlates of Mental Rehearsal in Dorsal Premotor Cortex. Nature 2004, 431, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Tkach, D.; Reimer, J.; Hatsopoulos, N.G. Congruent Activity during Action and Action Observation in Motor Cortex. J. Neurosci. 2007, 27, 13241–13250. [Google Scholar] [CrossRef] [PubMed]
- Nougaret, S.; Ferrucci, L.; Genovesio, A. Role of the Social Actor during Social Interaction and Learning in Human-Monkey Paradigms. Neurosci. Biobehav. Rev. 2019, 102, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Franch, M.; Yellapantula, S.; Parajuli, A.; Kharas, N.; Wright, A.; Aazhang, B.; Dragoi, V. Visuo-Frontal Interactions during Social Learning in Freely Moving Macaques. Nature 2024, 627, 174–181. [Google Scholar] [CrossRef]
- Stokes, M.G.; Kusunoki, M.; Sigala, N.; Nili, H.; Gaffan, D.; Duncan, J. Dynamic Coding for Cognitive Control in Prefrontal Cortex. Neuron 2013, 78, 364–375. [Google Scholar] [CrossRef]
- Rich, E.L.; Wallis, J.D. Decoding Subjective Decisions from Orbitofrontal Cortex. Nat. Neurosci. 2016, 19, 973–980. [Google Scholar] [CrossRef]
- Cirillo, R.; Fascianelli, V.; Ferrucci, L.; Genovesio, A. Neural Intrinsic Timescales in the Macaque Dorsal Premotor Cortex Predict the Strength of Spatial Response Coding. iScience 2018, 10, 203–210. [Google Scholar] [CrossRef]
- Fascianelli, V.; Ferrucci, L.; Tsujimoto, S.; Genovesio, A. Neural Correlates of Strategy Switching in the Macaque Orbital Prefrontal Cortex. J. Neurosci. 2020, 40, 3025–3034. [Google Scholar] [CrossRef] [PubMed]
- Fascianelli, V.; Tsujimoto, S.; Marcos, E.; Genovesio, A. Autocorrelation Structure in the Macaque Dorsolateral, But Not Orbital or Polar, Prefrontal Cortex Predicts Response-Coding Strength in a Visually Cued Strategy Task. Cereb. Cortex 2019, 29, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Benozzo, D.; Ferrucci, L.; Genovesio, A. Effects of Contraction Bias on the Decision Process in the Macaque Prefrontal Cortex. Cereb. Cortex 2023, 33, 2958–2968. [Google Scholar] [CrossRef]
- Ramawat, S.; Marc, I.B.; Ceccarelli, F.; Ferrucci, L.; Bardella, G.; Ferraina, S.; Pani, P.; Brunamonti, E. The Transitive Inference Task to Study the Neuronal Correlates of Memory-Driven Decision Making: A Monkey Neurophysiology Perspective. Neurosci. Biobehav. Rev. 2023, 152, 105258. [Google Scholar] [CrossRef]
- Ramawat, S.; Mione, V.; Di Bello, F.; Bardella, G.; Genovesio, A.; Pani, P.; Ferraina, S.; Brunamonti, E. Different Contribution of the Monkey Prefrontal and Premotor Dorsal Cortex in Decision Making During a Transitive Inference Task. Neuroscience 2022, 485, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Halliday, D.; Martinez-Trujillo, J.C. Neuronal Population Coding of Perceived and Memorized Visual Features in the Lateral Prefrontal Cortex. Nat. Commun. 2017, 8, 15471. [Google Scholar] [CrossRef]
- Sreenivasan, K.K.; D’Esposito, M. The What, Where and How of Delay Activity. Nat. Rev. Neurosci. 2019, 20, 466–481. [Google Scholar] [CrossRef]
- Lundqvist, M.; Brincat, S.L.; Rose, J.; Warden, M.R.; Buschman, T.J.; Miller, E.K.; Herman, P. Working Memory Control Dynamics Follow Principles of Spatial Computing. Nat. Commun. 2023, 14, 1429. [Google Scholar] [CrossRef]
- Fries, P.; Reynolds, J.H.; Rorie, A.E.; Desimone, R. Modulation of Oscillatory Neuronal Synchronization by Selective Visual Attention. Science 2001, 291, 1560–1563. [Google Scholar] [CrossRef]
- Sapountzis, P.; Paneri, S.; Papadopoulos, S.; Gregoriou, G.G. Dynamic and Stable Population Coding of Attentional Instructions Coexist in the Prefrontal Cortex. Proc. Natl. Acad. Sci. USA 2022, 119, e2202564119. [Google Scholar] [CrossRef]
- Messinger, A.; Cirillo, R.; Wise, S.P.; Genovesio, A. Separable Neuronal Contributions to Covertly Attended Locations and Movement Goals in Macaque Frontal Cortex. Sci. Adv. 2021, 7, eabe0716. [Google Scholar] [CrossRef]
- Di Bello, F.; Giamundo, M.; Brunamonti, E.; Cirillo, R.; Ferraina, S. The Puzzling Relationship between Attention and Motivation: Do Motor Biases Matter? Neuroscience 2019, 406, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Nieder, A.; Freedman, D.J.; Miller, E.K. Representation of the Quantity of Visual Items in the Primate Prefrontal Cortex. Science 2002, 297, 1708–1711. [Google Scholar] [CrossRef]
- Genovesio, A.; Tsujimoto, S.; Wise, S.P. Feature- and Order-Based Timing Representations in the Frontal Cortex. Neuron 2009, 63, 254–266. [Google Scholar] [CrossRef]
- Marcos, E.; Tsujimoto, S.; Genovesio, A. Independent Coding of Absolute Duration and Distance Magnitudes in the Prefrontal Cortex. J. Neurophysiol. 2017, 117, 195–203. [Google Scholar] [CrossRef]
- Merchant, H.; Harrington, D.L.; Meck, W.H. Neural Basis of the Perception and Estimation of Time. Annu. Rev. Neurosci. 2013, 36, 313–336. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, P.; Stein, A.M.; Nieder, A. Sequential Neuronal Processing of Number Values, Abstract Decision, and Action in the Primate Prefrontal Cortex. PLoS Biol. 2024, 22, e3002520. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, S.; Benna, M.K.; Rigotti, M.; Munuera, J.; Fusi, S.; Salzman, C.D. The Geometry of Abstraction in the Hippocampus and Prefrontal Cortex. Cell 2020, 183, 954–967.e21. [Google Scholar] [CrossRef]
- Fascianelli, V.; Battista, A.; Stefanini, F.; Tsujimoto, S.; Genovesio, A.; Fusi, S. Neural Representational Geometries Reflect Behavioral Differences in Monkeys and Recurrent Neural Networks. Nat. Commun. 2024, 15, 6479. [Google Scholar] [CrossRef]
- Costa, V.D.; Averbeck, B.B. Primate Orbitofrontal Cortex Codes Information Relevant for Managing Explore–Exploit Tradeoffs. J. Neurosci. 2020, 40, 2553–2561. [Google Scholar] [CrossRef]
- Nougaret, S.; Ferrucci, L.; Ceccarelli, F.; Sacchetti, S.; Benozzo, D.; Fascianelli, V.; Saunders, R.C.; Renaud, L.; Genovesio, A. Neurons in the Monkey Frontopolar Cortex Encode Learning Stage and Goal during a Fast Learning Task. PLoS Biol. 2024, 22, e3002500. [Google Scholar] [CrossRef]
- Kaufman, M.T.; Churchland, M.M.; Ryu, S.I.; Shenoy, K.V. Cortical Activity in the Null Space: Permitting Preparation without Movement. Nat. Neurosci. 2014, 17, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Gallego, J.A.; Perich, M.G.; Miller, L.E.; Solla, S.A. Neural Manifolds for the Control of Movement. Neuron 2017, 94, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Bardella, G.; Giuffrida, V.; Giarrocco, F.; Brunamonti, E.; Pani, P.; Ferraina, S. Response Inhibition in Premotor Cortex Corresponds to a Complex Reshuffle of the Mesoscopic Information Network. Netw. Neurosci. 2024, 8, 597–622. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Funahashi, S. Toward an Understanding of the Neural Mechanisms Underlying Dual-Task Performance: Contribution of Comparative Approaches Using Animal Models. Neurosci. Biobehav. Rev. 2018, 84, 12–28. [Google Scholar] [CrossRef]
- Asaad, W.F.; Rainer, G.; Miller, E.K. Neural Activity in the Primate Prefrontal Cortex during Associative Learning. Neuron 1998, 21, 1399–1407. [Google Scholar] [CrossRef]
- Brincat, S.L.; Miller, E.K. Frequency-Specific Hippocampal-Prefrontal Interactions during Associative Learning. Nat. Neurosci. 2015, 18, 576–581. [Google Scholar] [CrossRef]
- Nougaret, S.; Genovesio, A. Learning the Meaning of New Stimuli Increases the Cross-Correlated Activity of Prefrontal Neurons. Sci. Rep. 2018, 8, 11680. [Google Scholar] [CrossRef]
- Ceccarelli, F.; Ferrucci, L.; Londei, F.; Ramawat, S.; Brunamonti, E.; Genovesio, A. Static and Dynamic Coding in Distinct Cell Types during Associative Learning in the Prefrontal Cortex. Nat. Commun. 2023, 14, 8325. [Google Scholar] [CrossRef]
- Barraclough, D.J.; Conroy, M.L.; Lee, D. Prefrontal Cortex and Decision Making in a Mixed-Strategy Game. Nat. Neurosci. 2004, 7, 404–410. [Google Scholar] [CrossRef]
- Seo, H.; Barraclough, D.J.; Lee, D. Dynamic Signals Related to Choices and Outcomes in the Dorsolateral Prefrontal Cortex. Cereb. Cortex 2007, 17, i110–i117. [Google Scholar] [CrossRef] [PubMed]
- Walton, M.E.; Behrens, T.E.J.; Buckley, M.J.; Rudebeck, P.H.; Rushworth, M.F.S. Separable Learning Systems in the Macaque Brain and the Role of Orbitofrontal Cortex in Contingent Learning. Neuron 2010, 65, 927–939. [Google Scholar] [CrossRef]
- Mitchell, J.F.; Leopold, D.A. The Marmoset Monkey as a Model for Visual Neuroscience. Neurosci. Res. 2015, 93, 20–46. [Google Scholar] [CrossRef] [PubMed]
- Samandra, R.; Haque, Z.Z.; Rosa, M.G.P.; Mansouri, F.A. The Marmoset as a Model for Investigating the Neural Basis of Social Cognition in Health and Disease. Neurosci. Biobehav. Rev. 2022, 138, 104692. [Google Scholar] [CrossRef]
- Jendritza, P.; Klein, F.J.; Fries, P. Multi-Area Recordings and Optogenetics in the Awake, Behaving Marmoset. Nat. Commun. 2023, 14, 577. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.D.; MacLean, J.N.; Walker, J.D.; Hatsopoulos, N.G. A Dynamic Subset of Network Interactions Underlies Tuning to Natural Movements in Marmoset Sensorimotor Cortex. Nat. Commun. 2024, 15, 10517. [Google Scholar] [CrossRef]
- Tang, H.; Bartolo, R.; Averbeck, B.B. Reward-Related Choices Determine Information Timing and Flow across Macaque Lateral Prefrontal Cortex. Nat. Commun. 2021, 12, 894. [Google Scholar] [CrossRef]
- Tang, H.; Bartolo, R.; Averbeck, B.B. Ventral Frontostriatal Circuitry Mediates the Computation of Reinforcement from Symbolic Gains and Losses. Neuron 2024, 112, 3782–3795.e5. [Google Scholar] [CrossRef]
- Londei, F.; Arena, G.; Ferrucci, L.; Russo, E.; Ceccarelli, F.; Genovesio, A. Connecting the Dots in the Zona Incerta: A Study of Neural Assemblies and Motifs of Inter-Area Coordination in Mice. iScience 2024, 27, 108761. [Google Scholar] [CrossRef]
- Panichello, M.F.; Jonikaitis, D.; Oh, Y.J.; Zhu, S.; Trepka, E.B.; Moore, T. Intermittent Rate Coding and Cue-Specific Ensembles Support Working Memory. Nature 2024, 636, 422–429. [Google Scholar] [CrossRef]
- Arena, G.; Londei, F.; Ceccarelli, F.; Ferrucci, L.; Borra, E.; Genovesio, A. Disentangling the Identity of the Zona Incerta: A Review of the Known Connections and Latest Implications. Ageing Res. Rev. 2024, 93, 102140. [Google Scholar] [CrossRef] [PubMed]
- Londei, F.; Ceccarelli, F.; Arena, G.; Ferrucci, L.; Russo, E.; Brunamonti, E.; Genovesio, A. Out of the Single-Neuron Straitjacket: Neurons within Assemblies Change Selectivity and Their Reconfiguration Underlies Dynamic Coding. bioRxiv 2024. [Google Scholar] [CrossRef]
- Stoll, F.M.; Rudebeck, P.H. Decision-Making Shapes Dynamic Inter-Areal Communication within Macaque Ventral Frontal Cortex. Curr. Biol. 2024, 34, 4526–4538.e5. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Fifer, M.; Bickel, S.; Osborn, L.; Herrero, J.; Christie, B.; Xu, J.; Murphy, R.K.J.; Singh, S.; Glasser, M.F.; et al. Historical Perspectives, Challenges, and Future Directions of Implantable Brain-Computer Interfaces for Sensorimotor Applications. Bioelectron. Med. 2021, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Abibullaev, B.; Keutayeva, A.; Zollanvari, A. Deep Learning in EEG-Based BCIs: A Comprehensive Review of Transformer Models, Advantages, Challenges, and Applications. IEEE Access 2023, 11, 127271–127301. [Google Scholar] [CrossRef]
- Yao, M.; Richter, O.; Zhao, G.; Qiao, N.; Xing, Y.; Wang, D.; Hu, T.; Fang, W.; Demirci, T.; De Marchi, M.; et al. Spike-Based Dynamic Computing with Asynchronous Sensing-Computing Neuromorphic Chip. Nat. Commun. 2024, 15, 4464. [Google Scholar] [CrossRef]
- Zheng, H.; Feng, Y.; Tang, J.; Ma, S. Interfacing Brain Organoids with Precision Medicine and Machine Learning. Cell Rep. Phys. Sci. 2022, 3, 100974. [Google Scholar] [CrossRef]
- George, R.; Chiappalone, M.; Giugliano, M.; Levi, T.; Vassanelli, S.; Partzsch, J.; Mayr, C. Plasticity and Adaptation in Neuromorphic Biohybrid Systems. iScience 2020, 23, 101589. [Google Scholar] [CrossRef]
- Richards, B.A.; Lillicrap, T.P.; Beaudoin, P.; Bengio, Y.; Bogacz, R.; Christensen, A.; Clopath, C.; Costa, R.P.; De Berker, A.; Ganguli, S.; et al. A Deep Learning Framework for Neuroscience. Nat. Neurosci. 2019, 22, 1761–1770. [Google Scholar] [CrossRef]
- Gonçalves, P.J.; Lueckmann, J.-M.; Deistler, M.; Nonnenmacher, M.; Öcal, K.; Bassetto, G.; Chintaluri, C.; Podlaski, W.F.; Haddad, S.A.; Vogels, T.P.; et al. Training Deep Neural Density Estimators to Identify Mechanistic Models of Neural Dynamics. eLife 2020, 9, e56261. [Google Scholar] [CrossRef]
- Bardella, G.; Franchini, S.; Pani, P.; Ferraina, S. Lattice Physics Approaches for Neural Networks. iScience 2024, 27, 111390. [Google Scholar] [CrossRef] [PubMed]
- Candelori, B.; Bardella, G.; Spinelli, I.; Ramawat, S.; Pani, P.; Ferraina, S.; Scardapane, S. Spatio-Temporal Transformers for Decoding Neural Movement Control. J. Neural Eng. 2025, 22, 016023. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceccarelli, F.; Londei, F.; Arena, G.; Genovesio, A.; Ferrucci, L. Home-Cage Training for Non-Human Primates: An Opportunity to Reduce Stress and Study Natural Behavior in Neurophysiology Experiments. Animals 2025, 15, 1340. https://doi.org/10.3390/ani15091340
Ceccarelli F, Londei F, Arena G, Genovesio A, Ferrucci L. Home-Cage Training for Non-Human Primates: An Opportunity to Reduce Stress and Study Natural Behavior in Neurophysiology Experiments. Animals. 2025; 15(9):1340. https://doi.org/10.3390/ani15091340
Chicago/Turabian StyleCeccarelli, Francesco, Fabrizio Londei, Giulia Arena, Aldo Genovesio, and Lorenzo Ferrucci. 2025. "Home-Cage Training for Non-Human Primates: An Opportunity to Reduce Stress and Study Natural Behavior in Neurophysiology Experiments" Animals 15, no. 9: 1340. https://doi.org/10.3390/ani15091340
APA StyleCeccarelli, F., Londei, F., Arena, G., Genovesio, A., & Ferrucci, L. (2025). Home-Cage Training for Non-Human Primates: An Opportunity to Reduce Stress and Study Natural Behavior in Neurophysiology Experiments. Animals, 15(9), 1340. https://doi.org/10.3390/ani15091340






