Simple Summary
This study evaluated the anesthetic effect of eugenol and tricaine methanesulfonate (MS-222) on juvenile Peruvian grunt (Anisotremus scapularis), an important species for aquaculture in the southeastern Pacific. The objective was to compare induction and recovery times at different concentrations of both anesthetics. Fish were exposed to varying doses of eugenol (25, 50, 75, 100 mg/L) and MS-222 (50, 100, 150, 200 mg/L). Eugenol induced anesthesia faster and at lower concentrations than MS-222; however, recovery times were longer. These results suggest that while eugenol is effective for rapid induction, MS-222 may be preferable when faster recovery is needed. Further research is needed to evaluate the physiological effects and safety of these anesthetics in this species.
Abstract
Anisotremus scapularis is a commercially important species in Peru, where its cultivation and management require technological strategies to minimize stress during aquaculture and fishing practices. Fish handling and transport can induce adverse physiological responses, making anesthetic use a key tool to mitigate these effects and enhance animal welfare. However, information on optimal doses, safety margins, and induction and recovery times of anesthetics in this species remains limited. This study evaluated the effects of eugenol and tricaine (MS-222) on the sedation of A. scapularis juveniles. A total of 450 individuals (9–10 cm total length) were exposed to different concentrations of eugenol and MS-222 (20, 40, 60, 80, and 100 mg/L) via immersion. Induction and recovery times were recorded, determining that the lowest concentrations allowing an optimal induction time (<6 min) and adequate recovery (<16 min) were 20–60 mg/L for eugenol and 80 mg/L for MS-222. These results provide essential information to optimize sedation protocols for A. scapularis juveniles, promoting more efficient handling with minimal impact on fish welfare in production and fishery management systems.
1. Introduction
Aquaculture in Peru has experienced an annual growth rate of 20% in recent years, becoming a prominent activity due to its high production levels [1]. On the Peruvian coast, Anisotremus scapularis is a commercially valuable species because of its culinary qualities, and efforts are currently underway to achieve conditioning and captive breeding [2,3,4]. A. scapularis, commonly known as Peruvian grunt, belongs to the Haemulidae family, characterized by coastal marine species that emit characteristic sounds by contracting muscles associated with the swim bladder [5]. This species is distributed in the southeastern Pacific, mainly from northern Peru to northern Chile, and inhabits rocky areas and sandy bottoms, commonly between 5 and 50 m deep [6]. It reaches sexual maturity between 2 and 3 years of age, with an average size at sexual maturity of close to 20 cm in total length [7]. Its reproduction is seasonal, occurring mainly in spring and summer, when there are increases in temperature and productivity in the coastal zone [5]. Adults can reach sizes of up to 40 cm, with the commercial harvest size generally being between 25 and 30 cm [5]. It feeds on small benthic invertebrates, such as crustaceans, molluscs and polychaetes. A long-distance migratory pattern has not been described, although some seasonal displacement associated with environmental conditions has been observed [7]. Although successful reproduction has already been achieved under controlled conditions [8], further basic research is needed to optimize its management across different farming stages.
Among the key tools for proper management are anesthetics, which help reduce stress during common practices such as blood sampling, handling, vaccination, treatments, transportation, and induced spawning [9]. Stress in fish is often triggered during handling and transport [10,11], and can also be caused by physicochemical factors such as salinity, temperature, pH, and dissolved oxygen [12,13,14,15], negatively affecting their immune system and increasing mortality rates [16].
The use of anesthetics in aquaculture is regulated and must meet safety and efficacy standards [17]. These substances can be synthetic or plant-derived [16], with commonly used options including MS-222, benzocaine, quinaldine, 2-phenoxyethanol, and clove oil (whose active compound is eugenol) [15]. In aquaculture, anesthetics are used frequently because they considerably facilitate the various procedures mentioned earlier. These procedures have the potential to affect the commercial production of fish [18,19,20,21,22,23]. However, many of these compounds present limitations related to cost, availability, and commercial restrictions [24]. The limited availability of approved anesthetics has led to the frequent use of unauthorized products, posing risks to both fish and human health.
In Peru, there is currently no specific national regulation that governs the use of anesthetics in aquaculture. However, general guidelines for veterinary pharmaceutical products in aquaculture fall under the supervision of the Servicio Nacional de Sanidad Agraria del Perú (SENASA). In the absence of specific legislation, substances such as eugenol are used in experimental and production settings following international references and best practices, particularly those recommended by entities such as the Food and Agriculture Organization (FAO) and OIE (World Organisation for Animal Health). For approval, anesthetics must undergo rigorous evaluations, considering their effectiveness, toxicity, and adverse effects, including potential genotoxicity [25]. Their performance depends on biological (species, size, reproductive stage) and environmental (temperature, salinity, pH) variables, which influence induction and recovery times [26,27].
A. scapularis, commonly known as Peruvian grunt, belongs to the family Haemulidae, characterized by coastal marine species that emit characteristic sounds through the contraction of muscles associated with the swim bladder [5]. This species is distributed in the southeastern Pacific, mainly from northern Peru to northern Chile, and inhabits rocky areas and sandy bottoms, commonly between 5 and 50 m deep [6]. It reaches sexual maturity between 2 and 3 years of age, with an average size at sexual maturity close to 20 cm in total length [7]. Reproduction is seasonal, occurring mainly in spring and summer, when there are increases in temperature and productivity in the coastal zone [8].
This study evaluated the effects of eugenol and tricaine (MS-222) on juveniles of A. scapularis, focusing on procedures where anesthetic use can prevent physical damage and minimize stress and its physiological consequences. The appropriate use of anesthetics is essential for handling, transportation, and experimental procedures in fish, with stress control being a critical factor in aquaculture research [28].
2. Materials and Methods
The methodology used in this study has been previously validated in other works [24,29,30], where it demonstrated its effectiveness in the evaluation and management of the studied variables. Based on this background, the methodology was refined and adapted to meet our specific needs and the context in which the research was conducted, ensuring its applicability under the conditions of the study.
2.1. Conditioning of Juvenile A. scapularis
This study was conducted at the Morro Sama Aquaculture Center (CAMOSA), located 75 km from the Tacna–Ilo Coastal Highway, in the town of Morro Sama in the coastal district of Sama Las Yaras, in the Tacna region (18°00′08″ S–70°53′21″ W). The juvenile A. scapularis underwent a 24 h fasting period prior to biometric measurements, and individuals with a length between 9 and 10 cm were selected (length: 9.44 ± 0.37 cm, weight: 17.13 ± 2.02 g). They were then transferred to the laboratory, where they remained in a 1 m diameter, 800 L fiberglass circular tank, undergoing a two-week acclimatization period. During this acclimatization period, the water parameters were maintained within a specific range: pH between 7 and 8, temperature of 17 to 18 °C, and oxygen concentration of 7 to 8 mg/L. These conditions were established with the aim of minimizing stress, replicating normal conditions, and ensuring the validity of the experiment.
Seawater was used for the conditioning process. It was passed through a filtration system with 20, 10, and 5 μm filters. Additionally, the water used for induction and recovery underwent the same filtration process, followed by ultraviolet (UV) radiation treatment. The salinity of the seawater used throughout the experiment was approximately 35‰.
During the acclimatization period, the animals were fed handmade food three times a day. This food consisted of 50% protein. As part of the preparation for the experimental exposures, the fish were subjected to a 24 h fasting period.
In the experiments with eugenol (Prevest, 70%) and tricaine (Syndel, 100%), five different anesthetic doses (20, 40, 60, 80, and 100 mg/L) were used, each with three replicates, employing 6 L tanks with 15 individuals per tank (43 kg/m3). Eugenol was diluted with 90% ethanol in a 1:10 ratio, and this mixture was poured into the designated ‘anesthetic tanks’. The entire process was conducted under controlled conditions.
All animals were exposed to the anesthetic prior to water renewal, ensuring consistent exposure conditions across all replicates. The renewal process was carried out immediately after this initial exposure to maintain water quality and anesthetic concentration stability throughout the trial.
2.2. Determination of Anesthetic Induction Time
To determine the anesthetic induction time, different anesthetic stages were evaluated in the fish following established parameters based on those described by Ross & Ross [24] (Table 1).

Table 1.
Response to anesthetic induction and recovery at different stages in A. scapaularis. Adapted from Ross and Ross [24].
The induction time was defined as the period from the moment the fish encountered the anesthetic solution until it exhibited a loss of body movement. For this, the fish were transferred to the induction tank, which contained the water and anesthetic mixture. From this point onward, the changes in the behavior of the fish under study were closely observed, evaluating parameters such as swimming axis, body mobility, opercular frequency and rhythm, as well as responses to external mechanical stimuli and reflex responses. Anesthesia exposure durations were accurately recorded using a stopwatch.
2.3. Determination of Anesthetic Recovery Time
To determine the recovery time, the modified scale of Ross and Ross [24] (Table 1) was followed. This time was established as the interval from the removal of the fish from the anesthetic solution during the induction phase (I-3) to its placement in the recovery tank, considering the various stages of recovery. The time required for the fish to fully recover from the anesthetic effect was recorded.
2.4. Data Analysis
Statistical analyses were performed using Rstudio statistical software version 2024.04.2+764 from Rstudio, Inc (Boston, MA, USA). All data were subjected to normality tests using the Anderson–Darling test and variance homogeneity using Bartlett’s test. Data were evaluated with one-way analysis of variance (ANOVA) and Tukey’s post hoc test. When normality assumptions were not met, data were analyzed using the Kruskal–Wallis test to assess differences between treatments and the Dunnett test to determine homogeneous groups. The Pearson or Spearman correlation coefficient was also applied as appropriate. The experimental graphs were created using the ggplot2 package.
3. Results
During the experiment and two weeks post-anesthesia, no mortalities were recorded in A. scapularis.
3.1. Eugenol
Stage I-1 and R-1 showed a normal distribution (A = 0.599, p = 0.098 and A = 0.434, p = 0.262, respectively). The induction times during the anesthetic sedation stages in juvenile A. scapularis exhibited a dose-dependent variation with eugenol anesthetic, indicated by the correlation coefficient (Table 2), meaning that the induction time decreased with increasing eugenol concentration at each anesthetic stage. Furthermore, the animals exposed to this anesthetic reached accelerated anesthesia at 80 mg/L (96.00 s ± 14.41). However, at concentrations of 40 mg/L and 60 mg/L, no significant differences were observed among the three evaluation stages (I-1, I-2, and I-3).

Table 2.
Anesthetic induction and recovery times (s, mean ± SD), and correlation coefficient (r) in juvenile A. scapularis at different concentrations of eugenol. Different letters indicate significant differences (one-way ANOVA, p < 0.05).
Regarding recovery time, it can be observed that at concentrations of 40 mg/L and 60 mg/L, there was no significant difference during stages R-1 and R-2. However, at concentrations of 80 mg/L and 100 mg/L, there was a significant difference in each stage (R-1, R-2, and R-3).
Figure 1 shows that the fish subjected to the anesthetic induction process experience an alteration in their swimming ability, manifested by the loss of flotation axis, irregular movements, and a marked increase in opercular frequency (Figure 1a). In a more advanced phase, a progressive reduction in body mobility is observed along with a decrease in opercular activity, which remains in a stable pattern, as well as the absence of a response to external stimuli (Figure 1b). Finally, in the deepest stage of anesthesia, the fish present total immobility, accompanied by severe depression of opercular activity and an irregular opercular rhythm (Figure 1c).
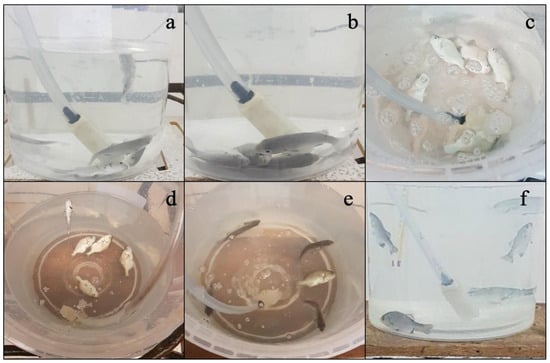
Figure 1.
Anesthetic induction response. (a) Stage I, (b) Stage II, and (c) Stage III and anesthetic recovery (d) Stage I, (e) Stage II, and (f) Stage III in A. scapularis.
During recovery, the fish maintained a state of immobility with a progressive increase in opercular frequency and lack of response to stimuli (Figure 1d). Recovery was defined as the reactivation of body movement and response to stimuli (Figure 1e). In the final stage of recovery (R-3), the fish took longer to recover their usual swimming behavior (Figure 1f).
Induction times during sedation and anesthesia stages in juvenile A. scapularis showed a dose-dependent effect of eugenol concentration, as shown in Figure 2a, where the 100 mg/L concentration presents the shortest induction time (8.3%, 79.67 s ± 6.66), while the induction time at 20 mg/L (27.1%, 237.67 s ± 43.36) shows the longest induction time, with a negative trend. Additionally, it is observed that at the 100 mg/L concentration, the recovery time (91.7%, 881.33 s ± 6.11) is higher than the recovery time at the 40 mg/L concentration (72.9%, 858.67 ± 23.35) with a positive trend.
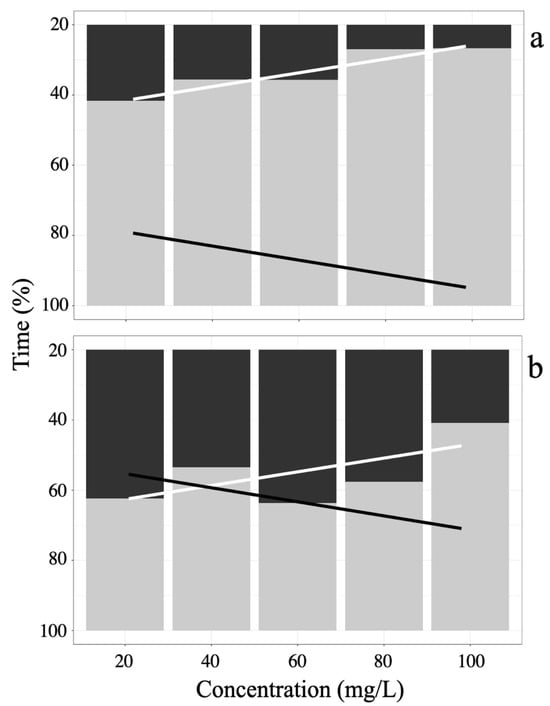
Figure 2.
Percentage anesthesia time in A. scapularis with (a) Eugenol, and (b) Tricaine MS-222. Induction state (black bars) and its trend line (black line), anesthetic recovery state (gray bars) and its trend line (white line).
3.2. Tricaine MS-222
The R-1 and R-3 stages followed a normal distribution (A = 0.413, p = 0.294 and A = 0.585, p = 0.107, respectively). The induction times in juvenile A. scapularis showed a dose-dependent variation of the anesthetic MS-222, indicated by the correlation coefficient (Table 3), with the concentrations of 20 mg/L and 40 mg/L showing no effect during the I-2 and I-3 stages, indicating no significant difference between these two stages. The 60 mg/L concentration showed the longest induction time (352.33 s ± 30.52), with a prolonged I-3 stage (204.00 ± 9.64). Recovery time in the R-1 stage showed no significant difference among treatments (F = 0.5362, p = 0.477).

Table 3.
Induction and recovery anesthesia times (s, mean ± SD), and correlation coefficient (r) in juvenile A. scapularis at different concentrations of MS-222. Different letters indicate significant differences (one-way ANOVA, p < 0.05).
As shown in Figure 1, the anesthetic induction process in fish generates a series of behavioral responses, initially characterized by the loss of postural control, erratic movements, and an increase in opercular frequency (Figure 1a). As the anesthetic effect progresses, there is a decrease in body mobility, along with a reduction in opercular frequency that maintains a stable pattern and a lack of response to stimuli (Figure 1b). In the I-3 stage, commonly referred to as the deep anesthesia phase, the fish reach complete immobility, accompanied by a marked reduction in opercular activity and altered respiratory rhythm (Figure 1c).
In the recovery phase (R-1), no differences were observed in the recovery patterns across all treatments, characterized by persistent immobility, a progressive increase in opercular frequency, and a lack of response to stimuli (Figure 1d). In the R-2 stage, late reactivation of mobility and recovery of response to mechanical stimuli were observed (Figure 1e). Finally, in the R-3 stage, the fish took longer to restore swimming axis control and to reach behavior like that observed before anesthesia (Figure 1f).
4. Discussion
The exposure of juvenile A. scapularis to the anesthetics eugenol and tricaine (MS-222) demonstrated a differential response in anesthetic induction and recovery. The evaluation of anesthetic stages was conducted following standardized methodologies described by McFarland [31], Hikasa et al. [32], Iwama [33], Cooke et al. [34], and Ross and Ross [24] with the anesthetic stage characteristics in A. scapularis being consistent with those previously reported by these and other authors (Table 4).

Table 4.
Anesthesia of other marine and freshwater fish.
4.1. Eugenol
Studies by Gomes et al. [46], Meinertz et al. [47], and Cupp et al. [48,49] highlight that eugenol, the main active component of clove oil (70–90% by weight), has been established as an effective anesthetic for numerous species of teleost fish [50]. The induction times for the sedation and anesthesia stages in juvenile A. scapularis showed a dose-dependent variation with eugenol (Figure 2).
These results suggest that higher concentrations of the anesthetic result in shorter induction times, a claim supported by previous research [51], which indicates that concentration is inversely proportional to induction time.
Moreover, the recovery time was greater than the anesthetic induction time, confirming that higher concentrations lead to a longer recovery time in fish. These findings are consistent with previous studies that highlight the relationship between eugenol concentration and recovery duration [50].
Other studies, such as those by He et al. [9], provided information on induction and recovery times for adult spotted sea bass (Lateolabrax maculatus), indicating that effective concentrations for eugenol were 60 mg/L. Similar results were obtained for A. scapularis in this study, with longer times for deep sedation (I-3) at concentrations of 20, 40, and 60 mg/L eugenol.
Additionally, Woody et al. [52] demonstrated that eugenol is useful as an anesthetic in red salmon (Oncorhynchus nerka), achieving anesthesia induction with 50 mg/L and surgical anesthesia with 80 mg/L in less than three minutes, with no mortality cases. Moreover, Cunha and Rosa [50] found that concentrations of 20 to 60 mg/L eugenol were effective for immobilizing seven species of tropical reef fish, with short induction times (<2 min) and recovery times of 2 to 4 min, directly related to anesthetic concentration. In our work, A. scapularis showed anesthetic induction times (<4 min) and recovery times ranging from 11 to 17 min.
He et al. [9] evaluated the acute toxicity of eugenol and benzocaine and the estimated induction and recovery times. Additionally, the anesthetic efficacy of different concentrations of eugenol at water temperatures of 20 and 30 °C was compared. They concluded that eugenol was an effective anesthetic, but its addition did not improve the transport of sea bass. Purbosari et al. [53] explored both natural and synthetic anesthetics derived from terrestrial and aquatic resources. This review of the different types, sources, and applications of natural and synthetic anesthetics was used to investigate the potential of marine algae as an anesthetic and the advantages and disadvantages of anesthetics. Uehara et al. [54] and Weber et al. [55] evaluated the efficacy of four anesthetic agents (including eugenol and MS-222) in Oligosarcus argenteus and Solea senegalensis. The conclusion of these two studies was that all the anesthetics tested effectively induced anesthesia in both fish species. Jerez-Cepa et al. [56] evaluated the effects of sedation doses of two anesthetics, clove oil (CO) and MS-222, on markers related to the regulation of the HPI (hypothalamic–pituitary–interrenal) axis and energy management in juvenile gilthead seabream (Sparus aurata) following simulated transport and subsequent recovery. Both anesthetics induced changes in the energy resource management of S. aurata.
Thus, eugenol presents itself as an alternative for the sedation and anesthesia of fish due to its low associated toxicity. However, it is important to determine the appropriate concentrations for the specific purpose of the experiment, as studies have reported toxicity and subsequent mortality in other teleosts when inappropriate concentrations were used [57]. Although eugenol proved effective as an anesthetic agent at low concentrations, several studies have reported potential toxic effects at higher concentrations or prolonged exposures, including damage to gill tissues, oxidative stress, and genotoxicity in teleost fish [25,49]. In addition, its use in fish intended for human consumption is not yet approved by regulatory agencies such as the FDA, which limits its commercial application in many countries. Therefore, it is essential to determine safe sublethal levels for this species through additional studies addressing both acute and chronic toxicity.
Although MS-222 is FDA approved for use in fish intended for human consumption, it requires a mandatory 21-day waiting period before slaughter [58,59], which may limit its applicability in certain production systems where rapid processing is needed.
4.2. MS-222 (Tricaine)
The results of this study support the assertion that higher concentrations of the anesthetic lead to shorter induction times, which is consistent with previous research [51] that suggest that the anesthetic concentration is inversely proportional to induction time.
In line with these findings, Uehara et al. [54] conducted studies demonstrating the efficacy of various anesthetics in inducing anesthesia in lambari-bocarra (Oligosarcus argenteus), highlighting that concentrations of 75 and 100 mg/L of MS-222 induced deep anesthesia. In our study, 60 and 80 mg/L induced a longer period of deep anesthesia (I-3).
On the other hand, ref. [60] simulated the transport of A. scapularis using tricaine (MS-222) for 24 h and recommended transporting them at 19 °C with MS-222 at 15 mg/L. In contrast, in our study, A. scapularis showed the longest deep sedation time at 60 mg/L. This difference in suggested concentrations is likely due to the exposure time to the anesthetic, as our experiment aimed for deep anesthesia, unlike the goal of Rosado et al. [60], who aimed for low sedation that would allow for transport and high survival of the species. However, other studies have established that the effective dose of MS-222 for fish is 100 mg/L [61,62,63,64,65,66]. Additionally, some fish species require higher concentrations (140–150 mg/L) of MS-222 to achieve the desired induction (≤3 min) and recovery (≤5 min) times [9,67,68], which are similar to those recorded in our study, with induction times 2.5–6 min and recovery times 4.3–7 min.
Of the two anesthetics studied, eugenol has a greater variability in effective concentrations according to the species of fish. Although MS-222 is FDA approved for use in fish intended for human consumption, it requires a mandatory 21-day waiting period before slaughter [58,59], which may limit its applicability in certain production systems where rapid processing is needed.
In addition, MS-222 has been considered more dangerous, with reports of potential ocular and neurological disorders in fish, amphibians, and humans with chronic exposure, although no recent incidents have been reported [69]. However, chemical anesthesia remains the only approved method for anesthetizing fish during sampling and surgical procedures according to various organizations [70]. Although MS-222 is one of the most used and approved anesthetics in aquaculture, its toxicity has also been widely documented, especially at high concentrations or following prolonged exposure. Adverse effects such as mucosal irritation, neurological alterations, and respiratory depression have been reported in several species [64,69]. Furthermore, its use requires a mandatory withdrawal period before the fish can be consumed, which adds complexity to its practical application. These aspects must be considered when selecting the most appropriate anesthetic according to the specific purpose of the aquaculture procedure.
4.3. Perspectives and Development of Anesthetics in Aquaculture
The use of anesthetics in modern aquaculture remains an area of ongoing research, driven by the need to optimize animal welfare. In the case of Anisotremus scapularis, anesthesia could be especially beneficial during routine aquaculture procedures, such as biometric measurements, vaccination, selection, transport, and tissue sampling. These activities, when performed without sedation, can trigger acute stress responses that affect survival, growth, and immune function. Applying effective anesthetic protocols during these procedures can significantly improve fish welfare and the overall success of aquaculture operations [71]. Traditional anesthetics such as eugenol and MS-222 have proven effective in various species, including Anisotremus scapularis. However, they have limitations in terms of regulation, toxicity, and variability in response depending on the species and environmental conditions.
One of the main lines of development in this field is the search for alternative anesthetics that are safer, more effective, and environmentally sustainable. Recent studies have explored the use of synthetic anesthetics and natural compounds derived from terrestrial and aquatic resources [53], which show potential as sedatives in fish due to their hypnotic properties and lower environmental impact. Some algae species, such as Ecklonia cava [72], Eucheuma cottonii [53], and Inyegaria estrellada [73], have been identified as sources of bioactive compounds with anesthetic effects. However, more research is needed to evaluate their efficacy, optimal dosages, and potential adverse effects in aquaculture species of interest.
On the other hand, the development of synthetic anesthetics with improved safety profiles is also a promising line of research. New compounds could be designed to minimize side effects and reduce post-anesthesia recovery time, thus improving the efficiency of fish handling. Furthermore, innovative approaches such as the use of nanoencapsulation to prolong the anesthetics’ effects and control their release have been proposed, enabling more precise and safe application in intensive production systems [74].
Finally, the future of anesthesia in aquaculture not only depends on the development of new compounds but also on optimizing their application. Combining anesthetics with management strategies, such as water temperature control or supplementation with anti-stress additives in feed, could enhance their effectiveness and reduce reliance on potentially harmful chemicals. In this regard, future studies will focus on identifying more effective, safer, and economical natural anesthetics for fish species of economic importance, both ornamental and for consumption, encompassing different sizes and ages [53].
Therefore, the development of anesthetics in aquaculture should be directed toward safer, more efficient, and sustainable alternatives. Research into natural compounds, the improvement of synthetic formulations, and the optimization of application protocols will be key to ensuring the welfare of fish and the economic and environmental viability of the aquaculture industry. Despite the results obtained in this study, we acknowledge that physiological health parameters of the fish following exposure to the anesthetics were not evaluated. Therefore, future studies will include the analysis of stress indicators such as cortisol and glucose, as well as hematological and genotoxic parameters, in order to establish the safety of these substances for sustained use in aquaculture. In addition, future research should evaluate the actual potential of anesthetics to modulate the hypothalamic–pituitary–interrenal (HPI) axis and reduce cortisol release, which plays a critical role in the fish stress response [75]. Understanding these physiological interactions will provide better guidance for selecting anesthetics that not only reduce behavioral indicators of stress, but also minimize the physiological impact on fish health.
5. Conclusions
- We determined that an effective concentration of eugenol in the range of 20 to 60 mg/L and 80 mg/L of MS-222 was sufficient to achieve induction and anesthetic recovery times of less than 6 and 16 min, respectively, with no observed mortality.
- In this study, eugenol was a more effective anesthetic than MS-222. This efficacy was defined as a combination of: (a) short induction times, (b) complete recovery with no visible adverse effects, and (c) an adequate duration of deep anesthesia (stage I-3) that allowed handling procedures to be performed with minimal interference from fish movement. In addition, eugenol showed better performance in terms of stability and duration of the deep anesthetic state.
Author Contributions
Conceptualization, L.A.E.-R. and R.P.-V.; Data curation, L.A.E.-R., R.P.-V. and J.I.H.; Formal analysis, L.A.E.-R., R.P.-V. and J.I.H.; Funding acquisition, L.A.E.-R.; Investigation, L.A.E.-R., R.P.-V., J.I.H. and Y.P.-V.; Methodology, R.P.-V., J.I.H. and Y.P.-V.; Resources, L.A.E.-R.; Visualization, L.A.E.-R.; Writing—original draft, L.A.E.-R., R.P.-V., J.I.H. and Y.P.-V.; Writing—review and editing, L.A.E.-R., R.P.-V. and J.I.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universidad Nacional Jorge Basadre Grohmann through the CANON contract “Investigación y desarrollo de las tecnologías de cultivo de peces marinos de importancia económica: corvina (Cilus gilberti) y sargo (Anisotremus scapularis) en la región Tacna” (Resolución Rectoral N° 3780-2014-UN/JBG). This article was carried out in the framework of the International Cooperation Network SEASOS (Euro-Latin Symbiosis for Sustainable Aquaculture) funded by program FORCYT (OEI-UE 2021-2023) and the Latin American Agro-Aquaculture Network (SIBIOLAT). The APC was funded by UNJBG.
Institutional Review Board Statement
This study was approved by the Ethics and Biosafety Committee of AQUAINNOVA (Code No. 07/2023—4 February 2023).
Informed Consent Statement
All animal procedures and handling as described in the experimental studies mentioned in this study were carried out according to the Peruvian law #30407 «Law on Animal Protection and Welfare» governed by The Ministry of Agriculture and Irrigation (MINAGRI, Peru) (https://www.leyes.congreso.gob.pe/Documentos/Leyes/30407.pdf) (accessed on 18 January 2019).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
The authors express their gratitude to Universidad Nacional Jorge Basadre Grohmann (UNJBG) for the financing of the project: “Investigación y desarrollo de las tecnologías de cultivo de peces marinos de importancia económica: corvina (Cilus gilberti) y sargo (Anisotremus scapularis) en la región Tacna” (Resolución Rectoral N° 3780-2014-UN/JBG), to Fondo Nacional de Desarrollo Pesquero (FONDEPES) by the rearing facilities provided in the Centro de Acuicultura de Morro Sama (CAMOSA). to the OPI Staff (Oficina de Proxectos Internacionais) and the ORI Staff (Oficina de Relaciones Internacionales) from Universidade de Vigo (Vigo, Spain), to the OEI Staff (Organización de Estados Iberoamericanos, Educación Superior y Ciencia), as well as to the Technical Staff from UNJBG and FONDEPES for their invaluable technical help during the experimentation. We thank the anonymous reviewers for their suggestions and recommendations to improve the quality of this manuscript. This article was carried out in the framework collaboration network SEASOS (Simbiosis Euro-Latina para la Acuicultura Sostenible (FORCYT, OEI) and the Latin American Agro-Aquaculture Network (SIBIOLAT).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Carrera, L.; Cota, N.; Linares, J.; Castro, A.; Orihuela, L.; Silva, E.; Montes, M. Manual Para Acondicionamiento de Reproductores de Chita Anisotremus scapularis. Inf. Inst. Mar Perú 2018, 45, 263–276. [Google Scholar]
- Ramos, L.A.E.; Layme, V.F.C.; Victoriano, R.G.P.; Choqueapaza, J.P.; Mamani, Z.C. Captura, Acondicionamiento y Primer Desove de Sargo Anisotremus scapularis en la Región Tacna. Cienc. Desarro. 2019, 25, 68–74. [Google Scholar] [CrossRef]
- Montes, M.; Castro, A.M.; Linares, J.F.; Orihuela, L.I.; Carrera, L.J. Embryonic Development of Peruvian Grunt Anisotremus scapularis (Perciformes: Haemulidae). Rev. Biol. Mar. Oceanogr. 2019, 54, 166–173. [Google Scholar] [CrossRef]
- Instituto del Mar del Perú: IMARPE. Ciclo de Vida de la Chita Anisotremus scapularis. Ser. Divulg. Científica IMARPE 2015, 1, 1–20. [Google Scholar]
- Chirichigno, N. Clave para Identificar los Peces Marinos del Perú; FAO: Lima, Peru, 1974; Volume 44. [Google Scholar]
- Froese, R.; Pauly, D. Species List: World Wide Web Electronic Publication. Available online: www.fishbase.org (accessed on 17 January 2025).
- IMARPE. Evaluación Pesquera y Perspectiva de Manejo de Chita Anisotremus scapularis en el Litoral Peruano; IMARPE: Lima, Peru, 2018. [Google Scholar]
- Pepe-Victoriano, R.; Huanacuni, J.I.; Presa, P.; Espinoza-Ramos, L.A. Reproductive Management: Conditioning, Spawning and Development of Peruvian Grunt Anisotremus scapularis in Southern Peru. PeerJ 2025, 13, e18655. [Google Scholar] [CrossRef]
- He, R.; Lei, B.; Su, Y.; Wang, A.; Cui, K.; Shi, X.; Chen, X. Effectiveness of Eugenol as an Anesthetic for Adult Spotted Sea Bass (Lateolabrax maculatus). Aquaculture 2020, 523, 735180. [Google Scholar] [CrossRef]
- Pepe-Victoriano, R.; Pepe-Vargas, P.; Yañez-Valenzuela, M.; Aravena-Ambrosetti, H.; Olivares-Cantillano, G.; Méndez-Abarca, F.; Huanacuni, J.I.; Méndez, S.; Espinoza-Ramos, L. Growth of Oncorhynchus mykiss (Rainbow Trout) through a Recirculation System in the Foothills of the Extreme North of Chile. Animals 2024, 14, 2567. [Google Scholar] [CrossRef]
- Pepe-Victoriano, R.; Aravena-Ambrosetti, H.; Merino, G.E. Breeding of a Wild Population of South Pacific Bonito Sarda Chiliensis Chiliensis (Cuvier 1832) Broodstock under Laboratory Conditions in Pisagua, Northern Chile. Animals 2021, 12, 24. [Google Scholar] [CrossRef]
- Bell, T.G.; Johnson, M.T.; Jickells, T.D.; Liss, P.S. Ammonia/Ammonium Dissociation Coefficient in Seawater: A Significant Numerical Correction. Environ. Chem. 2007, 4, 183–186, Erratum in Environ. Chem. 2008, 5, 258. [Google Scholar] [CrossRef]
- Kwasek, K.; Rimoldi, S.; Cattaneo, A.G.; Parker, T.; Dabrowski, K.; Terova, G. The Expression of Hypoxia-Inducible Factor-1α Gene Is Not Affected by Low-Oxygen Conditions in Yellow Perch (Perca flavescens) Juveniles. Fish Physiol. Biochem. 2017, 43, 849–862. [Google Scholar] [CrossRef]
- Mancera, J.M.; Martínez-Rodríguez, G.; Skrzynska, A.K.; Martos-Sitcha, J.A. Osmoregulatory Role of Vasotocinergic and Isotocinergic Systems in the Gilthead Sea Bream (Sparus aurata L). Gen. Comp. Endocrinol. 2018, 257, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Chacoff, L.; Ruiz-Jarabo, I.; Páscoa, I.; Gonçalves, O.; Mancera, J.M. Yearly Growth and Metabolic Changes in Earthen Pond-Cultured Meagre Argyrosomus regius. Sci. Mar. 2014, 78, 193–202. [Google Scholar] [CrossRef]
- Tacchi, L.; Lowrey, L.; Musharrafieh, R.; Crossey, K.; Larragoite, E.T.; Salinas, I. Effects of Transportation Stress and Addition of Salt to Transport Water on the Skin Mucosal Homeostasis of Rainbow Trout (Oncorhynchus mykiss). Aquaculture 2015, 435, 120–127. [Google Scholar] [CrossRef]
- Priborsky, J.; Velisek, J. A Review of Three Commonly Used Fish Anesthetics. Rev. Fish. Sci. Aquac. 2018, 26, 417–442. [Google Scholar] [CrossRef]
- Altun, T.; Bilgin, R.; Danabaş, D. Efectos del Bicarbonato de Sodio en la Anestesia de los Juveniles de Carpa Común (Cyprinus Carpio L., 1758). Rev. Turca Pesca Cienc. Acuáticas 2009, 9, 29–31. [Google Scholar]
- Hajek, G.J.; Kłyszejko, B.; Dziaman, R. The Anaesthetic Effect of Clove Oil on Common Carp, Cyprinus carpio L. Acta Ichthyol. Piscat. 2006, 36, 93–97. [Google Scholar] [CrossRef]
- Sneddon, L.U. Clinical Anesthesia and Analgesia in Fish. J. Exot. Pet Med. 2012, 21, 32–43. [Google Scholar] [CrossRef]
- Opiyo, M.A.; Ogello, E.O.; Charo-Karisa, H. Effectiveness of Sodium Bicarbonate as an Anaesthetic for Different Sizes of Nile Tilapia (Oreochromis niloticus L., 1758) Juveniles. Int. J. Aquat. Sci. 2013, 4, 14–22. [Google Scholar]
- Githuka, C.M. Anaesthetic Effects of Sodium Bicarbonate at Different Concentrations on African Catfish (Clarias gariepinus) Juveniles. J. Aquac. Eng. Fish. Res. 2016, 2, 151–158. [Google Scholar] [CrossRef]
- Hasimuna, O.J.; Maulu, S.; Monde, C.; Mweemba, M. Cage Aquaculture Production in Zambia: Assessment of Opportunities and Challenges on Lake Kariba, Siavonga District. Egypt. J. Aquat. Res. 2019, 45, 281–285. [Google Scholar] [CrossRef]
- Ross, L.G.; Ross, B. Anaesthetic and Sedative Techniques for Aquatic Animals, 3rd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2008; ISBN 978-1-405-14938-9. [Google Scholar]
- Nascimento, H.d.S.; Crispim, B.D.A.; Francisco, L.F.V.; Merey, F.M.; Kummrow, F.; Viana, L.F.; Inoue, L.A.K.A.; Barufatti, A. Genotoxicity Evaluation of Three Anesthetics Commonly Employed in Aquaculture Using Oreochromis niloticus and Astyanax lacustris. Aquac. Rep. 2020, 17, 100357. [Google Scholar] [CrossRef]
- Flores, C. Respuestas Neuroendócrinas al Estrés En Peces Teleósteos. Rev. Ictiol. 2002, 10, 57–78. [Google Scholar]
- Carter, K. The Effects of Dissolved Oxygen on Steelhead Trout, Coho Salmon, and Chinook Salmon Biology and Function by Life Stage. Quality 2005, 1–10. Available online: https://www.noaa.gov/sites/default/files/legacy/document/2020/Oct/0.7.115.29756-000004.pdf (accessed on 20 April 2025).
- Moraes, G.; Afonso, L.B.; Barton, B.A.; Iwama, G.K.; Mackinlay, D. Stress in Fish: Consequences to Performance. Symposium Proceedings; Physiology Section, Ed.; American Fisheries Society: Bethesda, MD, USA, 2004. [Google Scholar]
- Velisek, J.; Svobodova, Z.; Piackova, V.; Groch, L.; Nepejchalova, L. Effects of Clove Oil Anaesthesia on Common Carp (Cyprinus Carpio L.). Veter Med. 2005, 50, 269–275. [Google Scholar] [CrossRef]
- da Cunha, M.A.; Zeppenfeld, C.C.; Garcia, L.d.O.; Loro, V.L.; da Fonseca, M.B.; Emanuelli, T.; Veeck, A.P.d.L.; Copatti, C.E.; Baldisserotto, B. Anesthesia of Silver Catfish with Eugenol: Time of Induction, Cortisol Response and Sensory Analysis of Fillet. Cienc. Rural. 2010, 40, 2107–2114. [Google Scholar] [CrossRef]
- McFarland, W.N. A Study of the Effects of Anaesthetics on the Behaviour and Physiology of Fishes. Publ. Inst. Mar. Sci. 1959, 6, 22–55. [Google Scholar]
- Hikasa, Y.; Takase, K.; Ogasawara, T.; Ogasawara, S. Anesthesia and Recovery with Tricaine Methanesulfonate, Eugenol and Thiopental Sodium in the Carp, Cyprinus carpio. Jpn. J. Vet. Sci. 1986, 48, 341–351. [Google Scholar] [CrossRef]
- Iwama, G.K.; Thomas, P.T.; Forsyth, R.B.; Vijayan, M.M. Heat Shock Protein Expression in Fish. Rev. Fish Biol. Fish. 1998, 8, 35–56. [Google Scholar] [CrossRef]
- Cooke, S.J.; Suski, C.D.; Ostrand, K.G.; Tufts, B.L.; Wahl, D.H. Behavioral and Physiological Assessment of Low Concentrations of Clove Oil Anaesthetic for Handling and Transporting Largemouth Bass (Micropterus salmoides). Aquaculture 2004, 239, 509–529. [Google Scholar] [CrossRef]
- Park, I.-S. The Anesthetic Effects of Clove Oil and MS-222 on Far Eastern Catfish, Silurus asotus. Dev. Reprod. 2019, 23, 183–191. [Google Scholar] [CrossRef]
- Wang, W.; Dong, H.; Sun, Y.; Cao, M.; Duan, Y.; Li, H.; Liu, Q.; Gu, Q.; Zhang, J. The Efficacy of Eugenol and Tricaine Methanesulphonate as Anaesthetics for Juvenile Chinese Sea Bass (Lateolabrax maculatus) during Simulated Transport. J. Appl. Ichthyol. 2019, 35, 551–557. [Google Scholar] [CrossRef]
- Lee, S.; Nguyen, P.T.; Song, H.-K.; Hur, S.P.; Kim, J.-H. Effect of Water Temperature, Fish Age, and MS-222 Concentration on the Anesthetization of River Pufferfish, Takifugu obscurus. Korean J. Ichthyol. 2023, 35, 67–74. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Q.; Qiu, W.; Mei, J.; Xie, J. Transport and Recovery of Turbot (Scophthalmus Maximus) Sedated with MS-222 and Eugenol: Effects on Intermediary Metabolism and Osmoregulation. Animals 2021, 11, 2228. [Google Scholar] [CrossRef]
- Teles, M.; Oliveira, M.; Jerez-Cepa, I.; Franco-Martínez, L.; Tvarijonaviciute, A.; Tort, L.; Mancera, J.M. Transport and Recovery of Gilthead Sea Bream (Sparus aurata L.) Sedated With Clove Oil and MS222: Effects on Oxidative Stress Status. Front. Physiol. 2019, 10, 523. [Google Scholar] [CrossRef]
- Villamizar, N.; De Luque, A.; Gaitán-Ibarra, S. Evaluation of Eugenol as a Sedative for the Transportation of Common Snook Centropomus undecimalis (Bloch, 1792). Aquac. Res. 2021, 52, 5898–5902. [Google Scholar] [CrossRef]
- Honorato, L.; Batista, J.V.d.S.; Durigon, E.G.; de Mello, G.L.; Medeiros, M.V. Eugenol and Tricaine Methanesulfonate as Anhestesics for the Pearl Cichlid. Acta Sci. Biol. Sci. 2021, 43, e53422. [Google Scholar] [CrossRef]
- Nuanmanee, S.; Sriwanayos, P.; Boonyo, K.; Chaisri, W.; Saengsitthisak, B.; Tajai, P.; Pikulkaew, S. Synergistic Effect between Eugenol and 1,8-Cineole on Anesthesia in Guppy Fish (Poecilia reticulata). Veter Sci. 2024, 11, 165. [Google Scholar] [CrossRef]
- Vidal, L.V.O.; Albinati, R.C.B.; Albinati, A.C.L.; de Lira, A.D.; de Almeida, T.R.; Santos, G.B. Eugenol Como Anestésico Para a Tilápia-Do-Nilo. Pesqui. Agropecu. Bras. 2008, 43, 1069–1074. [Google Scholar] [CrossRef]
- Vidal, L.V.O.; Albinati, R.C.B.; Albinati, A.C.L.; de Mecêdo, G.R. Utilização Do Eugenol Como Anestésico Para o Manejo de Juvenis de Pintado (Pseudoplatystoma corruscans). Acta Sci. Biol. Sci. 2007, 28, 275–279. [Google Scholar] [CrossRef]
- Jia, Y.; Xie, T.; Gao, Y.; Qin, H.; Guan, C. Anesthetics Efficacy and Physiological Response of MS222 and Clove Oil in Spotted Knifejaw Oplegnathus punctatus. Aquac. Rep. 2022, 25, 101201. [Google Scholar] [CrossRef]
- Gomes, D.P.; Chaves, B.W.; Becker, A.G.; Baldisserotto, B. Water Parameters Affect Anaesthesia Induced by Eugenol in Silver Catfish, Rhamdia quelen. Aquac. Res. 2011, 42, 878–886. [Google Scholar] [CrossRef]
- Meinertz, J.R.; Schreier, T.M.; Porcher, S.T.; Smerud, J.R.; Gaikowski, M.P. Depletion of Eugenol Residues from the Skin-on Fillet Tissue of Rainbow Trout Exposed to 14C-Labeled Eugenol. Aquaculture 2014, 430, 74–78. [Google Scholar] [CrossRef]
- Cupp, A.R.; Hartleb, C.F.; Fredricks, K.T.; Gaikowski, M.P. Effectiveness of Eugenol Sedation to Reduce the Metabolic Rates of Cool and Warm Water Fish at High Loading Densities. Aquac. Res. 2016, 47, 234–242. [Google Scholar] [CrossRef]
- Cupp, A.R.; Schreier, T.M.; Schleis, S.M. Live Transport of Yellow Perch and Nile Tilapia in AQUI-S 20E (10% Eugenol) at High Loading Densities. N. Am. J. Aquac. 2017, 79, 176–182. [Google Scholar] [CrossRef]
- Cunha, F.E.A.; Rosa, I.L. Anaesthetic Effects of Clove Oil on Seven Species of Tropical Reef Teleosts. J. Fish Biol. 2006, 69, 1504–1512. [Google Scholar] [CrossRef]
- Roohi, Z.; Imanpoor, M.R. The Efficacy of the Oils of Spearmint and Methyl Salicylate as New Anesthetics and Their Effect on Glucose Levels in Common Carp (Cyprinus carpio L., 1758) Juveniles. Aquaculture 2015, 437, 327–332. [Google Scholar] [CrossRef]
- Woody, C.A.; Nelson, J.; Ramstad, K. Clove Oil as an Anaesthetic for Adult Sockeye Salmon: Field Trials. J. Fish Biol. 2002, 60, 340–347. [Google Scholar] [CrossRef]
- Purbosari, N.; Warsiki, E.; Syamsu, K.; Santoso, J. Natural versus Synthetic Anesthetic for Transport of Live Fish: A Review. Aquac. Fish. 2019, 4, 129–133. [Google Scholar] [CrossRef]
- Uehara, S.; Andrade, D.; Takata, R.; Júnior, A.G.; Vidal, M. The Effectiveness of Tricaine, Benzocaine, Clove Oil, and Menthol as Anesthetics for Lambari-Bocarra Oligosarcus argenteus. Aquaculture 2019, 502, 326–331. [Google Scholar] [CrossRef]
- Weber, R.; Peleteiro, J.; Martín, L.G.; Aldegunde, M. The Efficacy of 2-Phenoxyethanol, Metomidate, Clove Oil and MS-222 as Anaesthetic Agents in the Senegalese Sole (Solea senegalensis Kaup 1858). Aquaculture 2009, 288, 147–150. [Google Scholar] [CrossRef]
- Jerez-Cepa, I.; Fernández-Castro, M.; O’Neill, T.J.D.S.; Martos-Sitcha, J.A.; Martínez-Rodríguez, G.; Mancera, J.M.; Ruiz-Jarabo, I. Transport and Recovery of Gilthead Seabream (Sparus aurata L.) Sedated with Clove Oil and MS-222: Effects on Stress Axis Regulation and Intermediary Metabolism. Front. Physiol. 2019, 10, 612. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.M.A.; Wada, S.; Hatai, K.; Yamamoto, A. Antimycotic Activity of Eugenol against Selected Water Molds. J. Aquat. Anim. Health 2000, 12, 224–229. [Google Scholar] [CrossRef]
- Parker-Graham, C.A.; Lima, K.M.; Soto, E. The Effect of Anesthetic Time and Concentration on Blood Gases, Acid-Base Status, and Electrolytes in Koi (Cyprinus Carpio) Anesthetized with Buffered Tricaine Methanesulfonate (MS-222). J. Zoo Wildl. Med. 2020, 51, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Nochetto, C.B.; Reimschuessel, R.; Gieseker, C.; Cheely, C.S.; Carson, M.C. Determination of Tricaine Residues in Fish by Liquid Chromatography. J. AOAC Int. 2009, 92, 1241–1248. [Google Scholar] [CrossRef]
- Rosado, M.; Dionicio, J.; Aguirre-Velarde, A. Evaluación de Diferentes Concentraciones de Tricaína (MS-222) en el Transporte de Chitas (Anisotremus scapularis) Juveniles. Rev. Investig. Vet. Perú 2016, 27, 687. [Google Scholar] [CrossRef]
- Iwama, G.K.; Ackerman, P.A. Anaesthetics. In Biochemistry and Molecular Biology of Fishes; Elsevier: Amsterdam, The Netherlands, 1994; Volume 3, pp. 1–15. [Google Scholar]
- Kreiberg, H. Stress and Anesthesia. In The Laboratory Fish; Elsevier: Amsterdam, The Netherlands, 2000; pp. 503–511. [Google Scholar]
- Neiffer, D.L.; Stamper, M.A. Fish Sedation, Anesthesia, Analgesia, and Euthanasia: Considerations, Methods, and Types of Drugs. ILAR J. 2009, 50, 343–360. [Google Scholar] [CrossRef]
- Carter, K.M.; Woodley, C.M.; Brown, R.S. A Review of Tricaine Methanesulfonate for Anesthesia of Fish. Rev. Fish Biol. Fish. 2011, 21, 51–59. [Google Scholar] [CrossRef]
- Pelkowski, S.D.; Kapoor, M.; Richendrfer, H.A.; Wang, X.; Colwill, R.M.; Creton, R. A Novel High-Throughput Imaging System for Automated Analyses of Avoidance Behavior in Zebrafish Larvae. Behav. Brain Res. 2011, 223, 135–144. [Google Scholar] [CrossRef]
- Readman, G.D.; Owen, S.F.; Murrell, J.C.; Knowles, T.G. Do Fish Perceive Anaesthetics as Aversive? PLoS ONE 2013, 8, e73773. [Google Scholar] [CrossRef]
- Roubach, R.; Gomes, L.d.C.; Val, A.L. Safest Level of Tricaine Methanesulfonate (Ms-222) to Induce Anesthesia in Juveniles of Matrinxã, Brycon cephalus. Acta Amaz. 2001, 31, 159–163. [Google Scholar] [CrossRef]
- Mitjana, O.; Bonastre, C.; Insua, D.; Falceto, M.V.; Esteban, J.; Josa, A.; Espinosa, E. The Efficacy and Effect of Repeated Exposure to 2-Phenoxyethanol, Clove Oil and Tricaine Methanesulphonate as Anesthetic Agents on Juvenile Angelfish (Pterophyllum scalare). Aquaculture 2014, 433, 491–495. [Google Scholar] [CrossRef]
- Brown, L. Anaesthesia in fishes. J. Small Anim. Pract. 1981, 22, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Durhack, T.C.; Jeffrey, J.D.; Enders, E.C. In Search of an Anaesthesia Alternative for Field-Based Research. Aquaculture 2020, 525, 735285. [Google Scholar] [CrossRef]
- Minaz, M.; Er, A.; Ak, K.; Kurtoğlu, İ.Z.; Kayış, Ş. Determining the Appropriate Concentration of an Anesthetic Mixture in Three Different Fish Species with the PROMETHEE Decision Model. Front. Veter Sci. 2024, 11, 1492769. [Google Scholar] [CrossRef]
- Cho, S.; Yang, H.; Jeon, Y.-J.; Lee, C.J.; Jin, Y.-H.; Baek, N.-I.; Kim, D.; Kang, S.-M.; Yoon, M.; Yong, H.; et al. Phlorotannins of the Edible Brown Seaweed Ecklonia cava Kjellman Induce Sleep via Positive Allosteric Modulation of Gamma-Aminobutyric Acid Type A–Benzodiazepine Receptor: A Novel Neurological Activity of Seaweed Polyphenols. Food Chem. 2012, 132, 1133–1142. [Google Scholar] [CrossRef]
- Bushra, R.; Rahila, N.; Iqbal, A.; Somia, G. Neuropharmacological Screening of Iyengaria Stellata Revealed Its Memory Boosting, Anxiolytic and Antidepressant Effects. IRJP 2012, 10, 3. [Google Scholar]
- Charlie-Silva, I.; Feitosa, N.M.; Gomes, J.M.M.; Hoyos, D.C.d.M.; Mattioli, C.C.; Eto, S.F.; Fernandes, D.C.; Belo, M.A.d.A.; Silva, J.d.O.; de Barros, A.L.B.; et al. Potential of Mucoadhesive Nanocapsules in Drug Release and Toxicology in Zebrafish. PLoS ONE 2020, 15, e0238823. [Google Scholar] [CrossRef]
- Bertotto, D.; Poltronieri, C.; Negrato, E.; Richard, J.; Pascoli, F.; Simontacchi, C.; Radaelli, G. Whole Body Cortisol and Expression of HSP70, IGF-I and MSTN in Early Development of Sea Bass Subjected to Heat Shock. Gen. Comp. Endocrinol. 2011, 174, 44–50. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).