Prevalence and Progression of Resting ACTH, Insulin and Adiponectin Values as Indicators of Suspected Endocrine Diseases in Sport Horses and Ponies Compared to Non-Sport Horses, Ponies and Donkeys
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Examined
- -
- PPID likely, or positive cases in which the basal ACTH was found to be above the upper end of the grey zone of each specific weekly reference interval previously published (95% specific for the diagnosis of PPID) [32];
- -
- PPID unlikely, or negative cases in which basal ACTH was found to be below the lower end of the grey zone of the weekly reference intervals (95% sensitive for the exclusion of PPID diagnosis);
- -
- -
- ID unlikely, or negative cases when the resting insulin was below 32 mIU/L;
- -
- Metabolically obese likely, or positive cases in which the adiponectin concentration was found to be lower than 7.9 mIU/L [36] (animals at a higher risk of developing insulin dysregulation and laminitis);
- -
- Metabolically obese unlikely, or negative animals in which adiponectin concentrations were above 8 mIU/L.
2.2. Statistical Analysis
3. Results
3.1. Demographics
| Sport Horses | Non-Sport Horses | ||
|---|---|---|---|
| Warmblood types | 714 | Native ponies | 1976 |
| Baroque type sport horses | 68 | Heavy Draughts | 14 |
| Arabian horses | 142 | Cobs | 675 |
| American sport horses | 57 | Miniatures | 23 |
| Sport ponies | 187 | Heavy ponies | 44 |
| Racehorses | 312 | Donkeys | 94 |
| Cross breeds | 633 crossed from above | Cross breeds | 1109 crossed from above |
| Total horses | 2113 | Total horses | 3935 |
3.2. Submission Data
3.3. Endocrine Status
3.4. Analytes Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PPID | Pituitary pars intermedia dysfunction |
| ID | Insulin dysregulation |
| ACTH | Adrenocorticotropic hormone |
| EMS | Equine metabolic syndrome |
References
- Wylie, C.E.; Collins, S.N.; Verheyen, K.L.; Newton, J.R. Risk factors for equine laminitis: A case-control study conducted in veterinary-registered horses and ponies in Great Britain between 2009 and 2011. Vet. J. 2013, 198, 57–69. [Google Scholar] [CrossRef]
- McGowan, C.M. Endocrinopathic laminitis. Vet. Clin. N. Am. Equine Pract. 2010, 26, 233–237. [Google Scholar] [CrossRef]
- Durham, A.E. Endocrine Disease in Aged Horses. Vet. Clin. N. Am. Equine Pract. 2016, 32, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Ireland, J.L.; McGowan, C.M. Epidemiology of pituitary pars intermedia dysfunction: A systematic literature review of clinical presentation, disease prevalence and risk factors. Vet. J. 2018, 235, 22–33. [Google Scholar] [CrossRef]
- MacLeod, C. ECEIM consensus statement on equine metabolic syndrome. J. Vet. Intern. Med. 2019, 33, 1123–1124. [Google Scholar] [CrossRef] [PubMed]
- Muno, J.; Gallatin, L.; Geor, R. Prevalence and risk factors for hyperinsulinaemia in clinically normal horses in central ohio. J. Vet. Intern. Med. 2009, 23, 721. [Google Scholar]
- Jacob, S.I.; Geor, R.J.; Weber, P.S.D.; Harris, P.A.; McCue, M.E. Effect of age and dietary carbohydrate profiles on glucose and insulin dynamics in horses. Equine Vet. J. 2017, 50, 249–254. [Google Scholar] [CrossRef]
- Bamford, N.J.; Stewart, A.J.; El-Hage, C.M.; Bertin, F.R.; Bailey, S.R. Investigation of breed differences in plasma adrenocorticotropic hormone concentrations among healthy horses and ponies. Vet. J. 2023, 296–297, 105995. [Google Scholar] [CrossRef]
- Durham, A.E.; Potier, J.F.; Huber, L. The effect of month and breed on plasma adrenocorticotropic hormone concentrations in equids. Vet. J. 2022, 286, 105857. [Google Scholar] [CrossRef]
- Bamford, N.J.; Potter, S.J.; Harris, P.A.; Bailey, S.R. Breed differences in insulin sensitivity and insulinemic responses to oral glucose in horses and ponies of moderate body condition score. Domest. Anim. Endocrinol. 2014, 47, 101–107. [Google Scholar] [CrossRef]
- Bamford, N.J.; Baskerville, C.L.; Harris, P.A.; Bailey, S.R. Postprandial glucose, insulin, and glucagon-like peptide-1 responses of different equine breeds adapted to meals containing micronized maize. J. Anim. Sci. 2015, 93, 3377–3383. [Google Scholar] [CrossRef]
- Weckman, M.J.; Karikoski, N.P.; Raekallio, M.R.; Box, J.R.; Kvist, L. Genome-wide association study suggests genetic candidate loci of insulin dysregulation in Finnhorses. Vet. J. 2024, 303, 106063. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.L.; Holl, H.M.; Streeter, C.; Posbergh, C.; Schanbacher, B.J.; Place, N.J.; Mallicote, M.F.; Long, M.T.; Brooks, S.A. Genomewide association study reveals a risk locus for equine metabolic syndrome in the Arabian horse. J. Anim. Sci. 2017, 95, 1071–1079. [Google Scholar] [CrossRef]
- Patterson Rosa, L.; Mallicote, M.F.; Long, M.T.; Brooks, S.A. Metabogenomics reveals four candidate regions involved in the pathophysiology of Equine Metabolic Syndrome. Mol. Cell. Probes 2020, 53, 101620. [Google Scholar] [CrossRef]
- Durham, A.E.; Frank, N.; McGowan, C.M.; Menzies-Gow, N.J.; Roelfsema, E.; Vervuert, I.; Feige, K.; Fey, K. ECEIM consensus statement on equine metabolic syndrome. J. Vet. Intern. Med. 2019, 33, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Li, F.I.; Spence, R.J.; de Laat, M.A.; Harris, P.A.; Sonntag, J.; Menzies-Gow, N.J.; Durham, A.E.; Bailey, S.R.; Sillence, M.N. Association between insulin dysregulation and adrenocorticotropic hormone in aged horses and ponies with no clinical signs of pituitary pars intermedia dysfunction. Equine Vet. J. 2023, 55, 1003–1011. [Google Scholar] [CrossRef]
- de Laat, M.A.; Reiche, D.B.; Sillence, M.N.; McGree, J.M. Incidence and risk factors for recurrence of endocrinopathic laminitis in horses. J. Vet. Intern. Med. 2019, 33, 1473–1482. [Google Scholar] [CrossRef]
- Karikoski, N.P.; McGowan, C.M.; Singer, E.R.; Asplin, K.E.; Tulamo, R.M.; Patterson-Kane, J.C. Pathology of Natural Cases of Equine Endocrinopathic Laminitis Associated with Hyperinsulinemia. Vet. Pathol. 2015, 52, 945–956. [Google Scholar] [CrossRef] [PubMed]
- International Equestrian Federation (FEI). Event Paris 2024 Olympic Games. Paris 2024 Olympic Games: Schedule & Results. Available online: https://www.fei.org/events/paris-2024-olympic-games/results (accessed on 22 November 2024).
- Potter, K.; Stevens, K.; Menzies-Gow, N. Prevalence of and risk factors for acute laminitis in horses treated with corticosteroids. Vet. Rec. 2019, 185, 82. [Google Scholar] [CrossRef]
- Macon, E.L.; Harris, P.; Bailey, S.; Caldwell Barker, A.; Adams, A. Identifying possible thresholds for nonstructural carbohydrates in the insulin dysregulated horse. Equine Vet. J. 2023, 55, 1069–1077. [Google Scholar] [CrossRef]
- Treiber, K.H.; Boston, R.C.; Kronfeld, D.S.; Staniar, W.B.; Harris, P.A. Insulin resistance and compensation in Thoroughbred weanlings adapted to high-glycemic meals. J. Anim. Sci. 2005, 83, 2357–2364. [Google Scholar] [CrossRef]
- Bailey, S.R.; Menzies-Gow, N.J.; Harris, P.A.; Habershon-Butcher, J.L.; Crawford, C.; Berhane, Y.; Boston, R.C.; Elliott, J. Effect of dietary fructans and dexamethasone administration on the insulin response of ponies predisposed to laminitis. J. Am. Vet. Med. Assoc. 2007, 231, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Herbst, A.C.; Johnson, M.G.; Gammons, H.; Reedy, S.E.; Urschel, K.L.; Harris, P.A.; Adams, A.A. Development and Evaluation of a Muscle Atrophy Scoring System (MASS) for Horses. J. Equine Vet. Sci. 2022, 110, 103771. [Google Scholar] [CrossRef]
- Manfredi, J.M.; Jacob, S.I.; Boger, B.L.; Norton, E.M. A one-health approach to identifying and mitigating the impact of endocrine disorders on human and equine athletes. Am. J. Vet. Res. 2022, 84, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Frank, N. Endocrine Disorders of the Equine Athlete. Vet. Clin. N. Am. Equine Pract. 2018, 34, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Hofberger, S.; Gauff, F.; Licka, T. Suspensory ligament degeneration associated with pituitary pars intermedia dysfunction in horses. Vet. J. 2015, 203, 348–350. [Google Scholar] [CrossRef]
- Rohrbach, B.W.; Stafford, J.R.; Clermont, R.S.; Reed, S.M.; Schott, H.C., 2nd; Andrews, F.M. Diagnostic frequency, response to therapy, and long-term prognosis among horses and ponies with pituitary par intermedia dysfunction. J. Vet. Intern. Med. 2012, 26, 1027–1034. [Google Scholar] [CrossRef]
- International Equestrian Federation (FEI). 2025 Equine Prohibited Substances List. FEI Clean Sport Anti-Doping Rules. Available online: https://inside.fei.org/sites/default/files/2025%20Prohibited%20Substances%20List.pdf (accessed on 30 December 2024).
- Frank, N.; Bailey, S.; Bertin, F.R.; Burns, T.; de Laat, M.; Durham, A.E.; Kritchevsky, J.; Menzies-Gow, N. Recommendations for the Diagnosis and Management of Equine Metabolic Syndrome (EMS) and Insulin Dysregulation; Equine Endocrinology Group, 2022; Available online: https://static1.squarespace.com/static/65296d5988c69f3e85fa3653/t/667984057bceca614c913a83/1719239689544/2022-EMS-Recommendations.pdf (accessed on 30 December 2024).
- Hart, K.; Durham, A.; Frank, N.; McGowan, C.; Schott, H.; Stewart, A. Recommendations for the Diagnosis and Management of Pituitary Pars Intermedia Dysfunction (PPID); Equine Endocrinology Group, 2023; Available online: https://equineendocrinologygroup.org/ (accessed on 30 December 2024).
- Durham, A.E.; Clarke, B.R.; Potier, J.F.N.; Hammarstrand, R.; Malone, G.L. Clinically and temporally specific diagnostic thresholds for plasma ACTH in the horse. Equine Vet. J. 2021, 53, 250–260. [Google Scholar] [CrossRef]
- Dunbar, L.K.; Mielnicki, K.A.; Dembek, K.A.; Toribio, R.E.; Burns, T.A. Evaluation of Four Diagnostic Tests for Insulin Dysregulation in Adult Light-Breed Horses. J. Vet. Intern. Med. 2016, 30, 885–891. [Google Scholar] [CrossRef]
- Van Den Wollenberg, L.; Vandendriessche, V.; van Maanen, K.; Counotte, G.H.M. Comparison of Two Diagnostic Methods to Detect Insulin Dysregulation in Horses Under Field Conditions. J. Equine Vet. Sci. 2020, 88, 102954. [Google Scholar] [CrossRef]
- Delarocque, J.; Feige, K.; Carslake, H.B.; Durham, A.E.; Fey, K.; Warnken, T. Development of a Web App to Convert Blood Insulin Concentrations among Various Immunoassays Used in Horses. Animals 2023, 13, 2704. [Google Scholar] [CrossRef]
- Wooldridge, A.A.; Edwards, H.G.; Plaisance, E.P.; Applegate, R.; Taylor, D.R.; Taintor, J.; Zhong, Q.; Judd, R.L. Evaluation of high-molecular weight adiponectin in horses. Am. J. Vet. Res. 2012, 73, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Team, R.D.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Donaldson, M.T.; McDonnell, S.M.; Schanbacher, B.J.; Lamb, S.V.; McFarlane, D.; Beech, J. Variation in plasma adrenocorticotropic hormone concentration and dexamethasone suppression test results with season, age, and sex in healthy ponies and horses. J. Vet. Intern. Med. 2005, 19, 217–222. [Google Scholar] [CrossRef] [PubMed]
- de Laat, M.A.; Sillence, M.N.; Reiche, D.B. Phenotypic, hormonal, and clinical characteristics of equine endocrinopathic laminitis. J. Vet. Intern. Med. 2019, 33, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Menzies-Gow, N.J.; Stevens, K.; Barr, A.; Camm, I.; Pfeiffer, D.; Marr, C. MSeverity and outcome of equine pasture-associated laminitis managed in first opinion practice in the UK. Vet. Rec. 2010, 167, 364–369. [Google Scholar] [CrossRef]
- Pollard, D.; Wylie, C.E.; Newton, J.R.; Verheyen, K.L.P. Factors associated with euthanasia in horses and ponies enrolled in a laminitis cohort study in Great Britain. Prev. Vet. Med. 2020, 174, 104833. [Google Scholar] [CrossRef]
- Ursini, F.; Arturi, F.; D’Angelo, S.; Amara, L.; Nicolosi, K.; Russo, E.; Naty, S.; Bruno, C.; De Sarro, G.; Olivieri, I.; et al. High prevalence of Achilles tendon enthesopathic changes in patients with type 2 diabetes without peripheral neuropathy. J. Am. Podiatr. Med. Assoc. 2017, 107, 99–105. [Google Scholar] [CrossRef]
- Zhuo, Q.; Yang, W.; Chen, J.; Wang, Y. Metabolic syndrome meets osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 729–737. [Google Scholar] [CrossRef]
- Murray, R.C.; Dyson, S.J.; Tranquille, C.; Adams, V. Association of type of sport and performance level with anatomical site of orthopaedic injury diagnosis. Equine Vet. J. 2006, 38 (Suppl. S36), 411–416. [Google Scholar] [CrossRef]
- Rendle, D.; McGregor, C.A.; Bowen, M.; Carslake, H.; German, A.; Harris, P.; Knowles, E.; Menzies-Gow, N.; Morgan, R. Equine obesity: Current perspectives. UK-Vet. Equine 2018, 2 (Suppl. 5), 1–19. [Google Scholar] [CrossRef]
- Jeong, S.; Bond, S.L.; Sole-Guitart, A. Dynamic nasopharyngeal collapse in horses: What we know so far. Equine Vet. J. 2024, 56, 1129–1137. [Google Scholar] [CrossRef]
- Schwartz, A.R.; Patil, S.P.; Laffan, A.M.; Polotsky, V.; Schneider, H.; Smith, P.L. Obesity and obstructive sleep apnea: Pathogenic mechanisms and therapeutic approaches. Proc. Am. Thorac. Soc. 2008, 5, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Robin, C.A.; Ireland, J.L.; Wylie, C.E.; Collins, S.N.; Verheyen, K.L.; Newton, J.R. Prevalence of and risk factors for equine obesity in Great Britain based on owner-reported body condition scores. Equine Vet. J. 2015, 47, 196–201. [Google Scholar] [CrossRef]
- Bird, S.R.; Hawley, J.A. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc. Med. 2016, 2, e000143. [Google Scholar] [CrossRef]
- Dunger, D.B.; Salgin, B.; Ong, K. Muscle mass and insulin sensitivity in children and adolescents. Horm. Res. 2007, 66 (Suppl. 1), 79–84. [Google Scholar] [CrossRef]
- Noble, G.K.; Caldwell-Smith, A.J.; Bryden, W.l.; Sillence, M.N. Observations of Insulin Responses to Five Glucocorticoids Commonly Used in Equine Medicine. J. Equine Vet. Sci. 2011, 31, 303–304. [Google Scholar] [CrossRef]
- van Weeren, P.R.; Back, W. Musculoskeletal disease in aged horses and its management. Vet. Clin. Equine Pract. 2016, 32, 229–247. [Google Scholar] [CrossRef] [PubMed]
- Beaupere, C.; Liboz, A.; Fève, B.; Blondeau, B.; Guillemain, G. Molecular Mechanisms of Glucocorticoid-Induced Insulin Resistance. Int. J. Mol. Sci. 2021, 22, 623. [Google Scholar] [CrossRef]
- Ooi, T.; Twum-Barima, Y. Rebound elevation of cortisol following cyproheptadine withdrawal in Cushing’s disease from a pituitary macroadenoma. J. Endocrinol. Investig. 1987, 10, 107–109. [Google Scholar] [CrossRef]
- Charest, L.; Comtois, R.; Beauregard, H.; Serri, O. Growth hormone rebound after cessation of sms 201–995 treatment in acromegaly. Can. J. Neurol. Sci. 1989, 16, 442–445. [Google Scholar] [CrossRef]
- Petersenn, S.; Fleseriu, M.; Casanueva, F.F.; Giustina, A.; Biermasz, N.; Biller, B.M.K.; Bronstein, M.; Chanson, P.; Fukuoka, H.; Gadelha, M.; et al. Diagnosis and management of prolactin-secreting pituitary adenomas: A Pituitary Society international Consensus Statement. Nat. Rev. Endocrinol. 2023, 19, 722–740. [Google Scholar] [CrossRef] [PubMed]

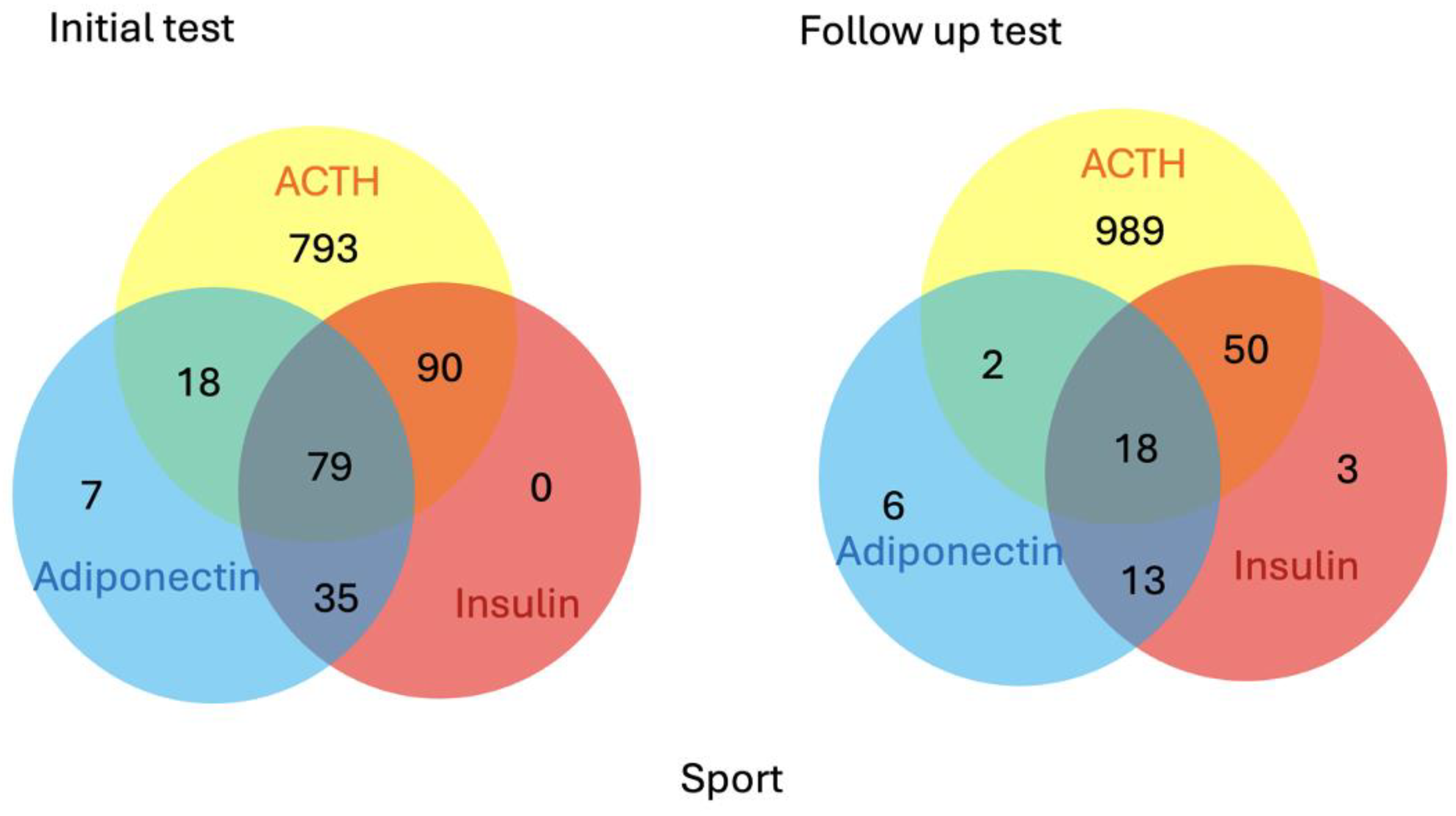
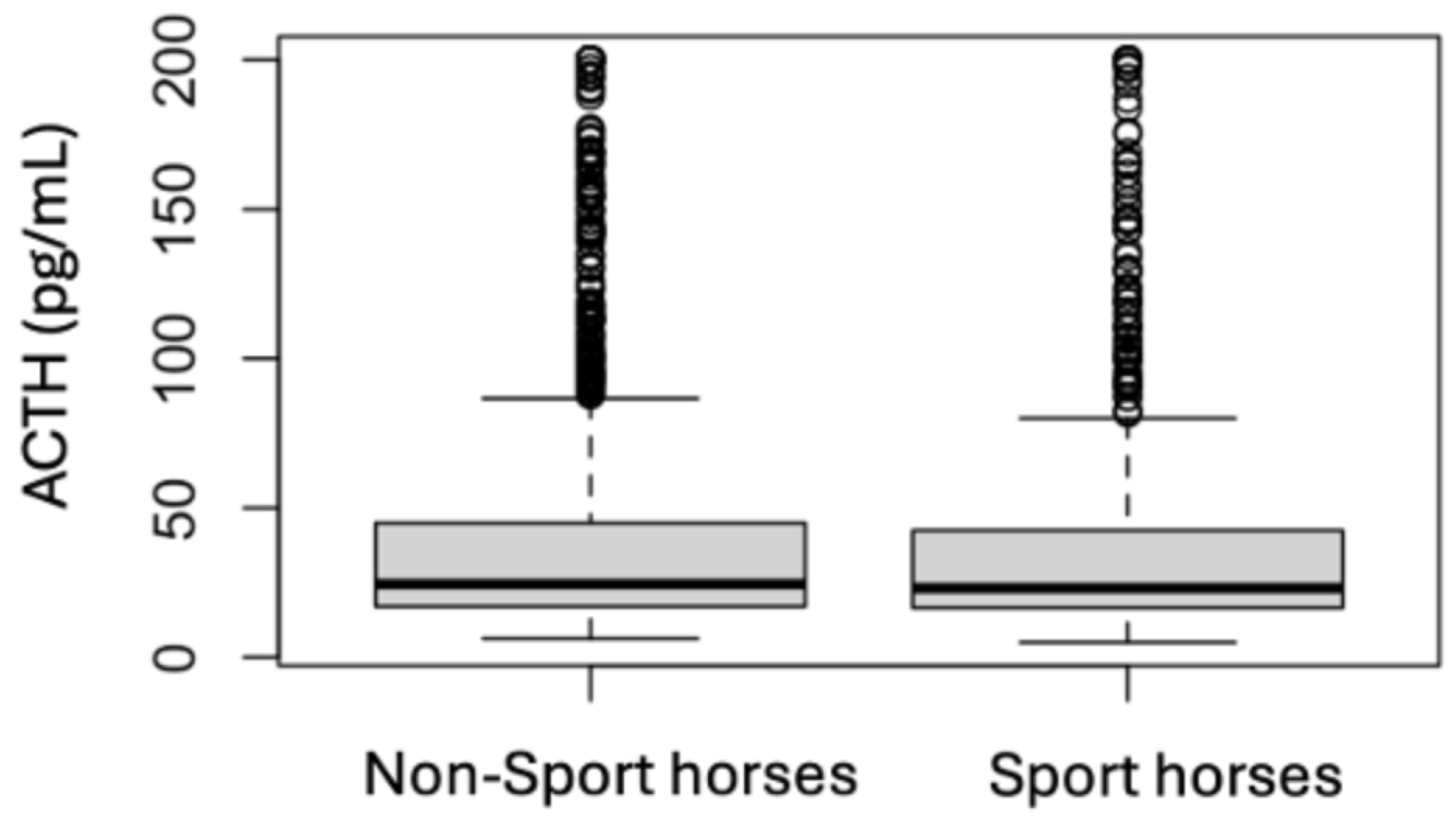
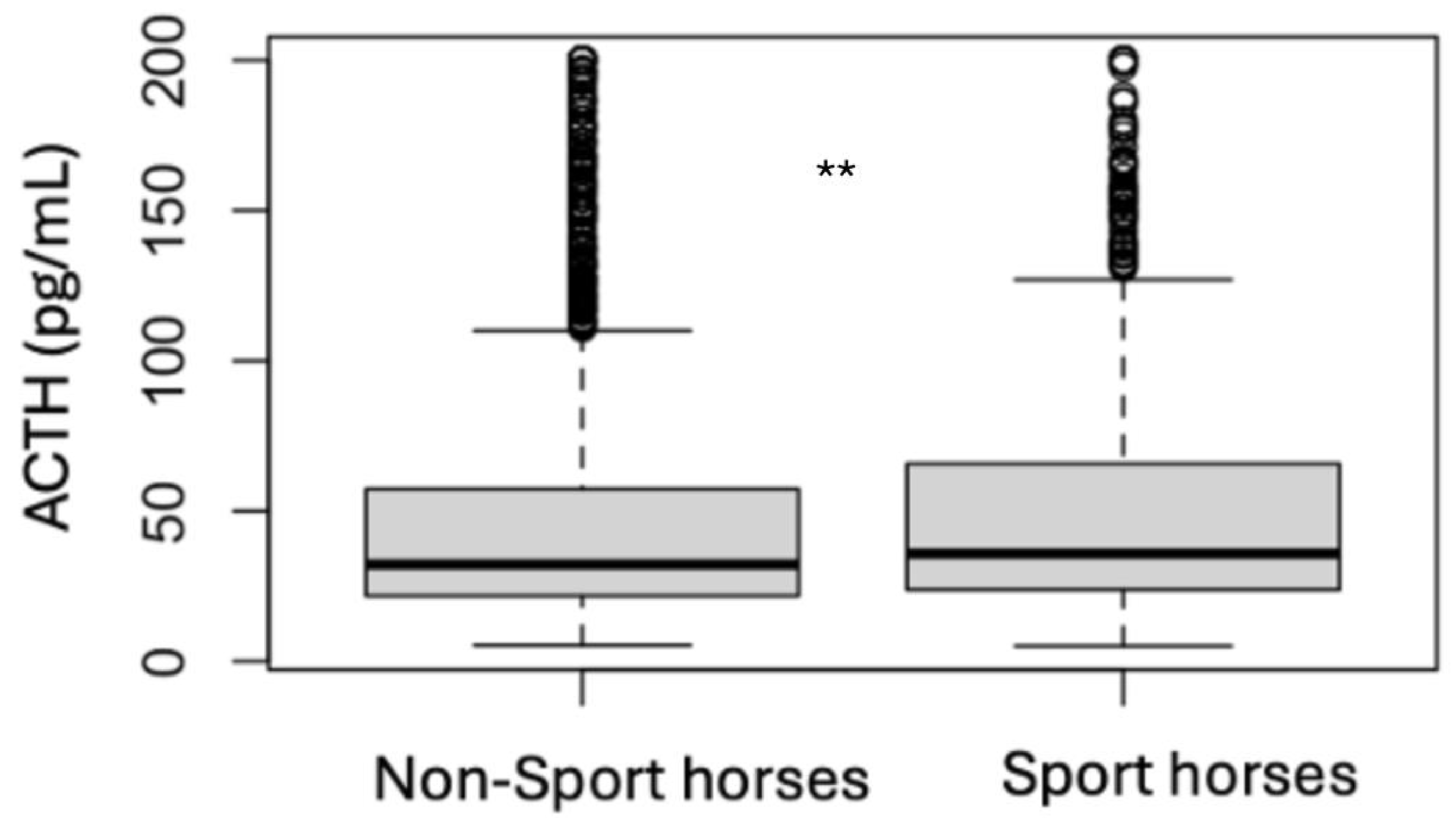
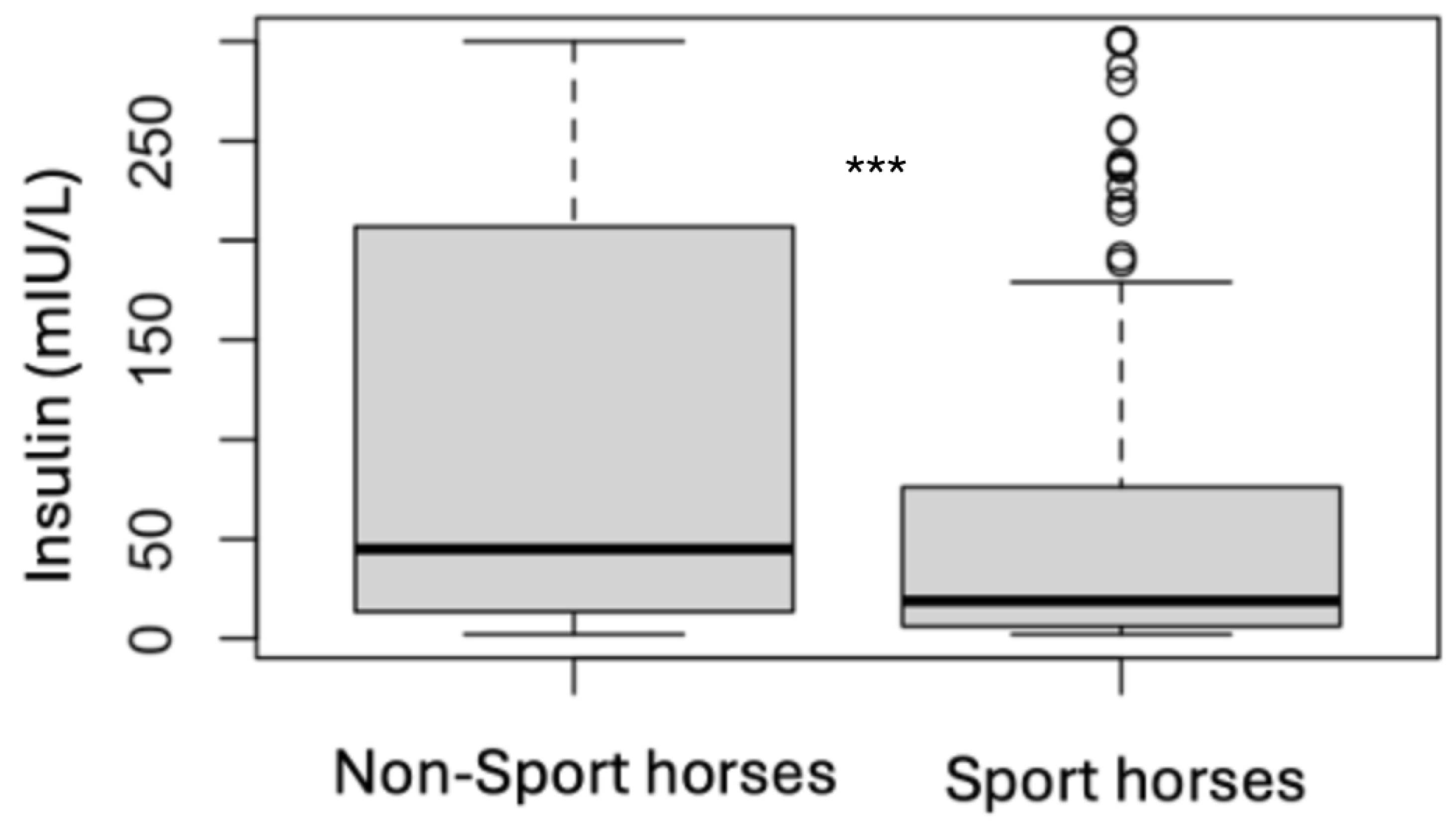
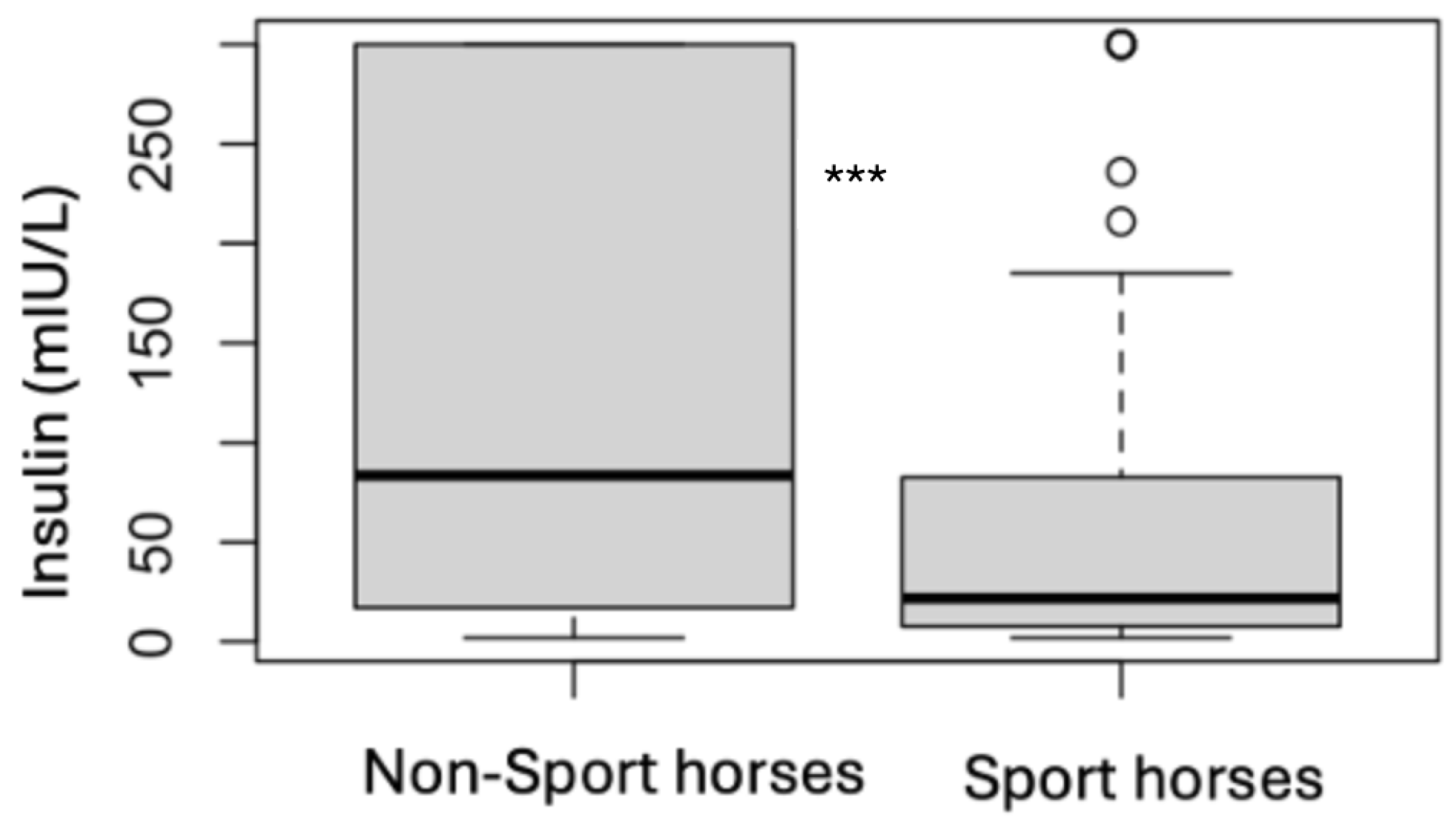
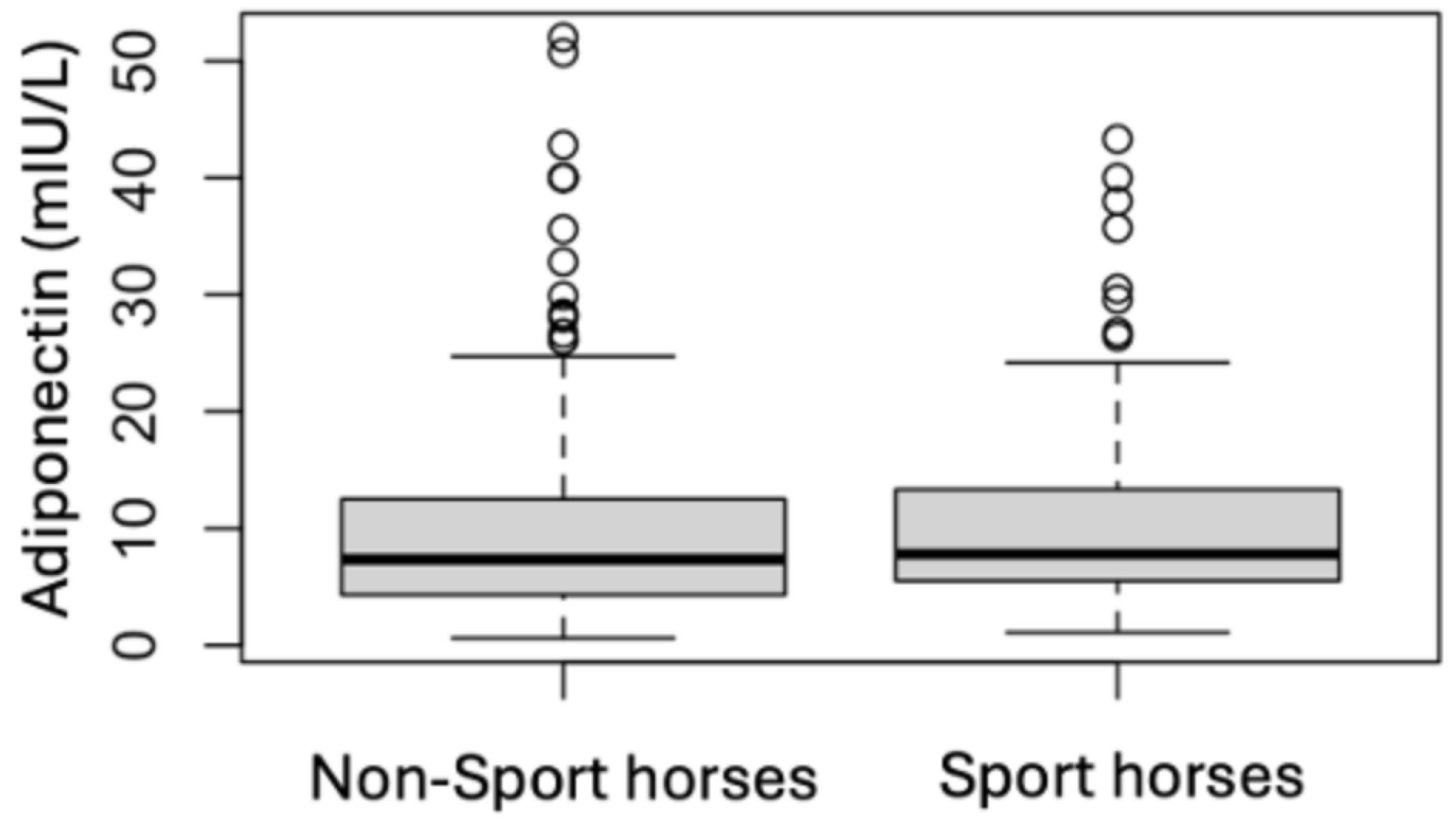
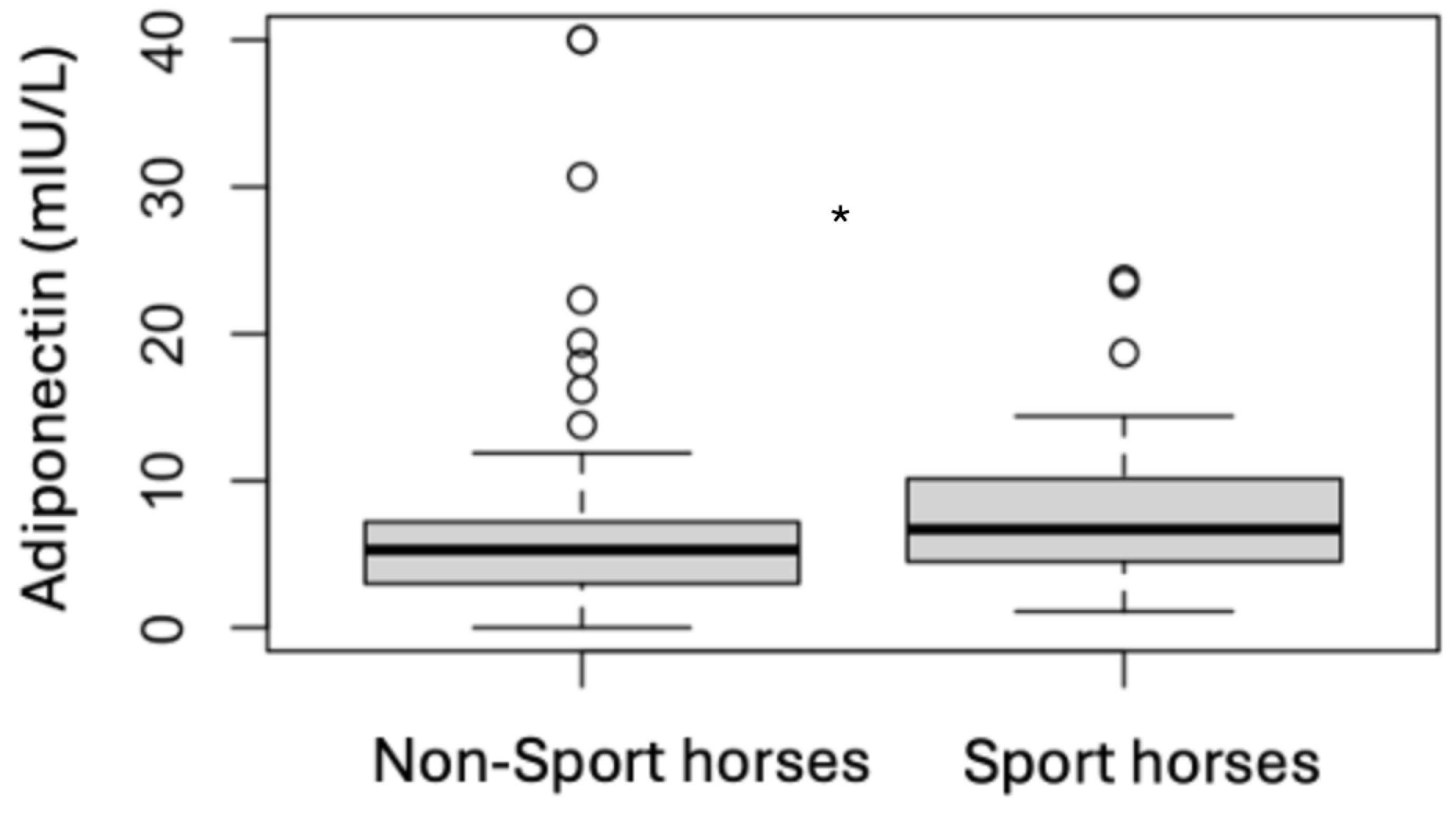
| Sport Horses | Non-Sport Horses | |||
|---|---|---|---|---|
| Initial | Follow-Up | Initial | Follow-Up | |
| PPID positive | 298 (30%) | 567 (54%) | 866 (66%) | 854 (47%) |
| PPID negative | 344 (35%) | 138 (13%) | 417 (32%) | 323 (18%) |
| ID positive | 68 (33%) | 31 (37%) | 173 (50%) | 105 (60%) |
| ID negative | 130 (64%) | 48 (57%) | 148 (43%) | 60 (34%) |
| Obesity positive | 73 (53%) | 17 (44%) | 86 (45%) | 16 (22%) |
| Obesity negative | 66 (47%) | 22 (56%) | 105 (55%) | 57 (78%) |
| Initial Tests | Follow-Up Tests | |||||
|---|---|---|---|---|---|---|
| ACTH (pg/mL) | Insulin (mIU/L) | Adiponectin (mIU/L) | ACTH (pg/mL) | Insulin (mIU/L) | Adiponectin (mIU/L) | |
| Median | 24.5 | 44.9 | 7.4 | 32.2 | 83.5 | 5.3 |
| IQR | 17.0–45.0 | 13.4–207.0 | 4.3–12.4 | 21.8–57.3 | 17.0–300.0 | 3.0–7.2 |
| Initial Tests | Follow-Up Tests | |||||
|---|---|---|---|---|---|---|
| ACTH (pg/mL) | Insulin (mIU/L) | Adiponectin (mIU/L) | ACTH (pg/mL) | Insulin (mIU/L) | Adiponectin (mIU/L) | |
| Median | 23.2 | 18.9 | 7.8 | 35.8 | 21.8 | 6.7 |
| IQR | 16.6–42.4 | 6.2–74.7 | 5.5–13.4 | 23.8–65.7 | 7.8–80.4 | 4.5–10.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, E.L.; Wood, A.D.; Potier, J.F.N. Prevalence and Progression of Resting ACTH, Insulin and Adiponectin Values as Indicators of Suspected Endocrine Diseases in Sport Horses and Ponies Compared to Non-Sport Horses, Ponies and Donkeys. Animals 2025, 15, 1316. https://doi.org/10.3390/ani15091316
Davis EL, Wood AD, Potier JFN. Prevalence and Progression of Resting ACTH, Insulin and Adiponectin Values as Indicators of Suspected Endocrine Diseases in Sport Horses and Ponies Compared to Non-Sport Horses, Ponies and Donkeys. Animals. 2025; 15(9):1316. https://doi.org/10.3390/ani15091316
Chicago/Turabian StyleDavis, Emma Louise, Andrew Douglas Wood, and Julie F. N. Potier. 2025. "Prevalence and Progression of Resting ACTH, Insulin and Adiponectin Values as Indicators of Suspected Endocrine Diseases in Sport Horses and Ponies Compared to Non-Sport Horses, Ponies and Donkeys" Animals 15, no. 9: 1316. https://doi.org/10.3390/ani15091316
APA StyleDavis, E. L., Wood, A. D., & Potier, J. F. N. (2025). Prevalence and Progression of Resting ACTH, Insulin and Adiponectin Values as Indicators of Suspected Endocrine Diseases in Sport Horses and Ponies Compared to Non-Sport Horses, Ponies and Donkeys. Animals, 15(9), 1316. https://doi.org/10.3390/ani15091316





