Improving Clinical Diagnosis of Transmissible Spongiform Encephalopathies in Sheep: Which Signs Are Important?
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Comparison of Clinical Signs Between TSE-Positive and -Negative Sheep
3.2. Presence of Two or More Clinical Signs in TSE-Positive and -Negative Sheep
3.3. Classification Tree Model to Determine Clinical Markers for TSEs
3.4. Classification Tree Model to Separate Different TSE Strains
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| APHA | Animal and Plant Health Agency |
| BSE | Bovine spongiform encephalopathy |
| CJD | Creutzfeldt–Jakob disease |
| NPV | Negative predictive value |
| PrPSc | Disease-associated prion protein |
| PPV | Positive predictive value |
| TSE | Transmissible spongiform encephalopathy |
| WOAH | World Organisation for Animal Health |
References
- Foster, J.D.; Hope, J.; Fraser, H. Transmission of bovine spongiform encephalopathy to sheep and goats. Vet. Rec. 1993, 133, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Del Rio Vilas, V.J.; Böhning, D.; Kuhnert, R. A comparison of the active surveillance of scrapie in the European Union. Vet. Res. 2008, 39, 37. [Google Scholar] [CrossRef] [PubMed]
- Eloit, M.; Adjou, K.; Coulpier, M.; Fontaine, J.J.; Hamel, R.; Lilin, T.; Messiaen, S.; Andréoletti, O.; Baron, T.; Bencsik, A.; et al. BSE agent signatures in a goat. Vet. Rec. 2005, 156, 523–524. [Google Scholar] [CrossRef]
- Benestad, S.L.; Sarradin, P.; Thu, B.; Schönheit, J.; Tranulis, M.A.; Bratberg, B. Cases of scrapie with unusual features in Norway and designation of a new type, Nor98. Vet. Rec. 2003, 153, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Benestad, S.L.; Arsac, J.N.; Goldmann, W.; Nöremark, M. Atypical/Nor98 scrapie: Properties of the agent, genetics, and epidemiology. Vet. Res. 2008, 39, 19. [Google Scholar] [CrossRef]
- Corona, C.; Vallino Costassa, E.; Iulini, B.; Caramelli, M.; Bozzetta, E.; Mazza, M.; Desiato, R.; Ru, G.; Casalone, C. Phenotypical variability in bovine spongiform encephalopathy: Epidemiology, pathogenesis, and diagnosis of classical and atypical forms. Prog. Mol. Biol. Transl. Sci. 2017, 150, 241–265. [Google Scholar]
- Simmons, M.M.; Moore, S.J.; Lockey, R.; Chaplin, M.J.; Konold, T.; Vickery, C.; Spiropoulos, J. Phenotype shift from atypical scrapie to CH1641 following experimental transmission in sheep. PLoS ONE 2015, 10, e0117063. [Google Scholar] [CrossRef]
- Betancor, M.; Marín, B.; Otero, A.; Hedman, C.; Romero, A.; Barrio, T.; Sevilla, E.; Douet, J.Y.; Huor, A.; Badiola, J.J.; et al. Detection of classical BSE prions in asymptomatic cows after inoculation with atypical/Nor98 scrapie. Vet. Res. 2023, 54, 89. [Google Scholar] [CrossRef]
- Konold, T.; Spiropoulos, J.; Hills, J.; Abdul, H.; Cawthraw, S.; Phelan, L.; McKenna, A.; Read, L.; Canoyra, S.; Marín-Moreno, A.; et al. Experimental transmission of ovine atypical scrapie to cattle. Vet. Res. 2023, 54, 98. [Google Scholar] [CrossRef]
- Kittelberger, R.; Chaplin, M.J.; Simmons, M.M.; Ramirez-Villaescusa, A.; McIntyre, L.; MacDiarmid, S.C.; Hannah, M.J.; Jenner, J.; Bueno, R.; Bayliss, D.; et al. Atypical scrapie/Nor98 in a sheep from New Zealand. J. Vet. Diagn. Investig. 2010, 22, 863–875. [Google Scholar] [CrossRef]
- Cook, R.; Bingham, J.; Besier, A.; Bayley, C.; Hawes, M.; Shearer, P.; Yamada, M.; Bergfeld, J.; Williams, D.; Middleton, D. Atypical scrapie in Australia. Aust. Vet. J. 2016, 94, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Ulvund, M.J. Clinical findings in scrapie. In Prions in Humans and Animals; Hörnlimann, B., Riesner, D., Kretzschmar, H., Eds.; de Gruyter: Berlin, Germany, 2006; pp. 398–407. [Google Scholar]
- Onodera, T.; Hayashi, T. Diversity of clinical signs in natural scrapie cases occurring in Japan. Jpn. Agr. Res. Q. 1994, 28, 59–61. [Google Scholar]
- Konold, T.; Davis, A.; Bone, G.; Bracegirdle, J.; Everitt, S.; Chaplin, M.; Saunders, G.C.; Cawthraw, S.; Simmons, M.M. Clinical findings in two cases of atypical scrapie in sheep: A case report. BMC Vet. Res. 2007, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Simmons, M.M.; Konold, T.; Thurston, L.; Bellworthy, S.J.; Chaplin, M.J.; Moore, S.J. The natural atypical scrapie phenotype is preserved on experimental transmission and sub-passage in PRNP homologous sheep. BMC Vet. Res. 2010, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Simmons, H.A.; Simmons, M.M.; Spencer, Y.I.; Chaplin, M.J.; Povey, G.; Davis, A.; Ortiz-Pelaez, A.; Hunter, N.; Matthews, D.; Wrathall, A.E. Atypical scrapie in sheep from a UK research flock which is free from classical scrapie. BMC Vet. Res. 2009, 5, 8. [Google Scholar] [CrossRef]
- Cassmann, E.D.; Mammadova, N.; Moore, S.J.; Benestad, S.; Greenlee, J.J. Transmission of the atypical/Nor98 scrapie agent to Suffolk sheep with VRQ/ARQ, ARQ/ARQ, and ARQ/ARR genotypes. PLoS ONE 2021, 16, e0246503. [Google Scholar] [CrossRef]
- Houston, E.F.; Gravenor, M.B. Clinical signs in sheep experimentally infected with scrapie and BSE. Vet. Rec. 2003, 152, 333–334. [Google Scholar] [CrossRef]
- Konold, T.; Bone, G.; Vidal-Diez, A.; Tortosa, R.; Davis, A.; Dexter, G.; Hill, P.; Jeffrey, M.; Simmons, M.M.; Chaplin, M.J.; et al. Pruritus is a common feature in sheep infected with the BSE agent. BMC Vet. Res. 2008, 4, 16. [Google Scholar] [CrossRef]
- Baron, T.G.M.; Madec, J.Y.; Calavas, D.; Richard, Y.; Barillet, F. Comparison of French natural scrapie isolates with bovine spongiform encephalopathy and experimental scrapie infected sheep. Neurosci. Lett. 2000, 284, 175–178. [Google Scholar] [CrossRef]
- Healy, A.M.; Weavers, E.; McElroy, M.; Gomez-Parada, M.; Collins, J.D.; O’Doherty, E.; Sweeney, T.; Doherty, M.L. The clinical neurology of scrapie in Irish sheep. J. Vet. Intern. Med. 2003, 17, 908–916. [Google Scholar] [CrossRef]
- Konold, T.; Bone, G.; Ortiz-Pelaez, A.; Tortosa, R.; Clifford, D.; Dexter, G.; Simmons, M.M.; Spiropoulos, J.; Berthelin-Baker, C.F. Associations of clinical signs and prion protein genotypes in British sheep with scrapie. Dtsch. Tierarztl. Wochenschr. 2009, 116, 380–388. [Google Scholar]
- D’Angelo, A.; Maurella, C.; Bona, C.; Borrelli, A.; Caramelli, M.; Elena, C.M.; Jaggy, A.; Ru, G. Assessment of clinical criteria to diagnose scrapie in Italy. Vet. J. 2007, 174, 106–112. [Google Scholar] [CrossRef]
- Vargas, F.; Lujan, L.; Bolea, R.; Monleon, E.; Martin-Burriel, I.; Fernandez, A.; De Blas, I.; Badiola, J.J. Detection and clinical evolution of scrapie in sheep by 3rd eyelid biopsy. J. Vet. Intern. Med. 2006, 20, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Konold, T.; Phelan, L. Clinical examination protocol to detect atypical and classical scrapie in sheep. J. Vis. Exp. 2014, e51101. [Google Scholar] [CrossRef]
- Ortiz-Pelaez, A.; Thompson, C.E.; Dawson, M. The impact of the National Scrapie Plan on the PRNP genotype distribution of the British national flock, 2002–2012. Vet. Rec. 2014, 174, 530. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.H.; Hawkins, S.A.C. Animal models of transmissible spongiform encephalopathies: Experimental infection, observation and tissue collection. In Techniques in Prion Research, 1st ed.; Lehmann, S., Grassi, J., Eds.; Birkhäuser Verlag: Basel, Switzerland, 2004; pp. 37–71. [Google Scholar]
- Bozzetta, E.; Nappi, R.; Crudeli, S.; Meloni, D.; Varello, K.; Loprevite, D.; Melis, P.G.; Mazza, M.; Colussi, S.; Ingravalle, F.; et al. Comparative performance of three TSE rapid tests for surveillance in healthy sheep affected by scrapie. J. Virol. Methods 2011, 173, 161–168. [Google Scholar] [CrossRef]
- Braun, U.; Amrein, E.; Estermann, U.; Pusterla, N.; Schonmann, M.; Schweizer, T.; Ehrensperger, F.; Vandevelde, M.; Kihm, U. Reliability of a diagnosis of BSE made on the basis of clinical signs. Vet. Rec. 1999, 145, 198–200. [Google Scholar] [CrossRef]
- Braun, U.; Schicker, E.; Hörnlimann, B. Diagnostic reliability of clinical signs in cows with suspected bovine spongiform encephalopathy. Vet. Rec. 1998, 143, 101–105. [Google Scholar] [CrossRef]
- González, L.; Horton, R.; Ramsay, D.; Toomik, R.; Leathers, V.; Tonelli, Q.; Dagleish, M.P.; Jeffrey, M.; Terry, L. Adaptation and evaluation of a rapid test for the diagnosis of sheep scrapie in samples of rectal mucosa. J. Vet. Diagn. Investig. 2008, 20, 203–208. [Google Scholar] [CrossRef]
- González, L.; Dagleish, M.P.; Bellworthy, S.J.; Sisó, S.; Stack, M.J.; Chaplin, M.J.; Davis, L.A.; Hawkins, S.A.; Hughes, J.; Jeffrey, M. Postmortem diagnosis of preclinical and clinical scrapie in sheep by the detection of disease-associated PrP in their rectal mucosa. Vet. Rec. 2006, 158, 325–331. [Google Scholar] [CrossRef]
- Huang, B.; Shafiian, N.; Masi, P.J.; Gordon, M.L.; Franceschi, A.M.; Giliberto, L. Creutzfeldt-Jakob disease presenting as psychiatric disorder: Case presentation and systematic review. Front. Neurol. 2024, 15, 1428021. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. The European Union summary report on surveillance for the presence of transmissible spongiform encephalopathies (TSE) in 2023. EFSA J. 2024, 22, e9097. [Google Scholar] [CrossRef]
- Vargas, F.; Bolea, R.; Monleon, E.; Acin, C.; Vargas, A.; De Blas, I.; Lujan, L.; Badiola, J.J. Clinical characterisation of natural scrapie in a native Spanish breed of sheep. Vet. Rec. 2005, 156, 318–320. [Google Scholar] [CrossRef]
- Konold, T.; Bone, G.E.; Phelan, L.J.; Simmons, M.M.; González, L.; Sisó, S.; Goldmann, W.; Cawthraw, S.; Hawkins, S.A.C. Monitoring of clinical signs in goats with transmissible spongiform encephalopathies. BMC Vet. Res. 2010, 6, 13. [Google Scholar] [CrossRef]
- Sharpe, A.; McElroy, M.; Bassett, H.; Sweeney, T. Clinical and pathological features of experimental scrapie in Irish Blackface Mountain sheep. Res. Vet. Sci. 2006, 80, 71–78. [Google Scholar] [CrossRef]
- Simmons, M.M.; Chaplin, M.J.; Konold, T.; Casalone, C.; Beck, K.E.; Thorne, L.; Everitt, S.; Floyd, T.; Clifford, D.; Spiropoulos, J. L-BSE experimentally transmitted to sheep presents as a unique disease phenotype. Vet. Res. 2016, 47, 112. [Google Scholar] [CrossRef] [PubMed]
- Saegerman, C.; Speybroeck, N.; Roels, S.; Vanopdenbosch, E.; Thiry, E.; Berkvens, D. Decision support tools for clinical diagnosis of disease in cows with suspected bovine spongiform encephalopathy. J. Clin. Microbiol. 2004, 42, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Abrahantes, J.C.; Aerts, M.; van Everbroeck, B.; Saegerman, C.; Berkvens, D.; Geys, H.; Mintiens, K.; Roels, S.; Cras, P. Classification of sporadic Creutzfeldt-Jakob disease based on clinical and neuropathological characteristics. Eur. J. Epidemiol. 2007, 22, 457–465. [Google Scholar] [CrossRef]
- Brandel, J.P.; Delasnerie-Lauprêtre, N.; Laplanche, J.L.; Hauw, J.J.; Alpérovitch, A. Diagnosis of Creutzfeldt-Jakob disease: Effect of clinical criteria on incidence estimates. Neurology 2000, 54, 1095–1099. [Google Scholar] [CrossRef]
- Calatayud, F.; Maublanc, M.L.; Col, E.; Lantier, F.; Bernadet, P.; Berthon, P.; Lantier, I.; Barc, C.; Sarradin, P.; Andréoletti, O.; et al. Behavioural study of sheep infected by bovine spongiform encephalopathy. Rev. Med. Vet. 2010, 161, 363–371. [Google Scholar]
- Bruce, M.E. TSE strain variation. Br. Med. Bull. 2003, 66, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Onodera, T.; Saeki, K. Japanese scrapie cases. Jpn. J. Infect. Dis. 2000, 53, 56–61. [Google Scholar] [CrossRef] [PubMed]
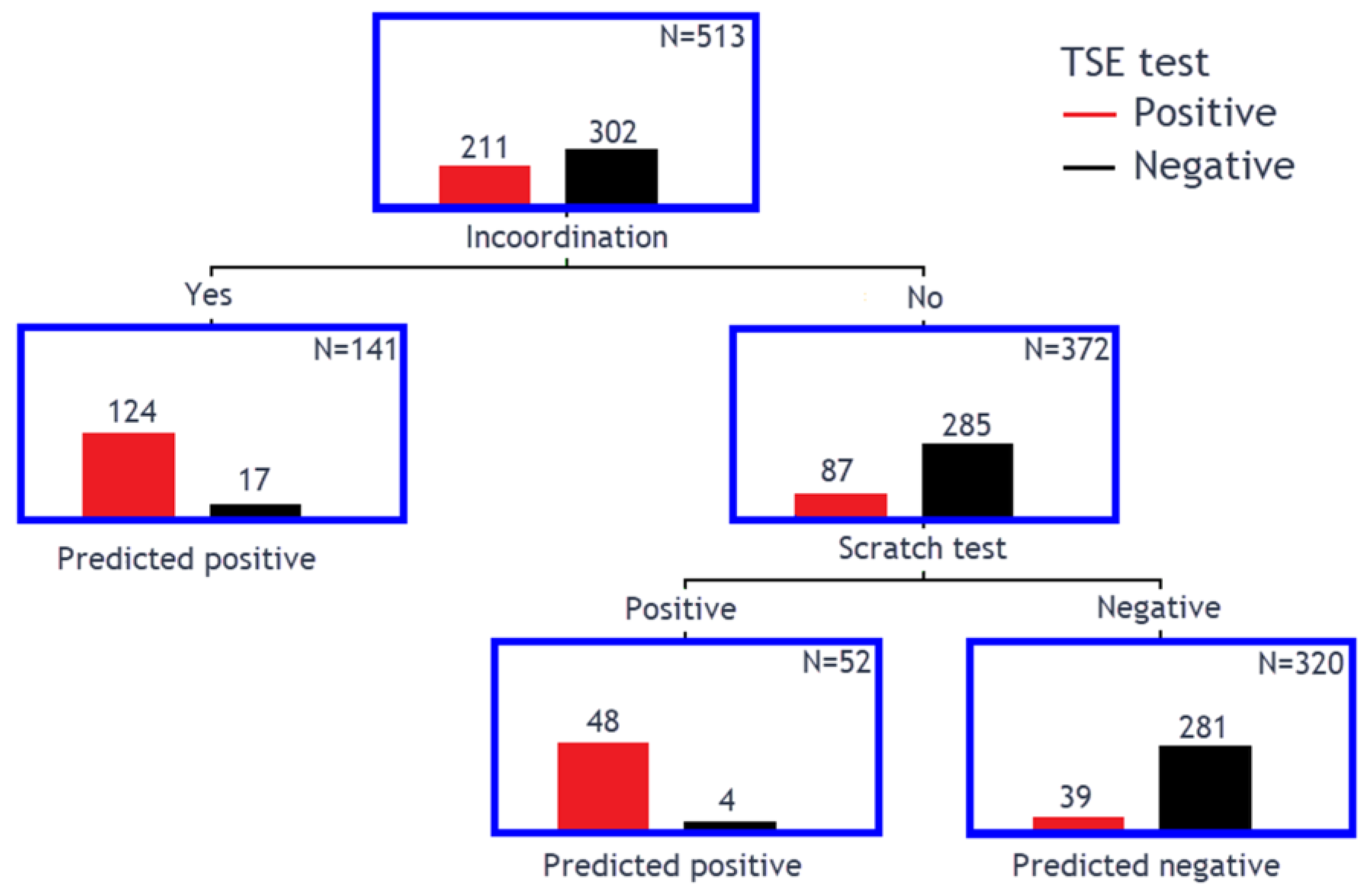
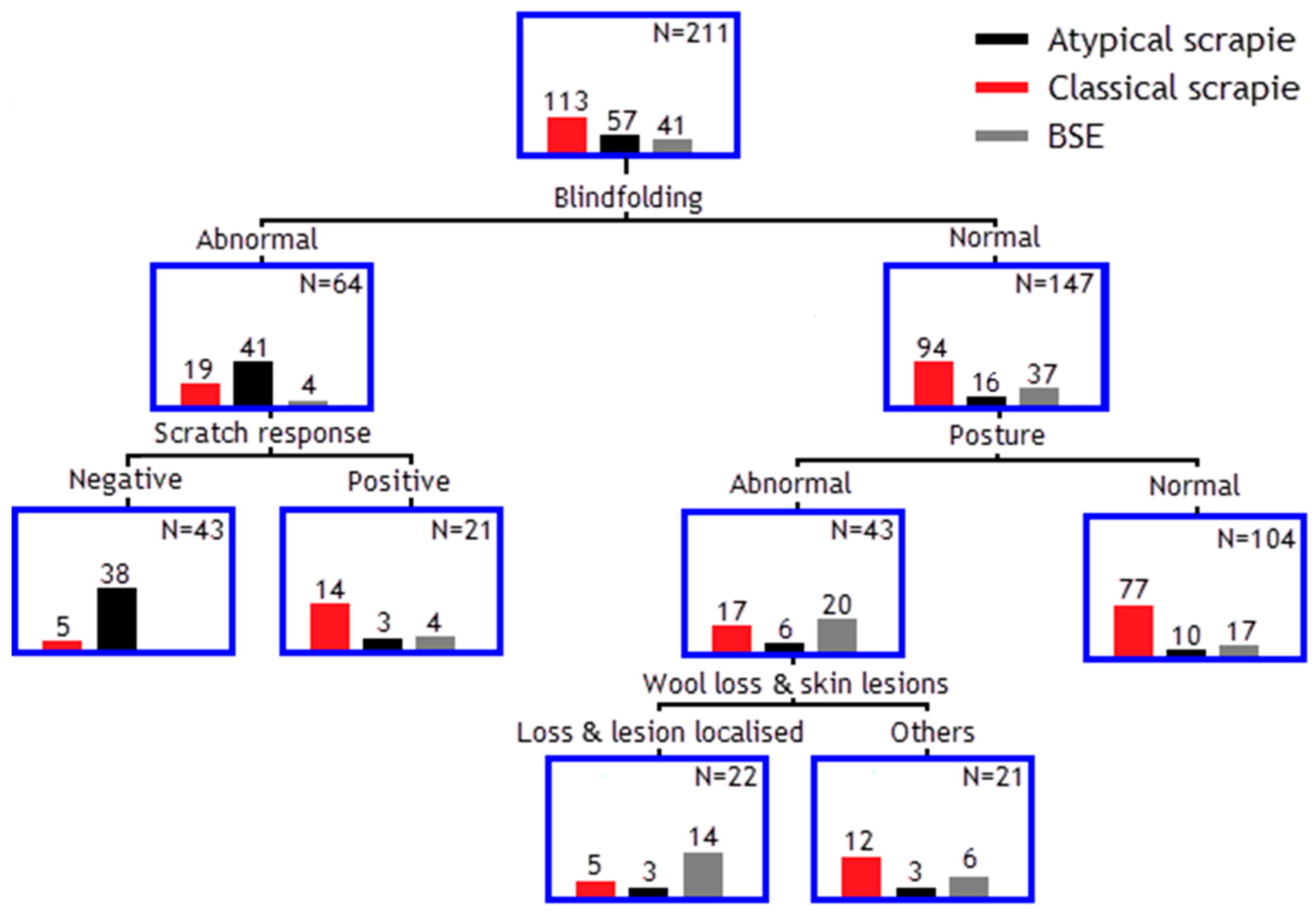

| TSE Result | Classical Scrapie | Atypical Scrapie | BSE | No Exposure or Infection | Total |
|---|---|---|---|---|---|
| Negative | 273 (39.6%) | 21 (3.0%) | 336 (48.7%) | 60 (8.7%) | 690 |
| Positive | 202 (64.7%) | 59 (18.9%) | 51 (16.3%) | 0 | 312 |
| Total | 475 (47.4%) | 80 (8.0%) | 387 (38.6%) | 60 (6.0%) | 1002 |
| Sign Category | Examples |
|---|---|
| Posture | Wide-based hind limbs or crossed legs, crouching, low head carriage |
| Behaviour | Collapsing when handled, teeth grinding, confusion, separation from others, spontaneous circling |
| Menace response | Unilaterally or bilaterally absent response |
| Scratch response | Only a repeatable head movement or nibble response counted as positive |
| Wool and skin changes | Wool loss or lesion at poll, back, rump, side of chest or abdomen, localised or generalised (>2 areas or wider area, e.g., both flanks) |
| Body condition score | Scored from 0.5 to 5, score of <2.5 was considered poor |
| Blindfolding response | Circling, swaying head or body, loss of balance |
| Incoordination | Wide-based movements of the hind limbs, hopping with both hind limbs, excessive swaying in the hind quarters, high stepping gait (hypermetria), drifting to one side |
| Tremor | Tremor when restrained or undisturbed, either localized (head or ear) or general (whole body) |
| Clinical Sign | TSE Positive | TSE Negative | Total Assessed |
|---|---|---|---|
| Positive scratch test | 158 (51.0%) | 18 (2.6%) | 999 |
| Incoordination | 156 (50.3%) | 20 (2.9%) | 1000 |
| Abnormal behaviour | 155 (49.8%) | 83 (12.0%) | 1001 |
| Tremor | 121 (38.9%) | 27 (3.9%) | 1001 |
| Abnormal blindfolding response | 65 (30.7%) | 17 (5.6%) | 515 |
| Poor body condition | 89 (28.7%) | 28 (4.1%) | 999 |
| Abnormal posture | 88 (28.2%) | 21 (3.0%) | 1001 |
| Wool loss & skin lesion | 78 (25.0%) | 27 (3.9%) | 1002 |
| Absent menace response | 59 (19.0%) | 27 (3.9%) | 1000 |
| Clinical Signs | Importance |
|---|---|
| Scratch test | 100% |
| Behaviour | 57% |
| Incoordination | 53% |
| Tremor | 37% |
| BCS | 34% |
| Wool loss and skin lesions | 26% |
| Posture | 26% |
| Abnormal blindfolding response | 21% |
| Menace response | 7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the Crown (United Kingdom). Submitted for possible open access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konold, T.; Phelan, L.J. Improving Clinical Diagnosis of Transmissible Spongiform Encephalopathies in Sheep: Which Signs Are Important? Animals 2025, 15, 1310. https://doi.org/10.3390/ani15091310
Konold T, Phelan LJ. Improving Clinical Diagnosis of Transmissible Spongiform Encephalopathies in Sheep: Which Signs Are Important? Animals. 2025; 15(9):1310. https://doi.org/10.3390/ani15091310
Chicago/Turabian StyleKonold, Timm, and Laura J. Phelan. 2025. "Improving Clinical Diagnosis of Transmissible Spongiform Encephalopathies in Sheep: Which Signs Are Important?" Animals 15, no. 9: 1310. https://doi.org/10.3390/ani15091310
APA StyleKonold, T., & Phelan, L. J. (2025). Improving Clinical Diagnosis of Transmissible Spongiform Encephalopathies in Sheep: Which Signs Are Important? Animals, 15(9), 1310. https://doi.org/10.3390/ani15091310







