Diversity, Endemism, and Conservation Status of the Herpetofauna of the Sierra Madre Occidental in Mexico with Comparison to Neighboring Biogeographic Provinces
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
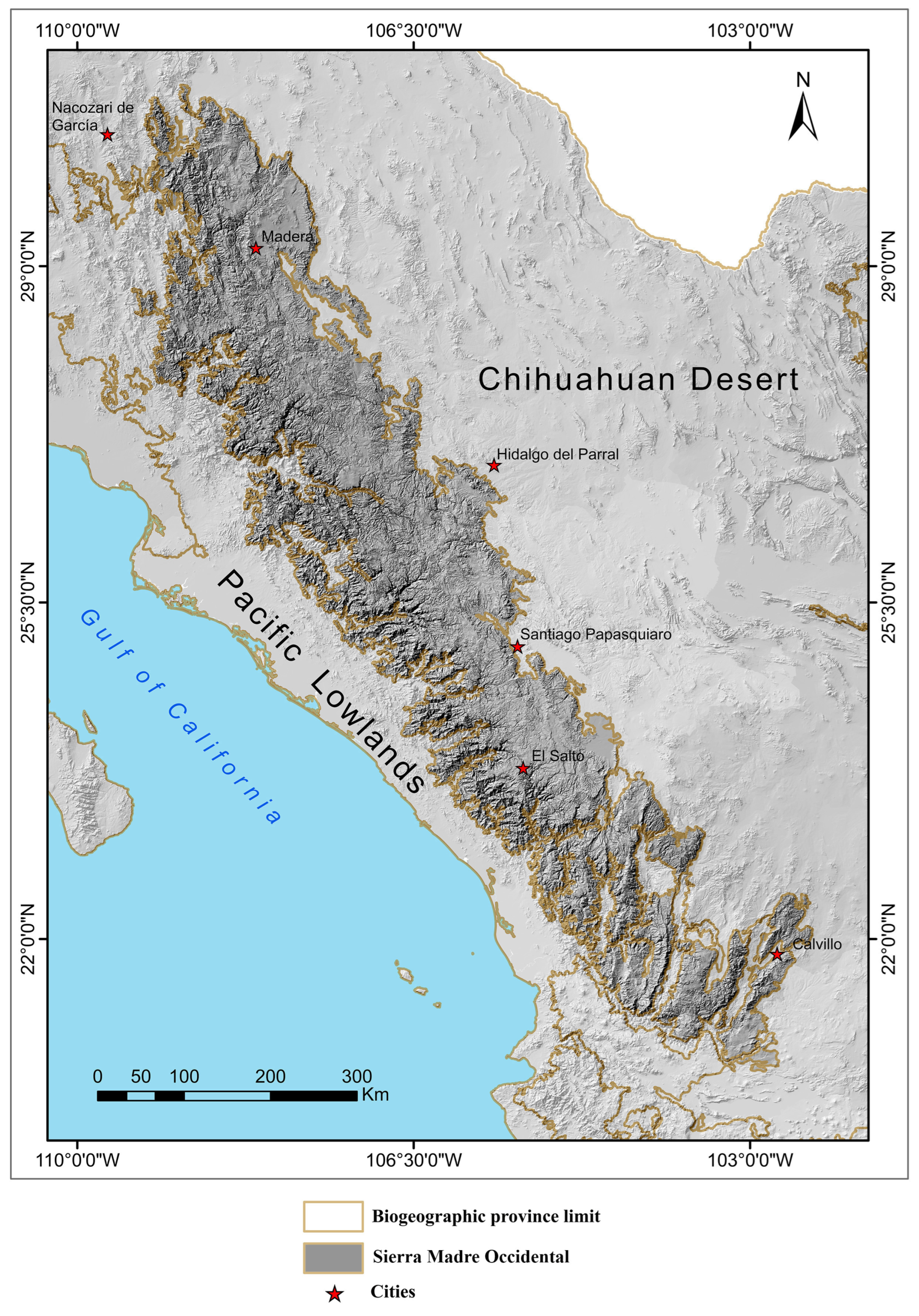
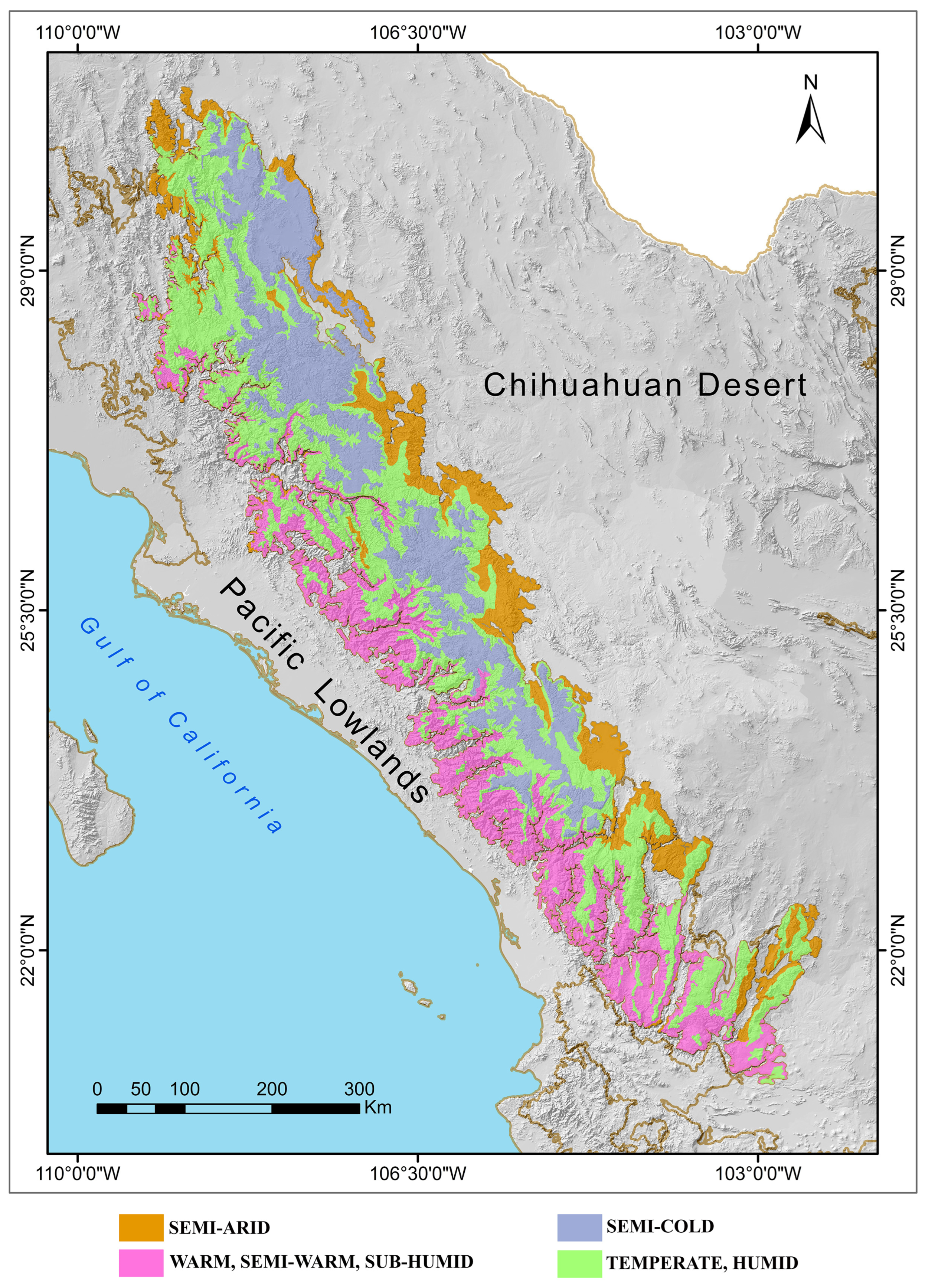
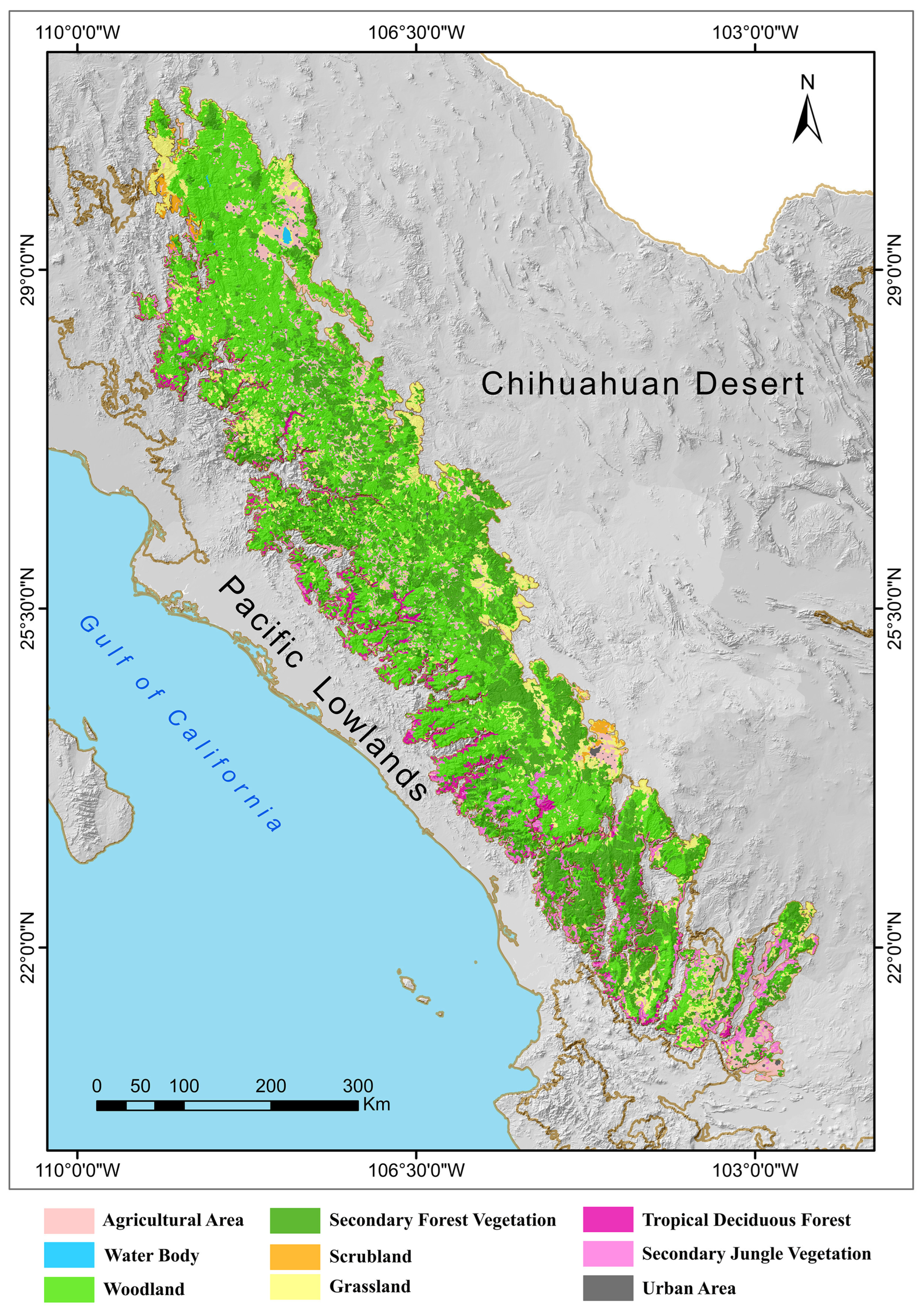
2.1. Physiographic Characteristics
2.2. Methodology
3. Results and Discussion
3.1. Species Richness
3.2. Endemism
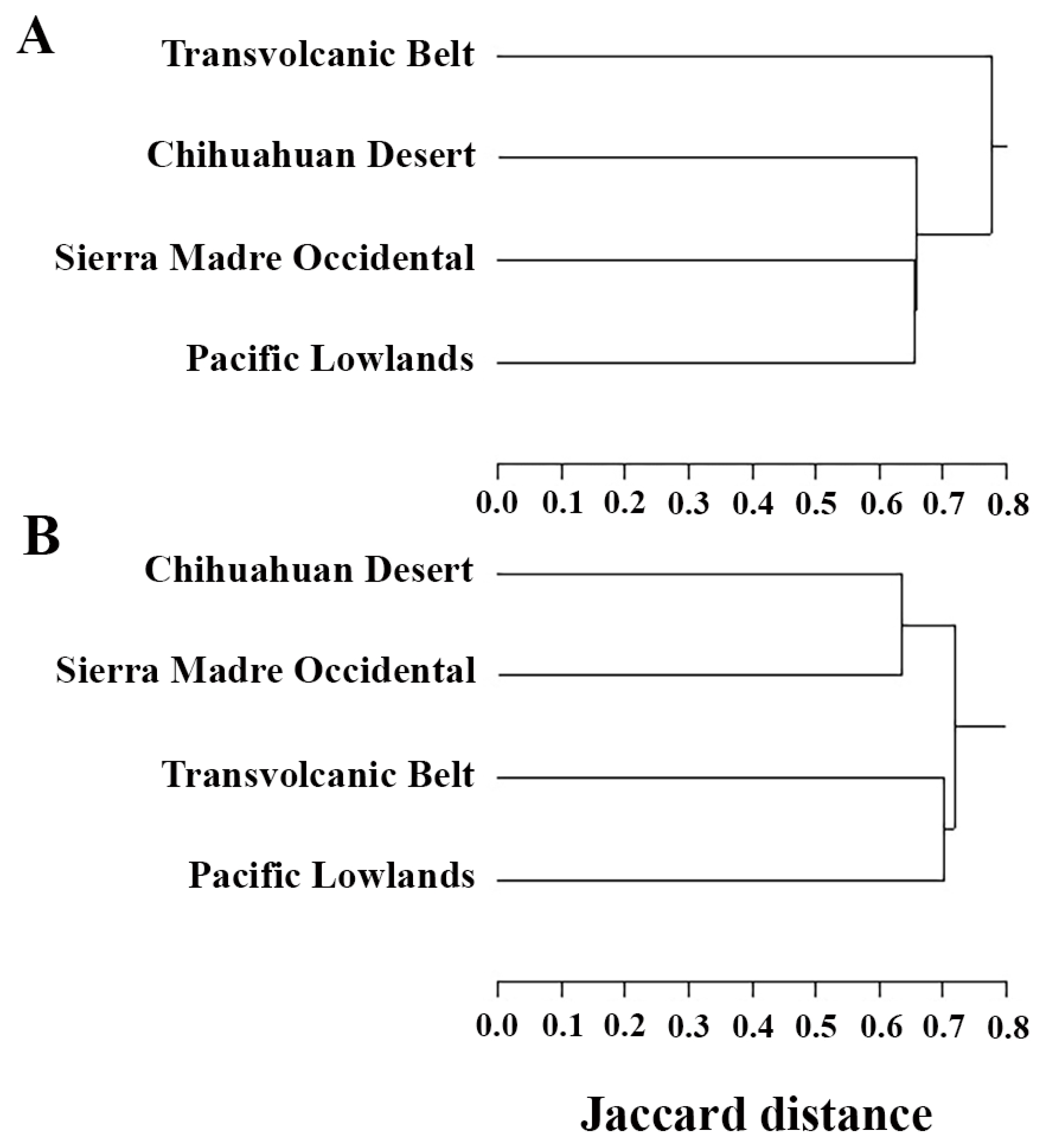
3.3. Comparison with Neighboring Provinces
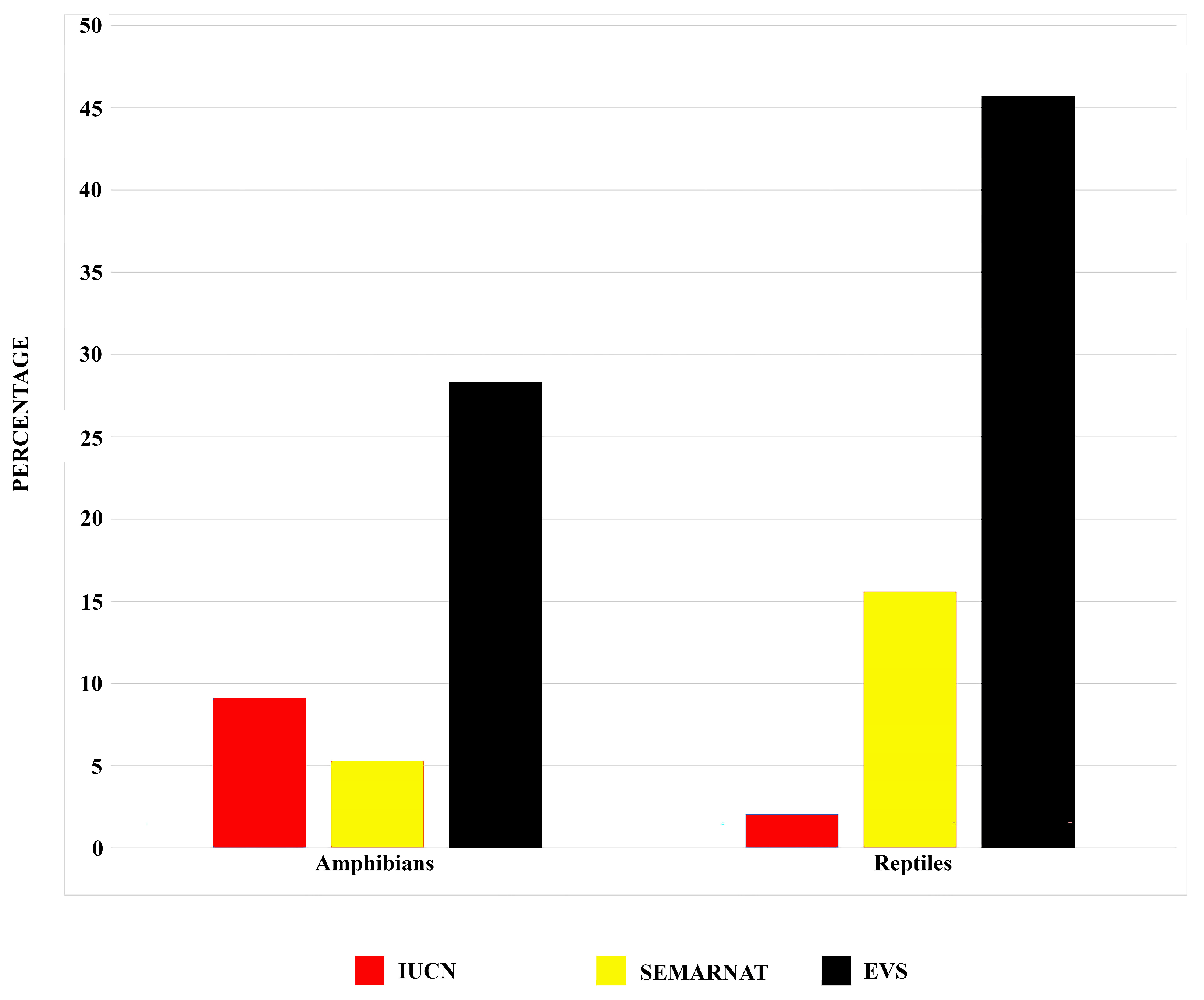
3.4. Conservation Status
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Felger, R.S.; Wilson, M.F. Northern Sierra Madre Occidental and Its Apachian Outliers: A Neglected Center of Biodiversity. In Biodiversity and Management of the Madrean Archipelago: The Sky Islands of Southwestern United States and Northwestern Mexico; DeBano, L., Gottfried, G.J., Hamre, R.H., Edminister, C.B., Eds.; Tech. Coord. USDA Forest Service General Technical Report RM-GTR-264; Rocky Mountain Forest and Range Experiment Station: Fort Collins, CO, USA, 1994; pp. 36–59. [Google Scholar]
- Lemos-Espinal, J.A.; Smith, H.M. Amphibians and Reptiles of the State of Chihuahua, Mexico; CONABIO: Ciudad de México, Mexico, 2007; p. 628. [Google Scholar]
- Lemos-Espinal, J.A.; Smith, H.M.; Cruz, A. Amphibians and Reptiles of the Sierra Tarahumara of Chihuahua; ECO Herpetological Publishing and Distribution: Rodeo, NM, USA, 2013; p. 445. [Google Scholar]
- Lemos-Espinal, J.A.; Smith, G.R.; Valdez-Lares, R. Amphibians and Reptiles of Durango, México; ECO-Herpetological Publishing and Distribution: Rodeo, NM, USA, 2019; p. 414. [Google Scholar]
- González-Elizondo, M.S.; González-Elizondo, M.; Tena-Flores, J.A.; Ruacho-González, L.; López-Enríquez, I.L. Vegetación de la Sierra Madre Occidental, México: Una síntesis. Acta Bot. Mex. 2012, 100, 351–405. [Google Scholar] [CrossRef]
- Morrone, J.J. Regionalización biogeográfica y evolución biótica de México: Encrucijada de la biodiversidad del Nuevo Mundo. Rev. Mex. Biodivers. 2019, 90, e902980. [Google Scholar] [CrossRef]
- López-González, C.; García-Mendoza, D.F.; Salas-H, T. Mammals of the Jesus Marie River basin, western Mexico: Alpha and beta diversity in an area of high environmental heterogeneity. West. N. Am. Nat. 2022, 82, 677–694. [Google Scholar] [CrossRef]
- Marshall, C.J.; Liebherr, J.K. Cladistic biogeography of the Mexican transition zone. J. Biogeogr. 2000, 27, 203–216. [Google Scholar] [CrossRef]
- Felger, R.S.; Johnson, M.B. Trees of the northern Sierra Madre Occidental and sky islands of southwestern North America. In Biodiversity and Management of the Madrean Archipelago: The Sky Islands of Southwestern United States and Northwestern Mexico; DeBano, L., Gottfried, G.J., Hamre, R.H., Edminister, C.B., Eds.; Tech. Coord. USDA Forest Service General Technical Report RM-GTR-264; Rocky Mountain Forest and Range Experiment Station: Fort Collins, CO, USA, 1994; pp. 71–83. [Google Scholar]
- López-Segoviano, G.; Díaz-Verduzco, L.; Arenas-Navarro, M.; Arizmendi, M.C. Diversidad estacional de aves en una region prioritaria para la conservacion en el centro oeste de la Sierra Madre Occidental. Rev. Mex. Biodivers. 2019, 90, e902754. [Google Scholar] [CrossRef]
- López-González, C.; García-Mendoza, D.F. Murciélagos de la Sierra Tarahumara, Chihuahua, México. Acta Zool. Mex. 2006, 22, 109–135. [Google Scholar] [CrossRef]
- Johnson, J.D.; Wilson, L.D.; Mata-Silva, V.; García-Padilla, E.; DeSantis, D.L. The endemic herpetofauna of Mexico: Organisms of global significance in severe peril. Mesoam. Herpetol. 2017, 4, 543–620. [Google Scholar]
- Wyndham, F.S. Spheres of relations, lines of interaction: Subtle ecologies of the Rarámuri landscape in northern Mexico. J. Ethnobiol. 2009, 29, 271–295. [Google Scholar] [CrossRef]
- Word Wildlife Fund (WWF). Sierra Tarahumara Forest Conservation Program Chihuahua, Mexico. First Year Work Plan; World Wildlife Fund México: Ciudad de México, Mexico, 2004; pp. 1–20. [Google Scholar]
- Windham, F.S. Learning Ecology: Ethnobotany in the Sierra Tarahumara, Mexico. Ph.D. Dissertation, University of Athens, Athens, GA, USA, 2004. [Google Scholar]
- Lumholtz, C. El México Desconocido: Cinco años de Exploración entre las tribus de la Sierra Madre Occidental; en la Tierra Caliente de Tepic y Jalisco y entre los Tarascos de Michoacán; Comisión Nacional para el Desarrollo de los Pueblos Indígenas. Tomo I/3a ed. Ilustrada: México City, Mexico, 2006; p. 336. [Google Scholar]
- Gingrich, R.W. The Political Ecology of Deforestation in the Sierra Madre Occidental of Chihuahua. Master’s Thesis, University of Arizona, Tucson, Arizona, 1993. [Google Scholar]
- Novo-Fernández, A.; Franks, S.; Wehenkel, C.; López-Serrano, P.M.; Molinier, M.; López-Sánchez, C.A. Landsat time series analysis for temperate forest cover change detection in the Sierra Madre Occidental, Durango, Mexico. Int. J. Appl. Earth Obs. Geoinf. 2018, 73, 230–244. [Google Scholar] [CrossRef]
- Heredia-Telles, A.; López-Serrano, P.M.; Molinier, M.; Wehenkel, C. Evaluation of forest cover loss in properties in the Sierra Madre Occidental, State of Durango, Mexico, certified by the Forest Stewardship Council. Trees For. People 2023, 14, 100454. [Google Scholar] [CrossRef]
- Santini, N.S.; Cuervo-Robayo, A.P.; Adame, M.F. Agricultural Land Degradation in Mexico. In Impact of Agriculture on Soil Degradation I: Perspectives from Africa, Asia, America and Oceania; Springer International Publishing: Cham, Switzerland, 2022; pp. 301–323. [Google Scholar]
- International Union for Conservation of Nature’s (IUCN). The IUCN Red List of Threatened Species. Version 2024-2. Available online: https://www.iucnredlist.org/ (accessed on 14 March 2025).
- Advanced Spaceborne Thermal Emission and Reflection Radiometer Global Digital Elevation Model Version 2 (ASTER GDEM2). In Modelo Digital de Elevación Global ASTER Versión 2. 1:50000; LP DACC: Sioux Falls, SD, USA, 2011. Available online: https://lpdaac.usgs.gov/documents/220/Summary_GDEM2_validation_report_final.pdf (accessed on 20 November 2024).
- García, E. “Climas (Clasificación de Köppen, modificado por García).” Escala 1:1 000 000; Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (CONABIO) México: México City, Mexico, 1998. [Google Scholar]
- Instituto Nacional de Estadística y Geografía [INEGI]. Conjunto de Datos Vectoriales de Uso de Suelo y Vegetación. Escala 1:250 000. Serie VI (Capa Unión), escala: 1:250 000, 1st ed.; Instituto Nacional de Estadística y Geografía: Aguascalientes, Mexico, 2016. [Google Scholar]
- Lemos-Espinal, J.A.; Smith, G.R. An analysis of the inter-state similarity of the herpetofaunas of Mexican states. Nat. Conserv. 2023, 53, 223–256. [Google Scholar] [CrossRef]
- Lemos-Espinal, J.A.; Smith, G.R. The distribution, diversity and conservation of the Mexican herpetofauna among its biogeographic provinces. J. Nat. Conserv. 2024, 82, 126714. [Google Scholar] [CrossRef]
- Frost, D.R. Amphibian Species of the World: An Online Reference, Version 6.2. Electronic Database. American Museum of Natural History: New York, NY, USA, 2025. Available online: https://amphibiansoftheworld.amnh.org/index.php (accessed on 17 March 2025). [CrossRef]
- AmphibiaWeb University of California, Berkeley, CA, USA. Available online: https://amphibiaweb.org (accessed on 17 March 2025).
- Uetz, P.; Freed, P.; Aguilar, R.; Reyes, F.; Kudera, J.; Hošek, J. (Eds.) The Reptile Database. Available online: http://www.reptile-database.org/ (accessed on 17 March 2025).
- Lemos-Espinal, J.A.; Smith, G.R. Amphibians and reptiles of the Transvolcanic Belt biogeographic provinces of Mexico: Diversity, solutions, and conservation. Nat. Conserv. 2024, 56, 37–76. [Google Scholar] [CrossRef]
- Lemos-Espinal, J.A.; Smith, G.R.; McCain, C.M. The herpetofauna of the Chihuahuan Desert Biogeographic Province of Mexico: Diversity, similarity to other provinces, and conservation status. Diversity 2024, 16, 771. [Google Scholar] [CrossRef]
- Lemos-Espinal, J.A.; Smith, G.R. Amphibians and Reptiles of the Pacific Lowlands Biogeographic Province of Mexico: Diversity, Similarities, and Conservation. Diversity 2024, 16, 735. [Google Scholar] [CrossRef]
- Morrone, J.J. Hacia una síntesis biogeográfica de México. Rev. Mex. Biodivers. 2005, 76, 207–252. [Google Scholar] [CrossRef]
- Morrone, J.J. Biogeographic areas and transition zones of Latin America and the Caribbean Islands based on panbiogeographic and cladistic analyses of the entomofauna. Annu. Rev. Entomol. 2006, 51, 467–494. [Google Scholar] [CrossRef]
- Morrone, J.J.; Escalante, T.; Rodríguez-Tapia, G. Mexican biogeographic provinces: Map and shapefiles. Zootaxa 2017, 4277, 277–279. [Google Scholar] [CrossRef]
- Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT). Modificación al anexo Normativo III, lista de Especies en riesgo de la Norma Oficial Mexicana NOM-059-Ecol-(2010) Protección Ambiental-Especies Nativas de México de Flora y Fauna silvestres-Categorías de riesgo y Especificaciones para su Inclusión, Exclusión o Cambio-Lista de Especies en Riesgo, Publicado el 30 de diciembre del 2010. 14 Noviembre 2019. Available online: https://normatecambiental.org/2020/03/05/se-actualiza-lista-de-especies-en-riesgo-de-nom-059-semarnat-2010/ (accessed on 14 November 2019).
- Wilson, L.D.; Johnson, J.D.; Mata-Silva, V. A conservation reassessment of the amphibians of Mexico based on the EVS measure. Amphib. Reptile Conserv. 2013, 7, 97–127. [Google Scholar]
- Wilson, L.D.; Mata-Silva, V.; Johnson, J.D. A conservation reassessment of the reptiles of Mexico based on the EVS measure. Amphib. Reptile Conserv. 2013, 7, 1–47. [Google Scholar]
- Ramírez-Bautista, A.; Torres-Hernández, L.A.; Cruz-Elizalde, R.; Berriozabal-Islas, C.; Hernández-Salinas, U.; Wilson, L.D.; Johnson, J.D.; Porras, L.W.; Balderas-Valdivia, C.J.; González-Hernández, A.J.; et al. An updated list of the Mexican herpetofauna: With a summary of historical and contemporary studies. ZooKeys 2023, 1166, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Berriozabal-Islas, C.; Ramírez-Bautista, A.; Cruz-Elizalde, R.; Hernández-Salinas, U. Modification of landscape as promoter of change in structure and taxonomic diversity of reptile’s communities: An example in tropical landscape in the central region of Mexico. Nat. Conserv. 2018, 28, 33–49. [Google Scholar] [CrossRef]
- Camarena-Hernández, A.; Ochoa-Ochoa, L.M.; Yáñez-Arenas, C. Quantifying the effects of Anhropocene activities in Mexican endemic amphibians. Anim. Conserv. 2024, 7, 449–460. [Google Scholar] [CrossRef]
- Cortés Montaño, C.; Fulé, P.Z.; Villanueva-Díaz, J.; Yocom, L.L. Linking old-growth forest composition, structure, fire history, climate and land-use in the mountains of northern Mexico. Ecosphere 2012, 3, 106. [Google Scholar] [CrossRef]
- Sáenz-Ceja, J.E.; Arenas-Navarro, M.; Torres-Miranda, A. Prioritizing conservation areas and vulnerability analyses of the genus Pinus L. (Pinaceae) in Mexico. J. Nat. Conserv. 2022, 67, 126171. [Google Scholar] [CrossRef]
- Villers-Ruíz, L.; Trejo-Vazquez, I. Impact of climatic change in forests and natural protected areas of Mexico. Interciencia 1998, 23, 10–19. [Google Scholar]
- Velasco, J.A.; Luna-Aranguré, C.; Calderón-Bustamante, O.; Mendoza-Ponce, A.; Estrada, F.; González-Salazar, C. Drivers of urban biodiversity in Mexico and joint risks from future urban expansion, climate change, and urban heat island effect. PLoS ONE 2024, 19, e0308522. [Google Scholar] [CrossRef]
- Ron, S.R. Predicting the distribution of the amphibian pathogen Batrachochytrium dendrobatidis in the New World. Biotropica 2005, 37, 209–221. [Google Scholar] [CrossRef]
- Íñiguez-Dávalos, L.I.; Jiménez-Sierra, C.L.; Sosa-Ramírez, J.; Ortega-Rubio, A. Categorías de las áreas naturales protegidas en México y una propuesta para la evaluación de su efectividad. Inv. y Cien. UAA 2014, 60, 65–70. [Google Scholar]
- Burke, R.A.; Frey, J.K.; Stoner, K.E. Using species distribution modeling to delineate richness patterns of Chiropterophilic plants and allocate conservation efforts in Mexico and the southwestern United States. Nat. Areas J. 2021, 41, 85–92. [Google Scholar] [CrossRef]
- D’Amore, A. Rana (Lithobates) catesbeiana Shaw (American Bullfrog). In A Handbook of Global Freshwater Invasive Species; Francis, R., Ed.; Earthscan: London, UK, 2012; pp. 321–330. [Google Scholar]
- Barragán-Ramírez, J.L.; Reyes-Luis, O.E.; Ascencio-Arrayga, J.J.; Navarrete-Heredia, J.L.; Vásquez-Bolaños, M. Diet and reproductive aspects of the exotic gecko Gehyra mutilata (Wiegmann, 1834) (Sauria: Gekkonidae) in the urban area of Chapala, Jalisco, Mexico. Acta Zool. Mex. 2015, 31, 67–73. [Google Scholar] [CrossRef]
- Rorabaugh, J.R. Brahminy Blindsnake (Indotyphlops braminus). Tucson Herpetological Society. Available online: https://tucsonherpsociety.org/amphibians-reptiles/snakes/brahminy-blindsnake-2/ (accessed on 27 December 2024).
- DeVos, T.B.; Giery, S.T. Establishment of the introduced Brahminy Blindsnakes (Indotyphlos braminus) on Abaco Island, The Bahamas, with notes on potential niche overlap with the native Cuban Brown Blindsnake (Typhlops lumbricalis). IRCF Amphib. Rept. 2021, 28, 555–557. [Google Scholar] [CrossRef]
- Ficetola, G.F.; Rödder, D.; Padoa-Schioppa, E. Trachemys scripta (Slider Terrapin). In A Handbook of Global Freshwater Invasive Species; Francis, R., Ed.; Earthscan: London, UK, 2012; pp. 331–339. [Google Scholar]


| IUCN | EVS | Mx | Global | Total | |
|---|---|---|---|---|---|
| Class Amphibia | |||||
| Order Anura | |||||
| Bufonidae | |||||
| Anaxyrus cognatus (Say, 1822) | LC (↓) | L (9) | NL | 2 | 5 |
| Anaxyrus compactilis (Wiegmann, 1833) | LC (?) | H (14) | NL | 1 | 6 |
| Anaxyrus debilis (Girard, 1854) | LC (=) | L (7) | Pr | 2 | 5 |
| Anaxyrus kelloggi (Taylor, 1938) | LC (=) | H (14) | NL | 1 | 3 |
| Anaxyrus mexicanus (Brocchi, 1879) | LC (↓) | M (13) | NL | 0 | EN |
| Anaxyrus punctatus (Baird & Girard, 1852) | LC (−) | L (5) | NL | 2 | 10 |
| Anaxyrus woodhousii (Girard, 1854) | LC (=) | M (10) | NL | 2 | 4 |
| Incilius alvarius (Girard, 1859) | LC (=) | M (11) | NL | 2 | 5 |
| Incilius marmoreus (Wiegmann, 1833) | LC (=) | M (11) | NL | 1 | 8 |
| Incilius mazatlanensis (Taylor, 1940) | LC (=) | M (12) | NL | 1 | 5 |
| Incilius mccoy Santos-Barrera & Flores-Villela, 2011 | LC (=) | H (14) | NL | 0 | EN |
| Incilius occidentalis (Camerano, 1879) | LC (=) | M (11) | NL | 1 | 7 |
| Rhinella horribilis (Wiegmann, 1833) | LC (↑) | L (3) | NL | 4 | 12 |
| Craugastoridae | |||||
| Craugastor augusti (Dugès, 1879) | LC (=) | L (8) | NL | 2 | 9 |
| Craugastor hobartsmithi (Taylor, 1937) | LC (=) | H (15) | NL | 1 | 5 |
| Craugastor occidentalis (Taylor, 1941) | LC (=) | M (13) | NL | 1 | 6 |
| Craugastor rubinus Jameson, Streicher, Manuelli, Head, & Smith, 2022 | NE | NE | NL | 0 | EN |
| Craugastor tarahumaraensis (Taylor, 1940) | LC (?) | H (17) | Pr | 0 | EN |
| Craugastor vocalis (Taylor, 1940) | LC (↓) | M (13) | NL | 1 | 5 |
| Eleutherodactylidae | |||||
| Eleutherodactylus interorbitalis (Langebartel & Shannon, 1956) | LC (=) | H (15) | Pr | 1 | 2 |
| Eleutherodactylus jamesdixoni Devitt, Tseng, Taylor-Adair, Koganti, Timugura, Cannatella, 2023 | NE | NE | NL | 1 | 4 |
| Eleutherodactylus pallidus (Duellman, 1958) | LC (=) | H (17) | Pr | 1 | 3 |
| Eleutherodactylus saxatilis (Webb, 1962) | NT (=) | H (17) | NL | 0 | EN |
| Eleutherodactylus teretistes (Duellman, 1958) | VU (?) | H (16) | NL | 1 | 3 |
| Eleutherodactylus wixarika Reyes-Velasco, Ahumada-Carrillo, Burkhardt, & Devitt, 2015 | EN (↓) | H (18) | NL | 0 | EN |
| Hylidae | |||||
| Agalychnis dacnicolor (Cope, 1864) | LC (↓) | M (11) | NL | 1 | 5 |
| Dryophytes arenicolor (Cope, 1886) | LC (=) | L (7) | NL | 2 | 8 |
| Dryophytes eximius (Baird, 1854) | LC (=) | M (10) | NL | 1 | 7 |
| Dryophytes wrightorum (Taylor, 1938) | LC (=) | L (9) | NL | 2 | 2 |
| Exerodonta smaragdina (Taylor, 1940) | LC (↓) | M (12) | Pr | 1 | 6 |
| Sarcohyla hapsa Campbell et al., 2018 | LC (?) | NE | NL | 1 | 5 |
| Smilisca baudinii (Duméril & Bibron, 1841) | LC (=) | L (3) | NL | 4 | 11 |
| Smilisca fodiens (Boulenger, 1882) | LC (=) | L (8) | NL | 2 | 7 |
| Tlalocohyla smithii (Boulenger, 1902) | LC (=) | M (11) | NL | 1 | 6 |
| Leptodactylidae | |||||
| Leptodactylus melanonotus (Hallowell) | LC (=) | L (6) | NL | 3 | 11 |
| Microhylidae | |||||
| Gastrophryne mazatlanensis (Taylor, 1943) | LC (?) | L (8) | NL | 2 | 3 |
| Hypopachus ustus (Cope, 1866) | LC (=) | L (7) | Pr | 3 | 8 |
| Hypopachus variolosus (Cope, 1866) | LC (=) | L (4) | NL | 4 | 11 |
| Ranidae | |||||
| Rana berlandieri Baird, 1854 | LC (=) | L (7) | Pr | 2 | 9 |
| Rana catesbeiana Shaw, 1802 | IN | ||||
| Rana chiricahuensis Platz & Mecham, 1979 | VU (↓) | M (11) | A | 2 | 2 |
| Rana forreri Boulenger, 1883 | LC (=) | L (3) | Pr | 3 | 8 |
| Rana lemosespinali Smith & Chiszar, 2003 | DD (?) | H (14) | NL | 0 | EN |
| Rana magnaocularis Frost & Bagnara, 1976 | LC (?) | M (12) | NL | 1 | 6 |
| Rana megapoda Taylor, 1942 | NT (↓) | H (14) | Pr | 1 | 5 |
| Rana montezumae Baird, 1854 | LC (↓) | M (13) | Pr | 1 | 6 |
| Rana neovolcanica Hillis & Frost, 1985 | LC (=) | M (13) | A | 1 | 6 |
| Rana psilonota Webb, 2001 | LC (?) | H (14) | NL | 1 | 4 |
| Rana pustulosa Boulenger, 1883 | LC (=) | L (9) | Pr | 1 | 5 |
| Rana tarahumarae Boulenger, 1917 | VU (↓) | L (8) | NL | 2 | 1 |
| Rana yavapaiensis Platz & Frost, 1984 | LC (↓) | M (12) | Pr | 2 | 3 |
| Scaphiopodidae | |||||
| Scaphiopus couchi Baird, 1854 | LC (=) | L (3) | NL | 2 | 10 |
| Spea multiplicata (Cope, 1863) | LC (=) | L (6) | NL | 2 | 9 |
| Order Caudata | |||||
| Ambystomatidae | |||||
| Ambystoma rosaceum Taylor, 1941 | LC (?) | H (14) | Pr | 0 | EN |
| Ambystoma silvense Webb, 2004 | DD (?) | H (14) | NL | 0 | EN |
| Ambystoma velasci (Dugès, 1888) | LC (?) | M (10) | Pr | 1 | 6 |
| Plethodontidae | |||||
| Isthmura belli (Gray, 1850) | LC (?) | M (12) | A | 1 | 5 |
| Isthmura sierraoccidentalis (Lowe, Jones & Wright, 1968) | VU (?) | NE | NL | 0 | EN |
| Class Reptilia | |||||
| Order Squamata | |||||
| Suborder Lacertilia | |||||
| Anguidae | |||||
| Barisia ciliaris (Smith, 1942) | NE | H (15) | NL | 1 | 3 |
| Barisia levicollis Stejneger, 1890 | DD (?) | H (15) | Pr | 0 | EN |
| Elgaria kingii Gray, 1838 | LC (=) | M (10) | Pr | 2 | 5 |
| Gerrhonotus infernalis Baird, 1859 | LC (=) | M (13) | NL | 2 | 4 |
| Gerrhonotus liocephalus Wiegmann, 1828 | LC (=) | L (6) | Pr | 4 | 9 |
| Anolidae | |||||
| Anolis nebulosus (Wiegmann, 1834) | LC (=) | M (13) | NL | 1 | 6 |
| Crotaphytidae | |||||
| Crotaphytus collaris (Say, 1823) | LC (=) | M (13) | A | 2 | 4 |
| Crotaphytus nebrius Axtell & Montanucci, 1977 | LC (=) | M (12) | NL | 2 | 2 |
| Eublepharidae | |||||
| Coleonyx fasciatus (Boulenger, 1885) | LC (↓) | H (17) | NL | 1 | 2 |
| Gekkonidae | |||||
| Gehyra mutilata (Wiegmann, 1834) | IN | ||||
| Helodermatidae | |||||
| Heloderma exasperatum Bogert & Martin del Campo, 1956 | LC (↓) | NE | NL | 1 | 2 |
| Heloderma horridum (Wiegmann, 1829) | LC (↓) | M (11) | A | 3 | 6 |
| Heloderma suspectum Cope, 1869 | NT (↓) | H (15) | A | 2 | 3 |
| Iguanidae | |||||
| Ctenosaura macrolopha Smith, 1972 | LC (↓) | H (19) | NL | 1 | 3 |
| Ctenosaura pectinata (Wiegmann, 1834) | LC (↓) | H (15) | A | 1 | 7 |
| Phrynosomatidae | |||||
| Cophosaurus texanus Troschel, 1852 | LC (=) | H (14) | A | 2 | 6 |
| Holbrookia approximans Baird, 1859 | NE | H (14) | NL | 1 | 2 |
| Holbrookia elegans Bocourt, 1874 | LC (=) | M (13) | NL | 2 | 4 |
| Phrynosoma cornutum (Harlan, 1825) | LC (=) | M (11) | NL | 2 | 5 |
| Phrynosoma ditmarsi Stejneger, 1906 | DD (?) | H (16) | NL | 0 | EN |
| Phrynosoma hernandesi Girard, 1858 | LC (=) | M (13) | NL | 2 | 2 |
| Phrynosoma orbiculare (Linnaeus, 1766) | LC (=) | M (12) | A | 1 | 6 |
| Phrynosoma ornatissimum (Girard, 1858) | NE | NE | NL | 2 | 2 |
| Phrynosoma solare Gray, 1845 | LC (=) | H (14) | NL | 2 | 4 |
| Sceloporus albiventris Smith, 1939 | NE | H (16) | NL | 1 | 3 |
| Sceloporus asper Boulenger, 1897 | LC (↓) | H (14) | Pr | 1 | 4 |
| Sceloporus aurantius Grummer & Bryson, 2014 | NE | H (16) | NL | 1 | 2 |
| Sceloporus brownorum Smith, Watkins-Colwell, Lemos-Espinal, & Chiszar, 1997 | NE | H (15) | NL | 0 | EN |
| Sceloporus bulleri Boulenger, 1894 | LC (=) | H (15) | NL | 1 | 4 |
| Sceloporus clarkii Baird & Girard, 1852 | LC (=) | M (10) | NL | 2 | 5 |
| Sceloporus dugesii Bocourt, 1874 | LC (=) | M (13) | NL | 1 | 4 |
| Sceloporus grammicus Wiegmann, 1828 | LC (=) | L (9) | Pr | 2 | 8 |
| Sceloporus heterolepis Boulenger, 1895 | LC (?) | H (14) | NL | 1 | 5 |
| Sceloporus horridus Wiegmann, 1834 | LC (=) | M (11) | NL | 1 | 6 |
| Sceloporus huichol Flores-Villela, Smith, Campillo-García, Martínez-Méndez, & Campbell, 2022 | NE | NE | NL | 1 | 2 |
| Sceloporus jarrovii Cope, 1875 | LC (=) | M (11) | NL | 2 | 3 |
| Sceloporus lemosespinali Lara-Góngora, 2004 | DD (?) | H (16) | NL | 0 | EN |
| Sceloporus melanogaster Cope, 1885 | NE | NE | NL | 1 | 3 |
| Sceloporus melanorhinus Bocourt, 1876 | LC (=) | L (9) | NL | 3 | 6 |
| Sceloporus nelsoni Cochran, 1923 | LC (=) | M (13) | NL | 1 | 4 |
| Sceloporus poinsettii Baird & Girard, 1852 | LC (=) | M (12) | NL | 2 | 4 |
| Sceloporus scalaris Wiegmann, 1828 | LC (=) | M (12) | NL | 1 | 6 |
| Sceloporus shannonorum Langebartel, 1959 | DD (?) | H (15) | NL | 1 | 2 |
| Sceloporus slevini Smith, 1937 | LC (↓) | M (11) | NL | 2 | 2 |
| Sceloporus spinosus Weigmann, 1828 | LC (=) | M (12) | NL | 1 | 7 |
| Sceloporus unicanthalis Smith, 1937 | NE | H (16) | NL | 1 | 4 |
| Sceloporus utiformis Cope, 1864 | LC (=) | H (15) | NL | 1 | 6 |
| Sceloporus virgatus Smith, 1938 | LC (=) | H (15) | NL | 2 | 1 |
| Urosaurus bicarinatus (Duméril, 1856) | LC (=) | M (12) | NL | 1 | 7 |
| Urosaurus ornatus (Baird & Girard, 1852) | LC (=) | M (10) | NL | 2 | 5 |
| Phyllodactylidae | |||||
| Phyllodactylus saxatilis Dixon, 1964 | NE | NE | NL | 1 | 2 |
| Phyllodactylus lanei Smith, 1935 | LC (=) | H (15) | NL | 1 | 5 |
| Scincidae | |||||
| Plestiodon bilineatus (Tanner, 1958) | NE | M (13) | NL | 0 | EN |
| Plestiodon callicephalus (Bocourt, 1879) | LC (=) | M (12) | NL | 2 | 4 |
| Plestiodon lynxe (Wiegmann, 1834) | LC (=) | M (10) | Pr | 1 | 6 |
| Plestiodon multilineatus (Tanner, 1957) | DD (?) | H (16) | Pr | 0 | EN |
| Plestiodon obsoletus (Baird & Girard, 1852) | LC (=) | M (11) | NL | 2 | 6 |
| Plestiodon parviauriculatus (Taylor, 1933) | DD (?) | H (15) | Pr | 1 | 2 |
| Plestiodon parvulus (Taylor, 1933) | DD (?) | H (15) | NL | 1 | 4 |
| Teiidae | |||||
| Aspidoscelis costatus (Cope, 1878) | LC (=) | M (11) | Pr | 1 | 8 |
| Aspidoscelis exsanguis (Lowe, 1956) | LC (=) | H (14) | NL | 2 | 2 |
| Aspidoscelis gularis (Baird & Girard, 1852) | LC (=) | L (9) | NL | 2 | 6 |
| Aspidoscelis lineattissimus (Cope, 1878) | LC (=) | H (14) | Pr | 1 | 5 |
| Aspidoscelis opatae (Wright, 1967) | DD (?) | H (16) | NL | 0 | EN |
| Aspidoscelis preopatae Barley, Reeder, Nieto-Montes de Oca, Cole & Thomson, 2021 | NE | NE | NL | 0 | EN |
| Aspidoscelis sonorae (Lowe & Wright, 1964) | LC (=) | M (13) | NL | 2 | 3 |
| Aspidoscelis stictogrammus (Burger, 1950) | LC (=) | H (14) | NL | 2 | 3 |
| Xantusidae | |||||
| Xantusia sanchezi Bezy & Flores-Villela, 1999 | LC (?) | H (16) | P | 1 | 2 |
| Order Squamata | |||||
| Suborder Serpentes | |||||
| Boidae | |||||
| Boa sigma (Smith, 1943) | NE | M (10) | NL | 1 | 6 |
| Colubridae | |||||
| Conopsis nasus (Günther, 1858) | LC (=) | M (11) | NL | 1 | 5 |
| Drymarchon melanurus (Duméril, Bibron & Duméril, 1854) | LC (=) | L (6) | NL | 4 | 12 |
| Drymobius margaritiferus (Schlegel, 1837) | LC (=) | L (6) | NL | 4 | 10 |
| Gyalopion canum Cope, 1861 | LC (=) | L (9) | NL | 2 | 4 |
| Gyalopion quadrangulare (Günther, 1893) | LC (=) | M (11) | Pr | 2 | 3 |
| Lampropeltis alterna (Brown, 1901) | LC (=) | H (14) | A | 2 | 3 |
| Lampropeltis californiae (Blainville, 1835) | LC (=) | M (10) | NL | 2 | 5 |
| Lampropeltis greeri Webb, 1961 | NE | NE | NL | 0 | EN |
| Lampropeltis knoblochi Taylor, 1940 | LC (=) | H (14) | NL | 2 | 1 |
| Lampropeltis mexicana (Garman, 1884) | LC (=) | H (15) | A | 1 | 5 |
| Lampropeltis polyzona Cope, 1860 | LC (?) | M (11) | NL | 1 | 9 |
| Lampropeltis splendida (Baird & Girard, 1853) | LC (=) | M (12) | NL | 2 | 3 |
| Lampropeltis webbi Bryson, Dixon & Lazcano, 2005 | DD | H (16) | NL | 0 | EN |
| Leptophis diplotropis (Günther, 1872) | LC (=) | H (14) | A | 1 | 8 |
| Masticophis bilineatus Jan, 1863 | LC (=) | M (11) | NL | 2 | 6 |
| Masticophis flagellum Shaw, 1802 | LC (=) | L (8) | A | 2 | 9 |
| Masticophis mentovarius (Duméril, Bibron & Duméril, 1854) | LC (=) | L (6) | A | 3 | 11 |
| Masticophis taeniatus (Hallowell, 1852) | LC (=) | M (10) | NL | 2 | 3 |
| Mastigodryas cliftoni (Hardy, 1964) | DD (?) | H (14) | NL | 1 | 4 |
| Opheodrys vernalis (Harlan, 1827) | LC (=) | H (14) | NL | 2 | 2 |
| Oxybelis microphthalmus Barbour & Amaral, 1926 | NE | NE | NL | 2 | 9 |
| Pituophis catenifer Blainville, 1835 | LC (=) | L (9) | NL | 2 | 8 |
| Pituophis deppei (Duméril, 1853) | LC (=) | H (14) | A | 1 | 7 |
| Pseudoficimia frontalis (Cope, 1864) | LC (=) | M (13) | NL | 1 | 7 |
| Rhinocheilus lecontei Baird & Girard, 1853 | LC (=) | L (8) | NL | 2 | 8 |
| Salvadora bairdii Jan & Sordelli, 1860 | LC (=) | H (15) | Pr | 1 | 8 |
| Salvadora deserticola Schmidt, 1940 | NE | H (14) | NL | 2 | 5 |
| Salvadora grahamiae Baird & Girard, 1853 | LC (=) | M (10) | NL | 2 | 6 |
| Salvadora mexicana (Duméril, Bibron & Duméril, 1854) | LC (=) | H (15) | Pr | 1 | 5 |
| Senticolis triaspis (Cope, 1866) | LC (=) | L (6) | NL | 4 | 11 |
| Sonora aemula (Cope, 1879) | NT (=) | H (16) | Pr | 1 | 2 |
| Sonora mutabilis Stickel, 1943 | LC (?) | H (14) | NL | 1 | 5 |
| Sonora semiannulata Baird & Girard, 1853 | LC (=) | L (5) | NL | 2 | 4 |
| Sympholis lippiens Cope, 1862 | DD (?) | H (14) | NL | 1 | 3 |
| Tantilla bocourti (Günther, 1895) | LC (?) | L (9) | NL | 1 | 8 |
| Tantilla hobartsmithi Taylor, 1936 | LC (=) | M (11) | NL | 2 | 4 |
| Tantilla wilcoxi Stejneger, 1902 | LC (=) | M (10) | NL | 2 | 3 |
| Tantilla yaquia Smith, 1942 | LC (=) | M (10) | NL | 2 | 4 |
| Trimorphodon lambda Cope, 1886 | LC (=) | M (13) | NL | 2 | 4 |
| Trimorphodon paucimaculatus Taylor, 1936 | NE | H (15) | NL | 1 | 5 |
| Trimorphodon tau Cope, 1870 | LC (=) | M (13) | NL | 1 | 8 |
| Trimorphodon vilkinsonii Cope, 1886 | LC (=) | H (15) | A | 2 | 3 |
| Dipsadidae | |||||
| Diadophis punctatus (Linnaeus, 1766) | LC (=) | L (4) | NL | 2 | 7 |
| Geophis dugesii Bocourt, 1883 | LC (?) | M (13) | NL | 1 | 3 |
| Hypsiglena affinis Boulenger, 1894 | NE | H (14) | Pr | 1 | 3 |
| Hypsiglena chlorophaea Cope, 1860 | LC (=) | L (8) | Pr | 2 | 3 |
| Hypsiglena jani Dugès, 1866 | LC (=) | L (6) | Pr | 2 | 6 |
| Hypsiglena torquata (Günther, 1860) | LC (=) | L (8) | Pr | 1 | 5 |
| Leptodeira maculata (Hallowell, 1861) | LC (=) | L (7) | Pr | 1 | 9 |
| Leptodeira punctata (Peters, 1866) | LC (?) | H (17) | NL | 1 | 4 |
| Leptodeira splendida Günther, 1895 | LC (?) | H (14) | NL | 1 | 6 |
| Manolepis putnami (Jan, 1863) | LC (=) | M (13) | NL | 1 | 6 |
| Rhadinaea hesperia Bailey, 1940 | LC (=) | M (10) | Pr | 1 | 7 |
| Rhadinaea laureata (Günther, 1868) | LC (?) | M (12) | NL | 1 | 3 |
| Rhadinaea taeniata (Peters, 1863) | LC (=) | M (13) | NL | 1 | 6 |
| Tropidodipsas repleta Smith, Lemos-Espinal, Hartman & Chiszar, 2005 | DD (?) | H (17) | NL | 1 | 2 |
| Elapidae | |||||
| Micruroides euryxanthus (Kennicott, 1860) | LC (=) | H (15) | A | 2 | 3 |
| Micrurus distans (Kennicott, 1860) | LC (=) | H (14) | Pr | 1 | 7 |
| Micrurus proximans Smith & Chrapliwy, 1958 | LC (?) | H (18) | Pr | 1 | 4 |
| Leptotyphlopidae | |||||
| Rena humilis Baird &Girard, 1853 | LC (=) | L (8) | NL | 2 | 9 |
| Natricidae | |||||
| Storeria storerioides (Cope, 1865) | LC (=) | M (11) | NL | 1 | 6 |
| Thamnophis cyrtopsis (Kennicott, 1860) | LC (=) | L (7) | A | 4 | 10 |
| Thamnophis elegans (Baird & Girard, 1853) | LC (=) | H (14) | A | 2 | 2 |
| Thamnophis eques (Reuss, 1834) | LC (=) | L (8) | A | 2 | 7 |
| Thamnophis errans Smith, 1942 | LC (?) | H (16) | NL | 0 | EN |
| Thamnophis foxi Rossman & Blaney, 1968 | DD (?) | H (16) | Pr | 0 | EN |
| Thamnophis marcianus (Baird & Girard, 1853) | LC (?) | M (10) | A | 4 | 9 |
| Thamnophis melanogaster (Peters, 1864) | EN (↓) | H (15) | A | 1 | 5 |
| Thamnophis nigronuchalis Thompson, 1957 | DD (?) | M (12) | Pr | 0 | EN |
| Thamnophis pulchrilatus (Cope, 1885) | LC (?) | H (15) | NL | 1 | 6 |
| Thamnophis scaliger (Jan, 1863) | VU (↓) | H (15) | A | 1 | 4 |
| Thamnophis sirtalis (Linnaeus, 1758) | LC (=) | H (14) | Pr | 2 | 2 |
| Thamnophis unilabialis Tanner, 1985 | NE | NE | NL | 1 | 2 |
| Thamnophis validus (Kennicott, 1860) | LC (=) | M (12) | NL | 1 | 5 |
| Typhlopidae | |||||
| Indotyphlops braminus (Daudin, 1803) | IN | ||||
| Viperidae | |||||
| Agkistrodon bilineatus (Günther, 1863) | NT (↓) | M (11) | Pr | 3 | 6 |
| Crotalus aquilus Klauber, 1952 | LC (↓) | H (16) | Pr | 1 | 4 |
| Crotalus atrox Baird & Girard, 1853 | LC (=) | M (9) | Pr | 2 | 9 |
| Crotalus basiliscus (Cope, 1864) | LC (=) | H (16) | Pr | 1 | 6 |
| Crotalus lepidus (Kennicott, 1861) | LC (=) | M (12) | Pr | 2 | 4 |
| Crotalus molossus Baird & Girard, 1853 | LC (=) | L (8) | Pr | 2 | 8 |
| Crotalus polystictus (Cope, 1865) | LC (↓) | H (16) | Pr | 1 | 4 |
| Crotalus pricei Van Denburgh, 1895 | LC (?) | H (14) | Pr | 2 | 2 |
| Crotalus scutulatus (Kennicott, 1861) | LC (=) | M (11) | Pr | 2 | 7 |
| Crotalus stejnegeri Kennicott, 1859 | VU (↓) | H (17) | A | 1 | 2 |
| Crotalus tigris Kennicott, 1859 | LC (=) | H (16) | Pr | 2 | 3 |
| Crotalus willardi Meek, 1905 | LC (=) | M (13) | Pr | 2 | 1 |
| Order Testudines | |||||
| Emydidae | |||||
| Chrysemys picta (Schneider, 1783) | LC (=) | H (14) | A | 2 | 2 |
| Terrapene nelsoni Stejneger, 1925 | DD | H (18) | Pr | 1 | 3 |
| Trachemys scripta (Thunberg, 1792) | IN | ||||
| Geoemydidae | |||||
| Rhinoclemmys pulcherrima (Gray, 1855) | NE | L (8) | A | 3 | 5 |
| Kinosternidae | |||||
| Kinosternon hirtipes (Wagler, 1830) | LC (↓) | M (10) | Pr | 2 | 6 |
| Kinosternon integrum LeConte, 1854 | LC (=) | M (11) | Pr | 1 | 9 |
| Kinosternon sonoriense LeConte, 1854 | NT (?) | H (14) | P | 2 | 3 |
| SMO | TVB | CD | Pacific | Total | |
|---|---|---|---|---|---|
| Class Amphibia | 57 | 36 (63.2) | 28 (49.1) | 34 (59.6) | 212 (26.9) |
| Order Anura | 52 | 34 (65.4) | 26 (50) | 34 (65.4) | 144 (36.1) |
| Bufonidae | 13 | 6 (46.2) | 8 (61.5) | 8 (61.5) | 22 (59.1) |
| Centrolenidae | - | - | - | - | 1 (0) |
| Craugastoridae | 6 | 4 (66.7) | 2 (33.3) | 4 (66.7) | 17 (35.3) |
| Eleutherodactylidae | 6 | 3 (50) | - | 4 (66.7) | 31 (19.4) |
| Hylidae | 9 | 8 (88.9) | 6 (66.7) | 7 (77.8) | 38 (23.7) |
| Leptodactylidae | 1 | 1 (100) | 1 (100) | 1 (100) | 3 (33.3) |
| Microhylidae | 3 | 2 (66.7) | 1 (33.3) | 3 (100) | 4 (75) |
| Ranidae | 12 | 8 (66.7) | 6 (50) | 6 (50) | 24 (50) |
| Rhinophrynidae | - | - | - | - | 1 (0) |
| Scaphiopodidae | 2 | 2 (100) | 2 (100) | 1 (50) | 3 (66.7) |
| Order Caudata | 5 | 2 (40) | 2 (40) | 66 (7.6) | |
| Ambystomatidae | 3 | 1 (33.3) | 1 (33.3) | - | 17 |
| Plethodontidae | 2 | 1 (50) | 1 (50) | - | 49 |
| Order Gymnophiona | 2 | ||||
| Dermophiidae | - | - | - | - | 2 |
| Class Reptilia | 160 | 95 (58.8) | 98 (61.3) | 85 (53.1) | 529 (30.2) |
| Order Crocodylia | - | - | - | - | 2 (0) |
| Alligatoridae | - | - | - | - | 1 (0) |
| Crocodylidae | - | - | - | - | 1 (0) |
| Order Squamata | 154 | 90 (58.4) | 94 (61) | 81 (52.6) | 490 (31.4) |
| Suborder Lacertilia | 67 | 32 (47.8) | 39 (58.2) | 32 (47.8) | 232 (28.9) |
| Anguidae | 5 | 3 (60) | 4 (80) | 2 (40) | 14 (35.7) |
| Anolidae | 1 | 1 (100) | 1 (100) | 1 (100) | 20 (5) |
| Bipedidae | - | - | - | - | 2 (0) |
| Corytophanidae | - | - | - | - | 3 (0) |
| Crotaphytidae | 2 | - | - | - | 4 (50) |
| Dibamidae | - | - | - | - | 1 (0) |
| Diploglossidae | - | - | - | - | 2 (0) |
| Eublepharidae | 1 | - | 1 (100) | 1 (100) | 5 (20) |
| Gymnophthalmidae | - | - | - | - | 1 (0) |
| Helodermatidae | 3 | 1 (33.3) | - | 3 (100) | 4 (75) |
| Iguanidae | 2 | 1 (50) | 1 (50) | 2 (100) | 8 (25) |
| Phrynosomatidae | 35 | 18 (51.4) | 25 (71.4) | 14 (40) | 101 (34.7) |
| Phyllodactylidae | 2 | 1 (50) | - | 2 (100) | 10 (20) |
| Scincidae | 7 | 3 (42.9) | 3 (42.9) | 4 (57.1) | 22 (31.8) |
| Sphaerodactylidae | - | - | - | - | 3 (0) |
| Teiidae | 8 | 3 (37.5) | 4 (50) | 3 (37.5) | 21 (28.6) |
| Xantusidae | 1 | 1 (100) | - | - | 10 (10) |
| Xenosauridae | - | - | - | - | 1 (0) |
| Suborder Serpentes | 87 | 58 (66.7) | 55 (63.2) | 49 (56.3) | 258 (33.7) |
| Boidae | 1 | 1 (100) | 1 (100) | 3 (33.3) | |
| Colubridae | 42 | 25 (59.5) | 29 (69) | 26 (61.9) | 93 (45.2) |
| Dipsadidae | 14 | 12 (85.7) | 9 (64.3) | 9 (64.3) | 70 (20) |
| Elapidae | 3 | 3 (100) | 1 (33.3) | 3 (100) | 15 (20) |
| Leptotyphlopidae | 1 | 1 (100) | 1 (100) | 1 (100) | 13 (7.7) |
| Loxocemidae | - | - | - | - | 1 (0) |
| Natricidae | 14 | 8 (57.1) | 9 (64.3) | 3 (21.4) | 24 (58.3) |
| Typhlopidae | - | - | - | - | 1 (0) |
| Viperidae | 12 | 8 (66.7) | 6 (50) | 6 (50) | 38 (31.6) |
| Order Testudines | 6 | 4 (66.7) | 4 (66.7) | 4 (66.7) | 37 (16.2) |
| Cheloniidae | - | - | - | - | 4 (0) |
| Dermochelyidae | - | - | - | - | 1 (0) |
| Emydidae | 2 | 1 (50) | 1 (50) | 1 (50) | 13 (15.4) |
| Geoemydidae | 1 | 1 (100) | - | 1 (100) | 2 (50) |
| Kinosternidae | 3 | 2 (66.7) | 3 (100) | 2 (66.7) | 13 (23.1) |
| Testudinidae | - | - | - | - | 3 (0) |
| Trionychidae | - | - | - | - | 1 (0) |
| Total | 217 | 131 (60.4) | 126 (58.1) | 119 (54.8) | 741 (29.3) |
| Scientific Name | Genera | Species | IUCN | EVS | SEMARNAT |
|---|---|---|---|---|---|
| DD, LC, NT, VU, EN, CR | NL, Pr, A, P | ||||
| Class Amphibia | |||||
| Order Anura | 17 | 52 | 1, 45, 2, 3, 1, 0 | 10.5 | 38, 12, 2, 0 |
| Bufonidae | 3 | 13 | 0, 13, 0, 0, 0, 0 | 10.3 | 12, 1, 0, 0 |
| Craugastoridae | 1 | 6 | 0, 6, 0, 0, 0, 0 | 12.5 | 5, 1, 0, 0 |
| Eleutherodactylidae | 1 | 6 | 0, 2, 1, 1, 1, 0 | 16.6 | 4, 2, 0, 0 |
| Hylidae | 5 | 8 | 0, 8, 0, 0, 0, 0 | 8.6 | 7, 1, 0, 0 |
| Leptodactylidae | 1 | 1 | 0, 1, 0, 0, 0, 0 | 6 | 1, 0, 0, 0 |
| Microhylidae | 2 | 3 | 0, 3, 0, 0, 0, 0 | 6.3 | 2, 1, 0, 0 |
| Phyllomedusidae | 1 | 1 | 0, 1, 0, 0, 0, 0 | 11 | 1, 0, 0, 0 |
| Ranidae | 1 | 12 | 1, 9, 1, 2, 0, 0 | 10.8 | 4, 6, 2, 0 |
| Scaphiopodidae | 2 | 2 | 0, 2, 0, 0, 0, 0 | 4.5 | 2, 0, 0, 0 |
| Order Caudata | 2 | 5 | 1, 3, 0, 1, 0, 0 | 12.5 | 2, 2, 1, 0 |
| Ambystomatidae | 1 | 3 | 1, 2, 0, 0, 0, 0 | 12.7 | 1, 2, 0, 0 |
| Plethodontidae | 1 | 2 | 0, 1, 0, 1, 0, 0 | 12 | 1, 0, 1, 0 |
| Subtotal | 19 | 57 | 2, 48, 2, 4, 1, 0 | 10.7 | 40, 14, 3, 0 |
| Class Reptilia | |||||
| Order Squamata | 43 | 154 | 14, 115, 3, 2, 1, 0 | 12.5 | 96, 36, 21, 1 |
| Suborder Lacertilia | 11 | 67 | 8, 46, 1, 0, 0, 0 | 13.1 | 50, 10, 6, 1 |
| Anguidae | 3 | 5 | 1, 3, 0, 0, 0, 0 | 11.8 | 2, 3, 0, 0 |
| Anolidae | 1 | 1 | 0, 1, 0, 0, 0, 0 | 13 | 1, 0, 0, 0 |
| Crotaphytidae | 1 | 2 | 0, 2, 0, 0, 0, 0 | 12.5 | 1, 0, 1, 0 |
| Eublepharidae | 1 | 1 | 0, 1, 0, 0, 0, 0 | 17 | 1, 0, 0, 0 |
| Helodermatidae | 1 | 3 | 0, 2, 1, 0, 0, 0 | 13 | 1, 0, 2, 0 |
| Iguanidae | 1 | 2 | 0, 2, 0, 0, 0, 0 | 17 | 1, 0, 1, 0 |
| Phrynosomatidae | 5 | 35 | 3, 24, 0, 0, 0, 0 | 13.1 | 31, 2, 2, 0 |
| Phyllodactylidae | 1 | 2 | 0, 1, 0, 0, 0, 0 | 15 | 2, 0, 0, 0 |
| Scincidae | 1 | 7 | 3, 3, 0, 0, 0, 0 | 13.1 | 4, 3, 0, 0 |
| Teiidae | 1 | 8 | 1, 6, 0, 0, 0, 0 | 13 | 6, 2, 0, 0 |
| Xantusidae | 1 | 1 | 0, 1, 0, 0, 0, 0 | 16 | 0, 0, 0, 1 |
| Suborder Serpentes | 32 | 87 | 6, 69, 2, 2, 1, 0 | 12 | 46, 26, 15, 0 |
| Boidae | 1 | 1 | 0, 0, 0, 0, 0, 0 | 10 | 1, 0, 0, 0 |
| Colubridae | 17 | 42 | 3, 34, 1, 0, 0, 0 | 11.5 | 31, 4, 7, 0 |
| Dipsadidae | 7 | 14 | 1, 12, 0, 0, 0, 0 | 11.1 | 8, 6, 0, 0 |
| Elapidae | 1 | 3 | 0, 3, 0, 0, 0, 0 | 15.7 | 0, 2, 1, 0 |
| Leptotyphlopidae | 1 | 1 | 0, 1, 0, 0, 0, 0 | 8 | 1, 0, 0, 0 |
| Natricidae | 3 | 14 | 2, 9, 0, 1, 1, 0 | 14 | 5, 3, 6, 0 |
| Viperidae | 2 | 12 | 0, 10, 1, 1, 0, 0 | 13.3 | 0, 11, 1, 0 |
| Order Testudines | 4 | 6 | 1, 3, 1, 0, 0, 0 | 12.5 | 0, 3, 2, 1 |
| Emydidae | 2 | 2 | 1, 1, 0, 0, 0, 0 | 16 | 0, 1, 1, 0 |
| Geoemydidae | 1 | 1 | 0, 0, 0, 0, 0, 0 | 8 | 0, 0, 1, 0 |
| Kinosternidae | 1 | 3 | 0, 2, 1, 0, 0, 0 | 11.7 | 0, 2, 0, 1 |
| Subtotal | 47 | 160 | 15, 118, 4, 2, 1, 0 | 12.5 | 95, 39, 23, 2 |
| Total | 66 | 217 | 17, 164, 6, 6, 2, 0 | 12 | 135, 53, 26, 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, G.R.; Lemos-Espinal, J.A. Diversity, Endemism, and Conservation Status of the Herpetofauna of the Sierra Madre Occidental in Mexico with Comparison to Neighboring Biogeographic Provinces. Animals 2025, 15, 1278. https://doi.org/10.3390/ani15091278
Smith GR, Lemos-Espinal JA. Diversity, Endemism, and Conservation Status of the Herpetofauna of the Sierra Madre Occidental in Mexico with Comparison to Neighboring Biogeographic Provinces. Animals. 2025; 15(9):1278. https://doi.org/10.3390/ani15091278
Chicago/Turabian StyleSmith, Geoffrey R., and Julio A. Lemos-Espinal. 2025. "Diversity, Endemism, and Conservation Status of the Herpetofauna of the Sierra Madre Occidental in Mexico with Comparison to Neighboring Biogeographic Provinces" Animals 15, no. 9: 1278. https://doi.org/10.3390/ani15091278
APA StyleSmith, G. R., & Lemos-Espinal, J. A. (2025). Diversity, Endemism, and Conservation Status of the Herpetofauna of the Sierra Madre Occidental in Mexico with Comparison to Neighboring Biogeographic Provinces. Animals, 15(9), 1278. https://doi.org/10.3390/ani15091278






