Aflatoxin M1 Content and Mastitis-Causing Bacteria in Milk from Skopelos Dairy Goats Reared in Extensive and Intensive Farming Systems
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Sample Processing
2.2. Analysis of Aflatoxin Content
2.3. Bacterial Cultures
2.4. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM1 | Aflatoxin M1 |
| AFB1 | Aflatoxin B1 |
| SCC | Somatic Cell Count |
| EFSA | European Food and Safety Authority |
| FDA | Food and Drug Administration |
| TBC | Total Bacterial Count |
| CNS | Coagulase-Negative Staphylococci |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| CNA | Columbia Naladixic Acid |
References
- Guevara-Gonzalez, R.G. (Ed.) Aflatoxins—Biochemistry and Molecular Biology; InTech: Toyama, Japan, 2011; ISBN 978-953-307-395-8. [Google Scholar]
- Ismail, A.; Akhtar, S.; Levin, R.E.; Ismail, T.; Riaz, M.; Amir, M. Aflatoxin M1: Prevalence and Decontamination Strategies in Milk and Milk Products. Crit. Rev. Microbiol. 2016, 42, 418–427. [Google Scholar] [CrossRef]
- Seid, A.; Mama, A. Aflatoxicosis and Occurrence of Aflatoxin M1 (AFM1) in Milk and Dairy Products: A Review. Austin J. Vet. Sci. Anim. Husb. 2019, 1, 1–12. [Google Scholar]
- Nguyen, T.; Flint, S.; Palmer, J. Control of Aflatoxin M1 in Milk by Novel Methods: A review. Food Chem. 2020, 311, 125984. [Google Scholar] [CrossRef] [PubMed]
- Vaz, A.; Cabral Silva, A.C.; Rodrigues, P.; Venâncio, A. Detection Methods for Aflatoxin M1 in Dairy Products. Microorganisms 2020, 8, 246. [Google Scholar] [CrossRef]
- Kolarič, L.; Minarovičová, L.; Lauková, M.; Kohajdová, Z.; Šimko, P. Elimination of Aflatoxin M1 from Milk: Current Status, and Potential Outline of Applicable Mitigation Procedures. Trends Food Sci. Technol. 2024, 150, 104603. [Google Scholar] [CrossRef]
- Setsetse, K.G. The Impact of Storage Facilities on Animal Feed Quality with Reference to Mycotoxin Contamination Around Ngaka Modiri Molema District, North West Province. Ph.D. Thesis, North-West University, Potchefstroom, South Africa, 2019. [Google Scholar]
- Rainard, P. Mammary Microbiota of Dairy Ruminants: Fact or Fiction? Vet. Res. 2017, 48, 25. [Google Scholar] [CrossRef]
- Neculai-Valeanu, A.-S.; Ariton, A.-M. Udder Health Monitoring for Prevention of Bovine Mastitis and Improvement of Milk Quality. Bioengineering 2022, 9, 608. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 Laying down Specific Hygiene Rules for Food of Animal Origin. Off. J. L 2004, 139, 30–205. [Google Scholar]
- European Commission. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. Off. J. Eur. Union 2005, 338, 1–26. [Google Scholar]
- van Egmond, H.P. (Ed.) Mycotoxins in Dairy Products; Elsevier: London, UK, 1989; ISBN 978-1-85166-369-9. [Google Scholar]
- Kos, J.; Lević, J.; Đuragić, O.; Kokić, B.; Miladinović, I. Occurrence and Estimation of Aflatoxin M1 Exposure in Milk in Serbia. Food Control 2014, 38, 41–46. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Jinap, S.; Pirouz, A.A.; Faizal, A.R.A. Aflatoxin M1 in Milk and Dairy Products, Occurrence and Recent Challenges: A Review. Trends Food Sci. Technol. 2015, 46, 110–119. [Google Scholar] [CrossRef]
- Silva, I.M.D.M.; Da Cruz, A.G.; Ali, S.; Freire, L.G.D.; Fonseca, L.M.; Rosim, R.E.; Corassin, C.H.; Oliveira, R.B.A.D.; Oliveira, C.A.F.D. Incidence and Levels of Aflatoxin M1 in Artisanal and Manufactured Cheese in Pernambuco State, Brazil. Toxins 2023, 15, 182. [Google Scholar] [CrossRef]
- Pietri, A.; Mulazzi, A.; Piva, G.; Bertuzzi, T. Fate of Aflatoxin M 1 During Production and Storage of Parmesan Cheese. Food Control 2016, 60, 478–483. [Google Scholar] [CrossRef]
- Elkak, A.; El Atat, O.; Habib, J.; Abbas, M. Occurrence of Aflatoxin M1 in Cheese Processed and Marketed in Lebanon. Food Control 2012, 25, 140–143. [Google Scholar] [CrossRef]
- Motawee, M.M.; McMahon, D.J. Fate of Aflatoxin M1 During Manufacture and Storage of Feta Cheese. J. Food Sci. 2009, 74, T42–T45. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006. Off. J. Eur. Union 2023, 119, 103–157. [Google Scholar]
- FDA. Drug Administration. Compliance Policy Guide (CPG) Sec 527.400 Whole Milk, Lowfat Milk, Skim Milk-Aflatoxin M1; Food and Drug Administration: Silver Spring, MD, USA, 2005.
- Gonzales-Barron, U.; Gonçalves-Tenório, A.; Rodrigues, V.; Cadavez, V. Foodborne Pathogens in Raw Milk and Cheese of Sheep and Goat Origin: A Meta-Analysis Approach. Curr. Opin. Food Sci. 2017, 18, 7–13. [Google Scholar] [CrossRef]
- Maréchal, L.; Loir, L.; Maréchal, C.L.; Thiéry, R.; Vautor, E.; Loir, Y.L.; Maréchal, C.L.; Loir, Y.L.; Thiéry, R. Mastitis Impact on Technological Properties of Milk and Quality of Milk Products—A Review. Dairy Sci. Technol. 2011, 91, 247–282. [Google Scholar] [CrossRef]
- Auldist, M.J.; Hubble, I.B. Effects of Mastitis on Raw Milk and Dairy Products. Aust. J. Dairy Technol. 1998, 53, 28. [Google Scholar]
- Akers, R.M.; Nickerson, S.C. Mastitis and Its Impact on Structure and Function in the Ruminant Mammary Gland. J. Mammary Gland Biol. Neoplasia 2011, 16, 275–289. [Google Scholar] [CrossRef]
- Nelli, A.; Voidarou, C.; Venardou, B.; Fotou, K.; Tsinas, A.; Bonos, E.; Fthenakis, G.C.; Skoufos, I.; Tzora, A. Antimicrobial and Methicillin Resistance Pattern of Potential Mastitis-Inducing Staphylococcus aureus and Coagulase-Negative Staphylococci Isolates from the Mammary Secretion of Dairy Goats. Biology 2022, 11, 1591. [Google Scholar] [CrossRef]
- Bhagya, J.N.; Tresamol, P.V.; Vijayakumar, K.; Shyma, V.H.; Mini, M. Molecular Detection of Methicillin Resistant Staphylococcus aureus Associated with Mastitis in Goats. J. Vet. Anim. Sci. 2022, 53, 189–194. [Google Scholar] [CrossRef]
- Rahimi, E.; Sepehri, S.; Safarpoor Dehkordi, F.; Shaygan, S.; Momtaz, H. Prevalence of Yersinia Species in Traditional and Commercial Dairy Products in Isfahan Province, Iran. Jundishapur J. Microbiol. 2014, 7, e9249. [Google Scholar] [CrossRef] [PubMed]
- Obaidat, M.M.; Salman, A.E.B.; Roess, A.A. High Prevalence and Antimicrobial Resistance of mecA Staphylococcus aureus in Dairy Cattle, Sheep, and Goat Bulk Tank Milk in Jordan. Trop. Anim. Health Prod. 2018, 50, 405–412. [Google Scholar] [CrossRef]
- Cavicchioli, V.Q.; Scatamburlo, T.M.; Yamazi, A.K.; Pieri, F.A.; Nero, L.A. Occurrence of Salmonella, Listeria monocytogenes, and Enterotoxigenic Staphylococcus in Goat Milk from Small and Medium-Sized Farms Located in Minas Gerais State, Brazil. J. Dairy Sci. 2015, 98, 8386–8390. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Zhang, Y.; Wu, Q.; Wang, X.; Ge, W.; Wu, C. Prevalence and Characterization of Staphylococcus aureus Isolated from Goat Milk Powder Processing Plants. Food Control 2016, 59, 644–650. [Google Scholar] [CrossRef]
- Yaniarti, M.N.; Amarantini, C.; Budiarso, T.Y. The Effect of Temperature and Pasteurization Time on Staphylococcus aureus Isolates from Dairy Products. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2017; Volume 1908. [Google Scholar] [CrossRef]
- Danmallam, F.A.; Pimenov, N.V. Study on Prevalence, Clinical Presentation, and Associated Bacterial Pathogens of Goat Mastitis in Bauchi, Plateau, and Edo States, Nigeria. Vet. World 2019, 12, 638. [Google Scholar] [CrossRef]
- Rovai, M.; Caja, G.; Salama, A.A.K.; Jubert, A.; Lázaro, B.; Lázaro, M.; Leitner, G. Identifying the Major Bacteria Causing Intramammary Infections in Individual Milk Samples of Sheep and Goats Using Traditional Bacteria Culturing and Real-Time Polymerase Chain Reaction. J. Dairy Sci. 2014, 97, 5393–5400. [Google Scholar] [CrossRef]
- Tzora, A.; Skoufos, J.; Tsinas, A.; Fotou, K.; Karamoutsios, A.; Kalyva, Z.; Nikolaou, K.; Fthenakis, G.C. The Bacterial Flora of the Udder of Goats. J. Hell. Vet. Med. Soc. 2016, 67, 99. [Google Scholar] [CrossRef]
- Abdelrahman, M.A.; Khadr, A.M.; Mahmoud, A.A.; Elsheimy, T.M.; Osman, A. Occurrence of Clinical and Subclinical Mastitis and Associated Risk Factors in Lactating Goats with Special Reference to Dry Period Infection and Teat Skin. Alex. J. Vet. Sci. 2020, 64, 95–101. [Google Scholar] [CrossRef]
- Tenhagen, B.-A.; Hansen, I.; Reinecke, A.; Heuwieser, W. Prevalence of Pathogens in Milk Samples of Dairy Cows with Clinical Mastitis and in Heifers at First Parturition. J. Dairy Res. 2009, 76, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Virdis, S.; Corgiolu, G.; Scarano, C.; Pilo, A.L.; De Santis, E.P.L. Occurrence of Aflatoxin M1 in Tank Bulk Goat Milk and Ripened Goat Cheese. Food Control 2008, 19, 44–49. [Google Scholar] [CrossRef]
- Waqas, M.; Pervaiz, W.; Zia, K.M.; Iqbal, S.Z. Assessment of Aflatoxin B1 in Animal Feed and Aflatoxin M1 in Raw Milk Samples of Different Species of Milking Animals from Punjab, Pakistan. Wiley Online Libr. 2021, 41, e12893. [Google Scholar] [CrossRef]
- Bingöl, N.T.; Dede, S.; Yuzuncu, V.; Ceylan, E. Influence of Aflatoxin Present in Forages and Concentrated Feding Stuffs on Milk and Some Serum Biochemical Parameters in Goats. Bull.-Vet. Inst. Pulawy 2007, 51, 65. [Google Scholar]
- de Matos, C.J.; Schabo, D.C.; do Nascimento, Y.M.; Tavares, J.F.; de Lima, E.O.; da Cruz, P.O.; de Souza, E.L.; Magnani, M.; Magalhães, H.I. Aflatoxin M 1 in Brazilian Goat Milk and Health Risk Assessment. J. Environ. Sci. Health 2021, 56, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Fallah, A.A.; Fazlollahi, R.; Emami, A. Seasonal Study of Aflatoxin M1 Contamination in Milk of Four Dairy Species in Yazd, Iran. Food Control 2016, 68, 77–82. [Google Scholar] [CrossRef]
- Asi, M.R.; Iqbal, S.Z.; Ariño, A.; Hussain, A. Effect of Seasonal Variations and Lactation Times on Aflatoxin M1 Contamination in Milk of Different Species from Punjab, Pakistan. Food Control 2012, 25, 34–38. [Google Scholar] [CrossRef]
- Manouras, A.; Malissiova, E. Occurrence of Aflatoxins in compound feeds and feed materials for dairy livestock in Central Greece. J. Hell. Vet. Med. Soc. 2018, 66, 169. [Google Scholar] [CrossRef]
- Chaisri, W.; Mongkon, W.; Sugita-Konishi, Y.; van Dam, D.; Huntley, I.; Suriyasathaporn, W. Feed and Feed Storage Factors in Relation to Aflatoxin M1 Contamination in Bulk Milk of Smallholder Dairy Farms. Mycotoxins 2017, 67, 85–88. [Google Scholar] [CrossRef]
- Makau, C.M.; Matofari, J.W.; Muliro, P.S.; Bebe, B.O. Aflatoxin B1 and Deoxynivalenol Contamination of Dairy Feeds and Presence of Aflatoxin M1 Contamination in Milk from Smallholder Dairy Systems in Nakuru, Kenya. Int. J. Food Contam. 2016, 3, 6. [Google Scholar] [CrossRef]
- Anyango, G.; Mutua, F.; Kagera, I.; AndangO, P.; Grace, D.; Lindahl, J.F. A Survey of Aflatoxin M1 Contamination in Raw Milk Produced in Urban and Peri-Urban Areas of Kisumu County, Kenya. Infect. Ecol. Epidemiol. 2018, 8, 1547094. [Google Scholar] [CrossRef]
- De Roma, A.; Rossini, C.; Ritieni, A.; Gallo, P.; Esposito, M. A Survey on the Aflatoxin M1 Occurrence and Seasonal Variation in Buffalo and Cow Milk from Southern Italy. Food Control 2017, 81, 30–33. [Google Scholar] [CrossRef]
- Ismaiel, A.A.; Tharwat, N.A.; Sayed, M.A.; Gameh, S.A. Two-Year Survey on the Seasonal Incidence of Aflatoxin M1 in Traditional Dairy Products in Egypt. J. Food Sci. Technol. 2020, 57, 2182–2189. [Google Scholar] [CrossRef]
- Marogna, G.; Pilo, C.; Vidili, A.; Tola, S.; Schianchi, G.; Leori, S.G. Comparison of Clinical Findings, Microbiological Results, and Farming Parameters in Goat Herds Affected by Recurrent Infectious Mastitis. Small Rumin. Res. 2012, 102, 74–83. [Google Scholar] [CrossRef]
- Gabli, Z.; Djerrou, Z.; Gabli, A.E.; Bensalem, M. Prevalence of Mastitis in Dairy Goat Farms in Eastern Algeria. Vet. World 2019, 12, 1563–1572. [Google Scholar] [CrossRef]
- Begum, M.; Hossain, M.; Ershaduzzaman, M.; Alam, M. Epidemiological Studies on Subclinical Mastitis in Dairy Goats in Northern Regions of Bangladesh. Bangladesh J. Livest. Res. 2016, 19, 112–122. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, H.; Zhao, X.; Gao, Y.; Zhang, M.; Chen, D. Prevalence and Pathogens of Subclinical Mastitis in Dairy Goats in China. Trop. Anim. Health Prod. 2015, 47, 429–435. [Google Scholar] [CrossRef]
- Ariffin, M.F.T.; Hasmadi, N.; Hian, C.M.; Ghazali, M.F.; Suhaili, Z.; Ariffin, S.M.Z. Prevalence and Antimicrobial Sensitivity Pattern of Staphylococcus aureus Isolated from Clinical and Subclinical Mastitis in Small Ruminant in Besut and Setiu, Terengganu, Malaysia. Malays. J. Microbiol. 2020, 16, 104. [Google Scholar] [CrossRef]
- Jabbar, A.; Saleem, M.H.; Iqbal, M.Z.; Qasim, M.; Ashraf, M.; Tolba, M.M.; Nasser, H.A.; Sajjad, H.; Hassan, A.; Imran, M.; et al. Epidemiology and Antibiogram of Common Mastitis-Causing Bacteria in Beetal goats. Vet. World 2020, 13, 2596–2607. [Google Scholar] [CrossRef]
- Widianingrum, D.C.; Silaban, D.G.; Fanata, W.I.D.; Khasanah, H. Identification of Antibiotic Resistance Genes in Escherichia coli from Subclinical Mastitis Milk in Dairy Cows and Goats, East Java Province. Vet. Med. 2024, 69, 35–41. [Google Scholar] [CrossRef]
- Moura, G.S.; Gebreyes, W.A.; Marques, M.F.S.; Stipp, D.T.; Souza, F.N.; Da Costa, L.B.; Oliveira, C.J.B. Short Communication: Occurrence of Methicillin-Resistant Staphylococcus aureus and Coagulase-Negative Staphylococci in Dairy Goat Herds in Ohio, United States. J. Dairy Sci. 2018, 101, 7804–7807. [Google Scholar] [CrossRef] [PubMed]
- Haggag, Y.; Nossair, M.; Naggar, A.; Abdallah, M.; Habib, H.; Farag, H. Microbiological Studies on Streptococcus Species Associated with Environmental Mastitis in Sheep and Goats Farms. Alex. J. Vet. Sci. 2019, 60, 155. [Google Scholar] [CrossRef]
- Mathewos, M.; Fesseha, H.; Tindashe, M.Y.; Markos, A.; Yirgalem, M. Study on Bovine Mastitis with Isolation, Identification and An-timicrobial Resistance Patterns of Streptococci Species from Raw Milk in Bishoftu Town, Ethiopia. SM Trop. Med. J. 2020, 5, 5. [Google Scholar] [CrossRef]
- Albenzio, M.; Santillo, A.; Caroprese, M.; Ciliberti, M.G.; Marino, R.; Sevi, A. Effect of Stage of Lactation on the Immune Competence of Goat Mammary Gland. J. Dairy Sci. 2016, 99, 3889–3895. [Google Scholar] [CrossRef] [PubMed]
- Souza, F.N.; Blagitz, M.G.; Penna, C.F.A.M.; Della Libera, A.M.M.P.; Heinemann, M.B.; Cerqueira, M.M.O.P. Somatic Cell Count in Small Ruminants: Friend or Foe? Small Rumin. Res. 2012, 107, 65–75. [Google Scholar] [CrossRef]
- Boyazoglu, J.; Morand-Fehr, P. Mediterranean Dairy Sheep and Goat Products and Their Quality: A Critical Review. Small Rumin. Res. 2001, 40, 1–11. [Google Scholar] [CrossRef]
- Desidera, F.; Skeie, S.B.; Devold, T.G.; Inglingstad, R.A.; Porcellato, D. Fluctuations in Somatic Cell Count and Their Impact on Individual Goat Milk Quality Throughout Lactation. J. Dairy Sci. 2025, 108, 152–163. [Google Scholar] [CrossRef]
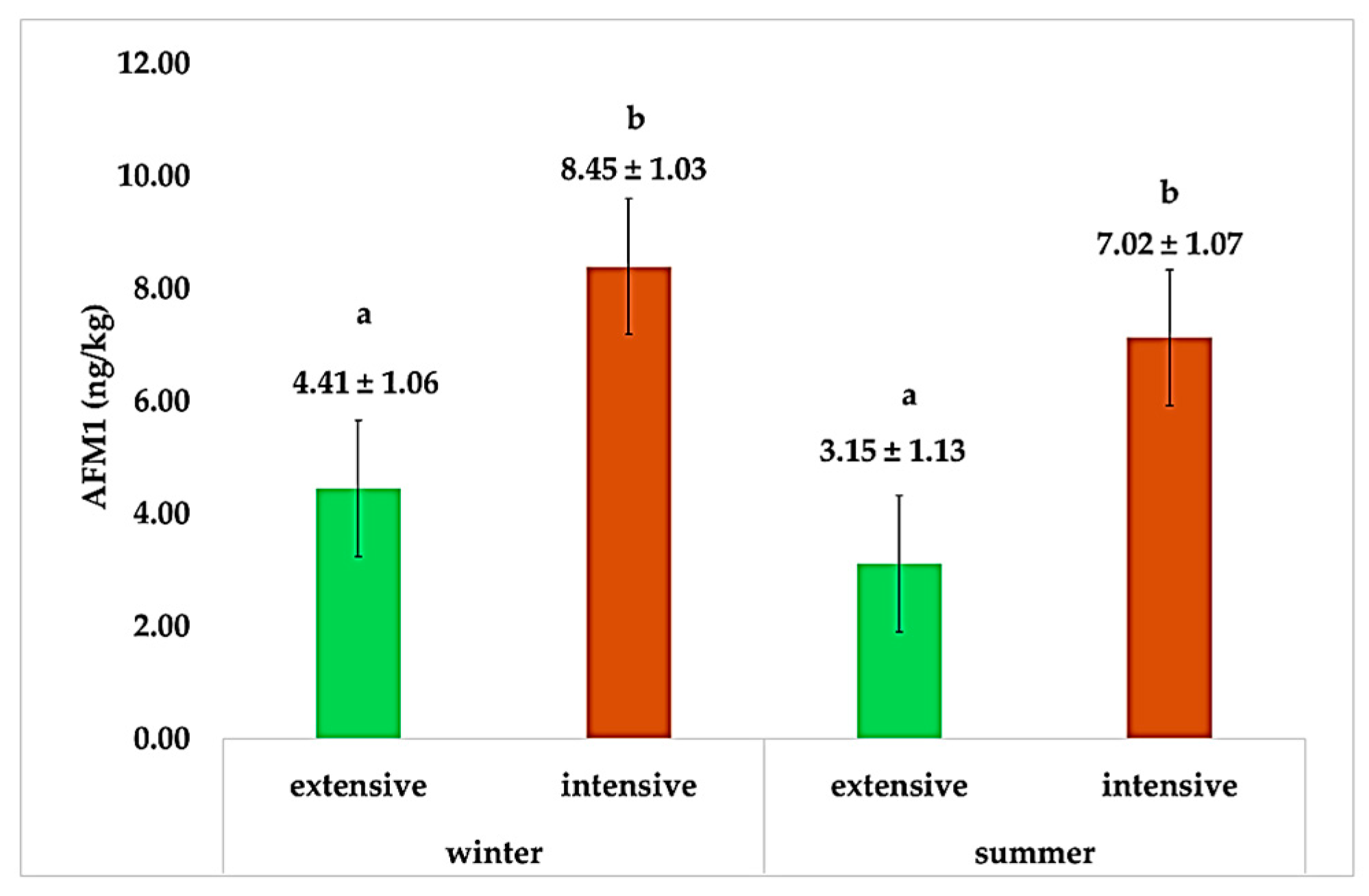
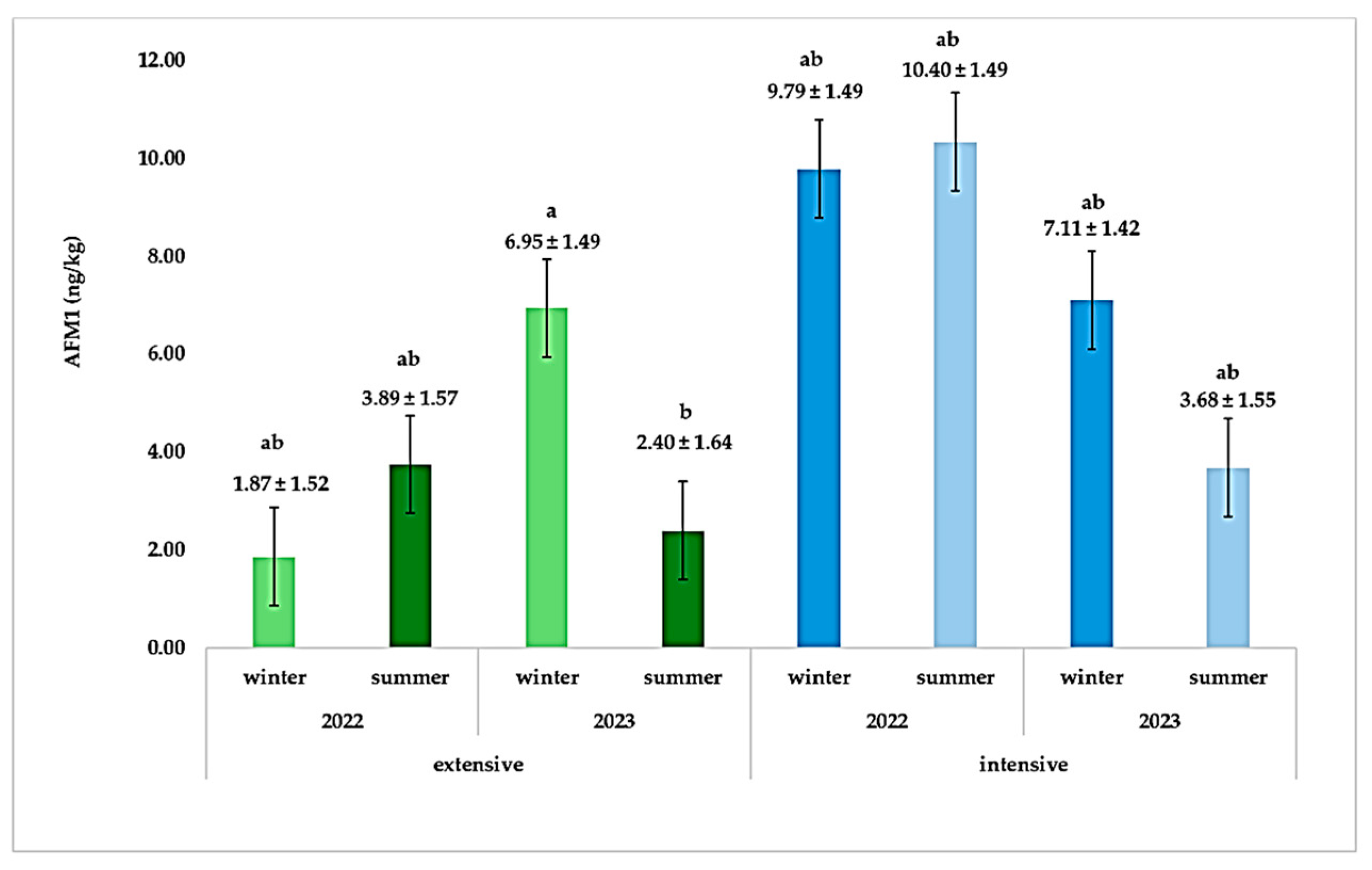
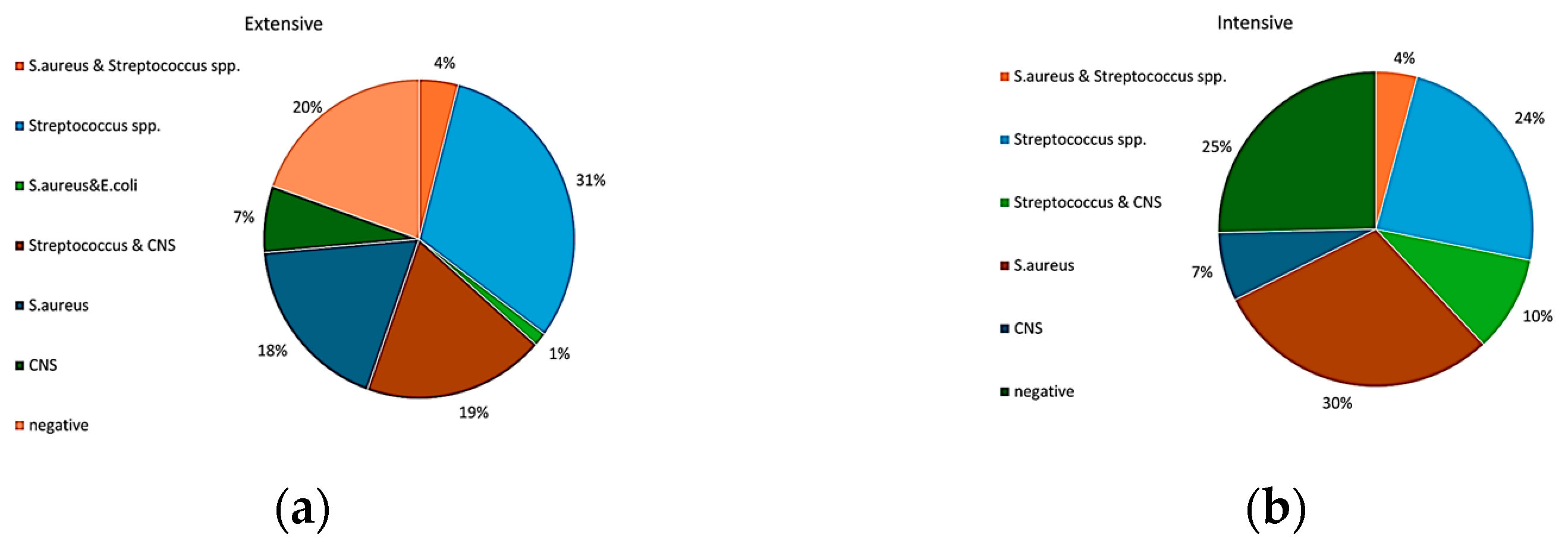
| Farming System | AFM1 Levels (ng/kg) | |||
|---|---|---|---|---|
| <5 | <25 | 25–50 | >50 | |
| Extensive | 81 | 31 | - | - |
| Intensive | 62 | 54 | 3 | 2 |
| FS | S | Y | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Aflatoxins | Extensive | Intensive | Winter | Summer | 2022 | 2023 | FS | S | Y | FS × S × Y |
| AFM1 (ng/kg) | 3.78 ± 0.79 | 7.76 ± 0.76 | 6.42 ± 1.10 | 5.12 ± 1.10 | 6.48 ± 0.76 | 5.04 ± 0.76 | <0.01 | 0.21 | 0.20 | 0.004 |
| Variable | B | S.E | Sig | Exp(B) |
|---|---|---|---|---|
| Farming system | 0.65 | 0.31 | 0.03 | 1.9 |
| Lactation stage | 0.98 | 0.35 | 0.005 | 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stavropoulos, I.; Basdagianni, Z.; Manessis, G.; Tsiftsi, A.; Bossis, I. Aflatoxin M1 Content and Mastitis-Causing Bacteria in Milk from Skopelos Dairy Goats Reared in Extensive and Intensive Farming Systems. Animals 2025, 15, 1238. https://doi.org/10.3390/ani15091238
Stavropoulos I, Basdagianni Z, Manessis G, Tsiftsi A, Bossis I. Aflatoxin M1 Content and Mastitis-Causing Bacteria in Milk from Skopelos Dairy Goats Reared in Extensive and Intensive Farming Systems. Animals. 2025; 15(9):1238. https://doi.org/10.3390/ani15091238
Chicago/Turabian StyleStavropoulos, Ioannis, Zoitsa Basdagianni, Georgios Manessis, Aikaterini Tsiftsi, and Ioannis Bossis. 2025. "Aflatoxin M1 Content and Mastitis-Causing Bacteria in Milk from Skopelos Dairy Goats Reared in Extensive and Intensive Farming Systems" Animals 15, no. 9: 1238. https://doi.org/10.3390/ani15091238
APA StyleStavropoulos, I., Basdagianni, Z., Manessis, G., Tsiftsi, A., & Bossis, I. (2025). Aflatoxin M1 Content and Mastitis-Causing Bacteria in Milk from Skopelos Dairy Goats Reared in Extensive and Intensive Farming Systems. Animals, 15(9), 1238. https://doi.org/10.3390/ani15091238








