Simple Summary
Gall mites (superfamily Eriophyoidea) are microscopic phytoparasites that transmit plant viruses and induce various abnormal growths, significantly affecting plant health and agricultural productivity. While most species within the Eriophyoidea superfamily are believed to have a simple life cycle, those with a complex life cycle often display seasonal dimorphism, characterized by two distinct female morphotypes: protogynes and deutogynes. In contrast, male dimorphism remains poorly understood due to the scarcity of males in populations and the historical focus on female morphology in Eriophyoidea systematics. Trisetacus kirghisorum (Nalepellidae), a conifer-associated species, is the only known eriophyoid taxon to exhibit seasonal dimorphism in both males and females. In this study, we analyzed morphological, molecular, and biological data from Austracus havrylenkonis, a gall mite species associated with Gondwanian relict host plants of the genus Nothofagus. Using material collected from Chile and Argentina, we demonstrated that this species exhibits two distinct forms of both males and females, marking it as the first known bisexually dimorphic taxon within the family Phytoptidae. We further discuss the gradual and discrete seasonal morphological changes observed in Eriophyoidea populations and emphasize the importance of additional studies on life cycles and faunistic surveys of gall mites associated with Nothofagus in Australasia.
Abstract
Acariform mites of the superfamily Eriophyoidea are permanent parasites of higher vascular plants. Seasonal morphological dimorphism in females has been documented across various eriophyoid taxa, while male dimorphism remains poorly understood. In this study, we analyzed morphological, molecular, and biological data from the genus Austracus Keifer 1944, with a particular focus on the type species, A. havrylenkonis Keifer 1944, associated with Nothofagus. Using new material collected from Chile and Argentina, we demonstrated that this species exhibits two distinct forms of both males and females, making it the first known bisexually dimorphic taxon within the family Phytoptidae. The summer form of A. havrylenkonis displays the unstable annulation of the dorsal opisthosoma, characterized by a significant variation in the number of thin, microtuberculated dorsal annuli interspersed among the broader, plate-like annuli typical of the winter form. This finding aligns with the previous observations of atypical deuterogyny in Eriophyoidea and leads us to hypothesize that gall mites employ diverse adaptive strategies—manifesting as either gradual or discrete morphological changes—to cope with seasonal environmental fluctuations. Investigating the genetic mechanisms underlying these adaptive strategies, along with further studies of eriophyoids associated with Nothofagus in the Southern Hemisphere, represents a promising direction for future research.
1. Introduction
Eriophyoid mites (Acariformes, Eriophyoidea) are an ancient group of microscopic chelicerates highly specialized for phytoparasitism [1]. Recent taxa of eriophyoids are permanently associated with three large groups of vascular plants (ferns, conifers, and angiosperms); although, in previous epochs, eriophyoids or their ancestors may have inhabited various extinct groups of land plants, including seed ferns, the extinct lineages of gymnosperms, and flowering plants [2,3]. Current consensus between morphological systematics and molecular phylogenetics implies that the superfamily Eriophyoidea comprises four distinct groups—Pentasetacidae, Phytoptidae s.str., Nalepellidae and Eriophyidae s.l.—the last including members of two families, Eriophyidae and Diptilomiopidae [4]. Among these groups, only Phytoptidae s.str. is restricted to angiosperms, with a distinct tendency for associations with endemic and relict hosts [5].
Current data on the biology of phytoptids suggest they lack pronounced seasonal dimorphism, since morphologically distinct seasonal forms have never been reported in Phytoptidae s.str. A remarkable phytoptid taxon, the hazelnut bud mite Phytoptus avellanae s.l. Nalepa from Corylus spp., has an atypical life cycle, including a morphologically aberrant Tegonotus-like form of nymphs [6,7,8]. These nymphs are strikingly different morphologically from the adults in the way typically observed between (a) vagrant and concealed ecological groups of gall mites as well as between (b) seasonal forms of females in the complex life cycle of some species from the family Eriophyidae Nalepa [9]. A recent molecular phylogenetic study inferred two phytoptid species, Austracus havrylenkonis Keifer 1944 and Sierraphytoptus alnivagrans Keifer 1939, to be the possible sister taxa of P. avellanae [5]. Unlike P. avellanae that is a worldwide pest [7,10,11,12], both A. havrylenkonis and S. alnivagrans are rarely encountered species, putatively restricted to local areas in southern part of South America and in North America (USA: CA, WV), respectively, where their host plants (Nothofagus spp. and Alnus spp.) are naturally distributed.
For a decade, we attempted to obtain material from Nothofagus in order to reinvestigate poorly studied Austracus mites. In 2017, we received ethanol material from San Martín de los Andes (Argentina) containing branches of Nothofagus antarctica with fruits infested by Austracus havrylenkonis Keifer 1944. Later, we investigated two DNA isolates (F234 and d96) of Austracus from this material and obtained sequences of the 28S gene. Morphologically, the mite specimens corresponding to different isolates were similar; however, their 28S sequences were not identical, and species delimitation analysis suggested possible cryptic species [5].
In this paper, we aimed to summarize morphological, molecular, and biological data on the genus Austracus and reinvestigate the type species of this genus, A. havrylenkonis Keifer 1944, based on the previously studied material from San Martín de los Andes [5]. Surprisingly, when we carefully examined this material, we found that two distinct morphotypes of Austracus were present within one infested fruit of Nothofagus. In order to test the conspecificity of these morphotypes, we sequenced three marker genes and performed molecular phylogenetic analysis. This led to the unexpected discovery of the first documented case of pronounced bisexual dimorphism in Phytoptidae s.str. and highlighted the need to revise the generic diagnosis of Austracus.
2. Materials and Methods
2.1. Collection and Morphological Measurements
Up to now, Austracus mites are known from four localities (a, b, c, d) in Argentina and Chile (Figure 1A, Table 1). This study is based mainly on the new material of A. havrylenkonis Keifer 1944 from the locality (a) near San Martín de los Andes (Argentina). This place is situated very close to the type locality (b) of A. havrylenkonis on the island Victoria in Nahuel Huapi National Park (Argentina), where in April 1943, this species was found for the first time inside the fruits of Nothofagus dombeyi [13]. For more than half of a century, no new records of this species were published. Recently, solitary females of Austracus cf havrylenkonis were found twice in southern Chile in region XII Magallanes (c): as accidental on a swamp sedge on 15 November 2015 in Laguna Parrillar National Reserve and as vagrant on very young leaves of sprouting N. antarctica on 15 November 2019 in Puerto Natales (Table 1). According to the official reports of Servicio Agricola y Ganadero (SAG) of the Ministry of Agriculture of Chile, mites of the genus Austracus have also been reported multiple times between 2016–2022 from central Chile (regions Araucania and Bio Bio); however, these reports did not include species-level identifications or confirm host associations, and the original material is unavailable for examination. We used the material from locality (a) for investigating morphology of A. havrylenkonis and the specimens from the locality (c) as additional material for comparison.
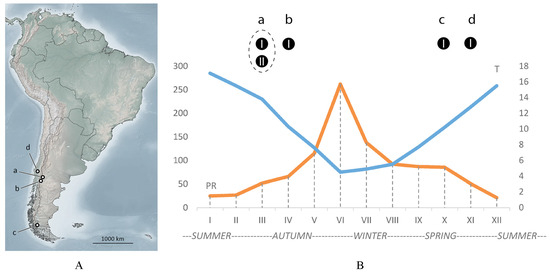
Figure 1.
Four localities (a, b, c, d) of Austracus mites in Argentina and Chile (A) mapped on a seasonal climate chart of San Martín de los Andes (B), where the main material for this study was collected. The two curves are daily mean temperature (T °C, blue) and average precipitation (PR, mm, orange), both reconstructed based on data from https://www.smn.gob.ar/estadisticas (accessed on 25 January 2025). Black circles and letters a, b, c, d in (B) correspond to findings of Austracus in the four localities (a, b, c, and d) shown in (A); I and II—two morphotypes of Austracus (see the Section 3 for details).

Table 1.
Published records of Austracus in Chile and Argentina. Asterisks (*) indicate the findings that are not shown in Figure 1 because of insufficient data.
The branches of N. antarctica (Forster) Oerst. with maturing fruits were collected on 23 March 2017 by the late Dr. Vladimir Žikić (Serbia) in San Martín de los Andes, Argentina (Figure 1A, a) and kept in a vial with 70% ethanol in a freezer (−20 °C). In 2018, the ethanol material was transferred to Saint Petersburg (Russia) for investigation. The fruits were dissected with a sterile blade, and mites were collected by a needle and slide-mounted in modified Hoyer medium [22]. The external morphology of the slide-mounted specimens was studied using conventional light microscopy (LM) using a Leica DM2500 (Leica Microsystems GmbH, Wetzlar, Germany). Morphological descriptions were based on phase contrast (PC) and differential interference contrast (DIC) LM observations. All measurements were obtained using ToupTek ToupView software x64 v. 4.11.19728.20211022 (Hangzhou ToupTek Photonics Co., Hangzhou 310030, China). The terminology of eriophyoid morphology and the classification of Eriophyoidea follow [23] and [24], respectively. The drawings of mites were sketched by pencil using a video projector [25], scanned, and finalized in Adobe Illustrator CC 2014 using a Wacom Intuos S (CTL-4100K-N) graphics tablet (Wacom Co., Ltd, Kazo, Saitama, Japan).
2.2. DNA Extraction and Sequencing
For DNA extraction, 1–2 females of morphotypes I and II, distinguished by coloration, were separately crushed with a fine pin in a 1.5 μL drop of distilled water on a cavity well microscope slide. The fragments of the posterior part of the mite bodies (including morphotype specific dorsal opisthosomal annuli) were pulled out of the drop and slide-mounted for morphotype confirmation. Each drop was pipetted into a thin-walled PCR tube with 30 μL of 6% solution of Chelex® 100 Resin Bio Rad before being heated three times (5 min at 95 °C) in a thermostat with intermediate short vortexing. The solution above the Chelex® granules was used as the DNA template for PCR to amplify the D1-D2 domains of the 28S rDNA, ITS1-5.8S-ITS2, and mitochondrial Cox1 genes. For the PCR and sequencing, we applied the protocols and primers detailed by [26]. Sequences were obtained using BigDye Terminator v.3.1 chemistry (Applied Biosystems, Foster City, CA, USA) and a 3500xl Genetic Analyzer (Applied Biosystems). Trace files were checked and edited using GeneStudioTM Professional 2.2.0.0 (www.genestudio.com, accessed on 20 May 2019). Overall, we obtained nine DNA isolates, six Cox1 sequences, eight ITS1-5.8S-ITS2 sequences, and four D1D2 28S sequences of A. havrylenkonis (Table 2).

Table 2.
Accession numbers of partial Cox1, ITS, and D1D2 28S sequences of mites Austracus havrylenkonis differing in color and external morphology (morphotypes I and II) from Argentina.
2.3. Sequence Alignment and Molecular Phylogenetic Analyses
Combined molecular phylogenetic analyses of Cox1 and D1D2 28S sequences were carried out to test the conspecificity of the morphotypes I and II of A. havrylenkonis. The ITS1–5.8S–ITS2 sequences were used only for intra-genus comparisons among Austracus DNA isolates. Sequences of 28S and Cox1 genes of Fragariocoptes setiger (JAIFTH010000001.1, JAIFTH010000002.1) and Phytoptus lineatus (MT712456, MT712746) were used as distant and close out-groups, respectively. We combined them with 28S and Cox1 sequences of Austracus listed in Table 2 and obtained the dataset for analyses. All sequences were aligned with the MAFFT algorithm [27] through the web-based program interface [28] using default settings. Maximum likelihood analyses were conducted in IQ-tree 2 [29]. For 28S/Cox1 gene evolution, the HKY+F+I/TPM2u+F+I models were selected using ModelFinder [30] as implemented in IQ-tree 2 based on the Bayesian information criterion. Branch support values were generated from Ultrafast bootstrap approximation (UFBoot) with 10,000 bootstrap alignments, 1000 maximum iterations, and a minimum correlation coefficient of 0.99. Values of a single branch test (SH-like approximate likelihood ratio test (SH-aLRT) with 1000 replicates [31]) were labeled on the maximum likelihood (ML) trees.
3. Results
3.1. Microscopic Observations and Morphotypes of Austracus havrylenkonis
The fruits of Nothofagus antarctica infested by A. havrylenkonis are swollen and globose. Numerous mites, ranging in color from white to bright or light orange, were observed clustered in groups between folds of the internal fruit tissues (Figure 2). Under a stereomicroscope, some of the white-colored adult mites appeared notably larger, being both longer and thicker, while most (though not all) of the orange mites were slightly shorter and slender. Immatures were significantly less numerous than adults, and all exhibited a whitish or semi-translucent appearance. Microscopic observations of slide-mounted adult mites revealed two morphotypes (MT-I and MT-II, see below) in both females and males. While these morphotypes were not perfectly correlated with color, all larger white mites corresponded to morphotype I, and all smaller bright orange mites belonged to morphotype II.
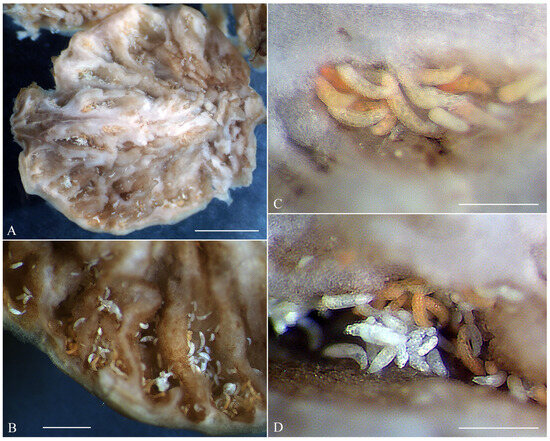
Figure 2.
Dissected fruit of Nothofagus antarctica infested by Austracus havrylenkonis Keifer 1944. (A,B)—fragments of the dissected fruit with numerous mites; (C)—cluster of light and bright orange mites (mostly of the morphotype I); (D)—mixed group of mites of the morphotypes I and II. Scale bar: (A)—25 mm, (B)—1 mm, (C,D)—300 μm.
3.1.1. Female and Male Morphotype I (MT-I)
These specimens are morphologically similar to those described by Keifer [13] from locality b and later recorded in localities c and d (Figure 1A). They exhibit a light-to-bright orange coloration, with smooth, broad, and slightly overlapping plate-like dorsal annuli, as well as microtuberculated narrow ventral annuli that are significantly more numerous than the dorsal ones (Figure 3A).
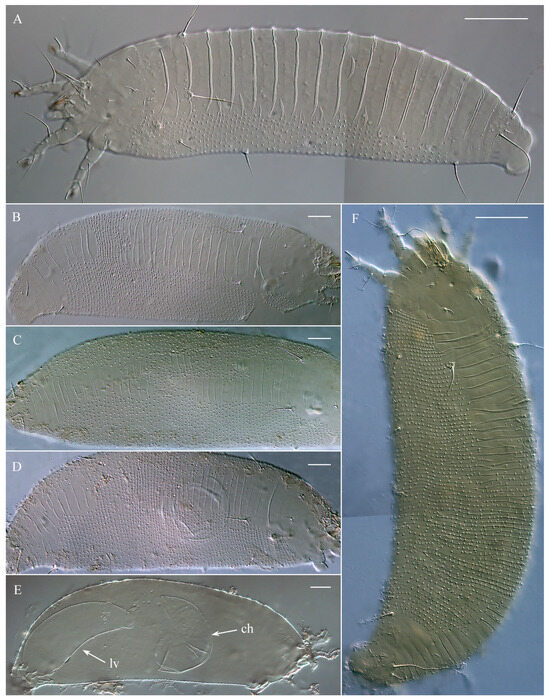
Figure 3.
DIC LM microphotographs of morphotypes MT-I (A) and MT-II (B–F) of Austracus havrylenkonis Keifer 1944 (females) from fruits of Nothofagus antarctica from San Martín de los Andes (Argentina). Note: ruptured chorion (ch) and larva (lv) inside a female of morphotype II are shown in (E). Scale bar: (A,F)—40 μm; (B–E)—20 μm.
3.1.2. Female and Male Morphotype II (MT-II)
This new form of Austracus was found for the first time in this study in locality a (Figure 1A). These mites were predominantly white, with some individuals displaying a light orange coloration. They had irregular annulation of the dorsal opisthosoma, which shows a wide range of shapes from minimal differentiation of the dorsal opisthosomal annuli into broader and narrower annuli to distinctly heterogeneous patterns, where some dorsal annuli are as thin and microtuberculated as the ventral ones, while others are significantly wider and smooth (Figure 3B–D,F).
3.2. Molecular Phylogenetics
To test the genetic identity of the morphotypes I and II, we obtained sequences of three marker genes—Cox1, ITS, and D1D2 28S (Table 2). The Cox1 and ITS sequences from the mites of different morphotypes were identical. D1D2 28S sequences were almost identical except one nucleotide position in the middle of D2 region of 28S gene: “G” in isolates d488 (MT-I), d492 (MT-II), and d493 (MT-II) vs. “A” in isolate d490 (MT-I). This mononucleotide variation in the 28S gene does not correlate with mite morphotype. Overall, a sequence comparison indicates that the morphotypes I and II are conspecific.
Only one Cox1 sequence, two 28S sequences, and no ITS sequences of Austracus are present in GenBank (accessed on 25 February 2025). Nucleotide blast (BLASTN) for our longest Cox1 sequence (PQ406503, isolate d488) revealed three Cox1 sequences of phytoptids to be the closest: MT712721 (A. havrylenkonis isolate F234, 86.9% identity, 100% coverage), MT712756 (Solenocristus searsius, 86.3% identity, 100% coverage), and MT712739 (Phytoptus chamaebatiae, 86.4% identity, 99% coverage). Calculated Kimura two parameter (K2P) genetic distance between sequences PQ406503 and MT712721 of A. havrylenkonis is equal to 16% ± 2%.
Protein blast (BLASTX) for the same Cox1 sequence (PQ406503) revealed one 100% identical sequence (QLD94595 of A. havrylenkonis isolate F234) and two sequences with 97–98% identity (ATY50385 of Novophytoptus rostratae and QLD94620 of Phytoptus lineatus), all of them with 99% coverage. The 100% identity of amino acid sequences PQ406503 and QLD94595 indicates that the differences between the Cox1 nucleotide sequences of Austracus from our material (PQ406503) and the sequence of Austracus isolate F234 from GenBank (MT712721) are due to synonymous substitutions.
Blast search for our four new 28S sequences of Austracus mites of different morphotypes revealed the three most similar sequences of phytoptids with 99–100% coverage: MT712437 (Austracus sp. isolate d96, 99.9–100% identity), MT712438 (A. havrylenkonis isolate F234, 99.0–99.1% identity), and KT070303 (Sierraphytoptus alnivagrans, 93.9–94.1% identity).
A combined (Cox1 + D1D2 28S) molecular phylogenetic analysis revealed monophyletic Austracus diverging into two clades, one including a single isolate F234 from GenBank and the other comprising all the other isolates (Figure 4). Isolates representing different morphotypes do not form monophyletic groups, indicating that MT-I and MT-II are two morphological forms but not different species.
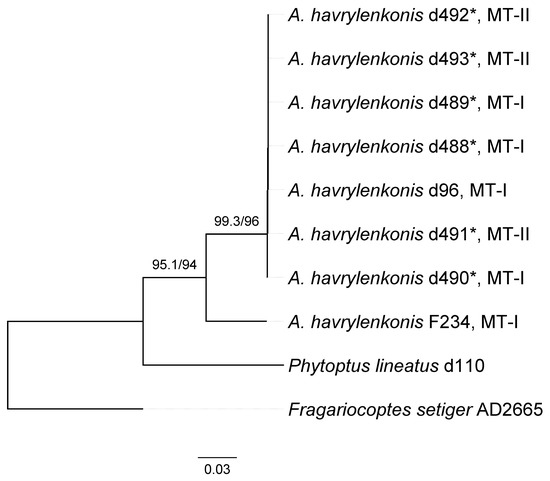
Figure 4.
Combined Cox1 + D1D2 28S ML tree of eight isolates of Austracus havrylenkonis Keifer 1944. Values of SH-aLRT /UF support are indicated above branches. MT-I and MT-II—morphological morphotypes I and II. Asterisks indicate new isolates obtained in this study (Table 2).
3.3. Seasonal Findings of Austracus and Data on Molting, Sperm Storage, and Putative Dispersal
Seasonal changes in temperature and precipitation in San Martín de los Andes are shown in Figure 1B. In this area, winter (May–July) is cold and wet, but summer (December–February) is warm and dry. In Argentina (localities a and b, Figure 1A), Austracus mites were found in autumn. Findings in Chile in localities c and d were made at the end of spring.
Morphotype MT-I was recorded in all four localities (a, b, c, d) inside fruits (a, b) and as vagrant (c, d), whereas morphotype MT-II was found only in fruits in the locality (a).
In the mixed population from San Martín de los Andes (autumn sample), females of the morphotype MT-II often contained developing eggs and larvae eclosing inside (Figure 3D,E), which is a clear indication of female senility [32]. In the spermathecae of most partially cleared MT-I females from that sample, we observed clusters of sperm cells; however, no MT-I females with developing eggs were found, suggesting no reproduction in that period.
Remarkably, those were MT-I females that were detected in early spring in the Chilean samples from Magallanes (Figure 1A, c) as vagrants and as accidental on atypical host, probably in the period of dispersal after overwintering in fruits.
Overall, our limited seasonal data combined with the results of the sequence comparison and molecular phylogenetic analyses (reported above) indicate conspecificity of MT-I and MT-II and suggest that MT-II is a summer form (protogyne) and MT-I is a winter form (deutogyne) of A. havrylenkonis. The form MT-I was described by Keifer [13], and MT-II is a newly discovered seasonal form.
3.4. Morphological Differences Between Seasonal Morphotypes of Austracus havrylenkonis
The summer and winter forms of A. havrylenkonis are very similar in most morphometric characters, except for the dimensions of the body and prodorsal shield, distances between prodorsal shield setae, and the number of opisthosomal annuli (Table 3). Although the values of all these morphometrics exhibit marginal overlap, the summer forms of both sexes generally display a longer and broader body, a wider prodorsal shield, greater pairwise distances between setae ve and sc, and a higher number of opisthosomal annuli compared to the winter forms. The topography of the dorsal opisthosoma serves as the most distinctive qualitative trait differentiating these forms (Figure 3, Figure 5 and Figure 6). In the winter form (MT-I), both females and males exhibit a slightly flattened dorsal opisthosoma, characterized by broad, partially overlapping plate-like dorsal annuli. In contrast, the summer form (MT-II) of both sexes also displays plate-like annuli, but these are notably narrower and are often interrupted in the mid-region of the opisthosoma by thinner, microtuberculated annuli. These thin annuli in summer forms vary significantly in number, resulting in a continuum of numerous intermediate forms (Figure 3B–D,F).

Table 3.
Measurements (means followed by ranges) of summer and winter forms of Austracus havrylenkonis Keifer 1944. Characters that distinguish between forms are highlighted in gray. Remarks: only numbers of wider plate-like dorsal annuli are given for summer females and males, whereas highly variable numbers of narrower microtuberculated dorsal annuli situated between them are not given.
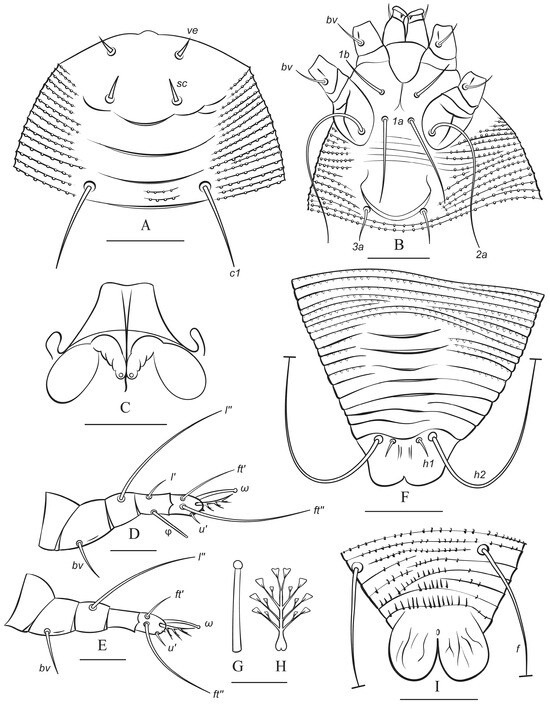
Figure 5.
Drawings of protogyne female (summer form) of Austracus havrylenkonis Keifer 1944. (A)—prodorsal shield and dorsal view of anterior part of opisthosoma; (B)—coxigenital area; (C)—internal genitalia; (D,E)—legs I and II; (F)—dorsal view of rear part of opisthosoma; (G,H)—tarsal solenidion I (G) and tarsal empodium I (H); (I)—ventral view of telosoma. Scale bar: (A,F,I)—30 µm; (B)—25 µm; (C)—1 µm 5; (D,E)—10 µm; (G,H)—5 µm.
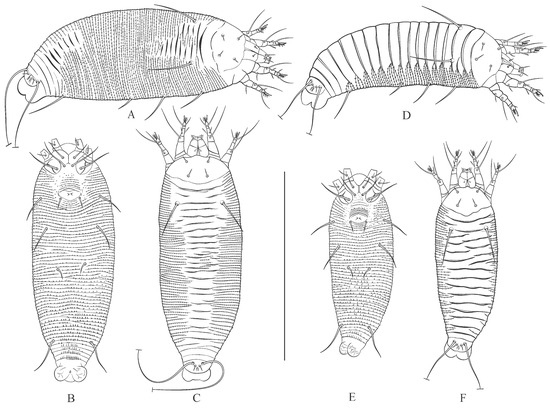
Figure 6.
Drawings of summer (A–C) and winter (D–F) forms of females (A,D) and males (B,C,E,F) of Austracus havrylenkonis Keifer 1944. Scale bar: (A–F)—250 µm.
4. Discussion
Seasonal dimorphism in populations of Eriophyoidea: gradual vs. discrete morphological changes. Seasonal morphological dimorphism in the females of Eriophyoidea was first reported by Putman [33]. Subsequently, this phenomenon was documented by Keifer [34], who coined the term “deuterogeny”, designating summer females as “protogynes” and winter females as “deutogynes”. In this study, we show that Austracus havrylenkonis Keifer 1944, which inhabits fruits of Nothofagus spp., has a complex life cycle with seasonal dimorphism present in both sexes, making it the first bisexually dimorphic species associated with an angiosperm host within the family Phytoptidae s. str. Until now, seasonal dimorphism in both sexes has only been documented in Trisetacus kirghisorum Shevchenko 1962 (Nalepellidae), infesting the seeds of junipers [35]. Males of eriophyoid mites have received limited attention from acarologists for two primary reasons: (a) males are typically far less abundant than females in populations, and (b) male morphology offers limited diagnostic value, as the systematics of Eriophyoidea has historically relied on female morphological traits. While male dimorphism remains poorly understood across most eriophyoid genera, further research into the life cycles of gall mites from diverse taxa is necessary to determine whether bisexual seasonal dimorphism represents a lineage-specific homoplastic adaptation or a symplesiomorphic trait of Eriophyoidea.
There is significant variation in the extent to which seasonal forms differ morphologically across various eriophyoid taxa [36]. In some cases, the differences are nearly imperceptible, while in others, the seasonal forms may be so morphologically distinct that they could be classified as separate species or even genera. Seasonal dimorphism in Austracus is particularly notable, as the summer form exhibits the unstable annulation of the dorsal opisthosoma. There is considerable variation in the number of thin, microtuberculated dorsal annuli interspersed between the broader plate-like annuli (Figure 3B–D,F), which are characteristic of the winter form (Figure 3A). Interestingly, Keifer [37] (p. 2) observed that, in a population of the North American species Aculops rhoicecis (Eriophyidae) from leaf galls of Rhus trilobata Nutt. (Anacardiaceae), some females with “intermediate characteristics” were present alongside morphologically distinct protogynes and deutogynes. A somewhat similar case was reported in Oziella atherodes Chetverikov, 2011 (Phytoptidae s.str.), found inside leaf sheaths of the sedge Carex atherodes Spreng. (Cyperaceae) in Northwestern Russia. Alongside larger protogynes with a higher number of empodial rays and smaller deutogynes with fewer empodial rays, there was a series of differently sized females with intermediate numbers of empodial rays representing a gradual morphological transition from protogynes to deutogynes [38]. In contrast to these cases (corresponding to “atypical deuterogeny” sensu Manson and Oldfield [36]), dimorphism in some species of Eriophyoidea is distinctly non-gradual but discrete (“typical deuterogeny”). For example, in the genus Shevchenkella, protogynes and deutogynes exhibit entirely distinct morphologies with no intermediate forms [34,39]. We hypothesize that these “gradual” and “discrete” seasonal morphological differences reflect different adaptive strategies of gall mites to their environment. Investigating the genetic mechanisms underlying these strategies represents a compelling direction for future research.
Is Austracus havrylenkonis a complex of cryptic species? Cryptic speciation is a widespread evolutionary phenomenon observed across diverse groups of living organisms, including arthropods, amphibians, fish, and plants [40,41]. Among these, mites (Acari) represent a particularly notable example, as their high morphological uniformity and small size often obscure significant genetic divergence, leading to the discovery of numerous cryptic species within traditionally recognized taxa [42,43]. Because of the unique ecological traits and population structures of Eriophyoidea (as discussed by Sabelis and Bruin [44]), cryptic speciation is considered especially common in this group of mites [43]. Among the most striking examples are species complexes within the genera Aceria and Abacarus from herbaceous monocots extensively studied in the first decades of the XXI century [45,46,47,48]. Some grass-inhabiting species in these genera show significant genetic divergence (up to ~20% in Cox1 sequences), yet they are so morphologically similar that they can only be differentiated through comprehensive multivariate statistical analyses [43].
In this study, we encountered an intriguing case where nucleotide sequences of the Cox1 gene from specimens of A. havrylenkonis from different fruits of the same host-plant individual showed significant differences (K2P = 16%; PQ406503, isolate d488 vs MT712721, isolate F234). Remarkably, all these differences represented synonymous substitutions, resulting in no changes to the amino acid sequences. This pattern may reflect the clonal structure of A. havrylenkonis and the isolated evolution of its populations within the fruits, where genetic differences accumulate due to the prolonged absence of gene flow, despite the likely absence of reproductive barriers. On the other hand, biogeographic data on the host plants of Austracus suggest an alternative scenario. The genus Nothofagus is an ancient Gondwanan plant lineage, now distributed in Australasia and South America [49,50]. A series of ten vicariant species of Nothofagus is distributed across a vast area of central and southern Chile, as well as in Argentina [51], the regions characterized by diverse geographical barriers and climatic conditions [52,53,54]. As a result, the formation of host-specific races and complexes of genetically diverging species of gall mites associated with Nothofagus is highly probable. This question could be addressed through a longitudinal screening in Chile, examining different South American species of Nothofagus. Additionally, it would be interesting to search for phytoptid mites in the eastern part of the Southern Hemisphere—specifically, in New Zealand, New Guinea, New Caledonia, and Australia—regions rich in endemic species of Nothofagus. Such a study could help determine whether Austracus or other phytoptid mites are present in these regions, how they have evolved morphologically compared to South American phytoptids associated with Nothofagus, and what genetic differences they have acquired over the long period of isolation.
The problematic diagnosis of the genus Austracus. Keifer [13] described the monotypic genus Austracus based on females showing deutogyne characters, which contradicts current tradition to compose a generic diagnosis based on morphology of protogyne females [24]. Based on our new morphological data on summer females of A. havrylenkonis, the diagnosis of Austracus should incorporate the distinctive opisthosomal features of protogynes—specifically, the presence of two types of dorsal annuli: broad plate-like annuli interspersed with thin, microtuberculated annuli. However, this proposed amendment requires further validation, as undescribed Austracus species may exhibit protogyne opisthosomal morphology that differs from the pattern observed in A. havrylenkonis.
Among other phytoptid taxa, the protogynes of A. havrylenkonis are closest to the species of the group I of the genus Phytoptus Dujardin 1851 (Phytoptidae, Phytoptinae) that retain φ I. Contrary to Phytoptus characterized by opisthosomal annuli that are not differentiated dorso-ventrally, protogynes of A. havrylenkonis have several dorsal plates (wider opisthosomal annuli) on their dorsal opisthosoma along with the typical narrow dorsal opisthosomal annuli.
Deutogynes of A. havrylenkonis are very close to a phytoptid genus Solenocristus Chetverikov et al., 2018 (tribe Sierraphytoptini). They differ in the position of setae ve (displaced before the antero-lateral margin of prodorsal shield in Solenocristus) and the presence of a large subtriangular frontal lobe of prodorsal shield and middorsal opisthosomal ridge (both are absent in A. havrylenkonis). The deutogynes of A. havrylenkonis are also similar to the genera Fragariocoptes Roivainen 1951 and Sierraphytoptus Keifer 1939 (tribe Sierraphytoptini), but these two genera lack tibial solenidion φ I (present in A. havrylenkonis) and have tubercles of setae ve displaced forward and positioned just before the antero-lateral margin of prodorsal shield (ve not displaced in A. havrylenkonis). Finally, deutogynes of A. havrylenkonis also bear a distant resemblance with some vagrant phytoptids associated with palms, e.g., Mackiella Keifer 1939 and Retracrus Keifer 1965 (tribe Mackiellini), but these phytoptids lack opisthosomal setae c1, which are present in A. havrylenkonis.
5. Conclusions
Taxonomic research on Eriophyoidea is advancing rapidly, with numerous new species being described annually. Beyond these taxonomi developments, studies on the life cycles of Eriophyoidea, combined with DNA genotyping, are of critical importance, particularly for evaluating the conspecificity of morphologically dissimilar sympatric mite individuals that correspond to different morphotaxa [39,53]. Our research on A. havrylenkonis revealed that morphologically distinct seasonal forms of this species share identical sequences across three marker genes. We also identified A. havrylenkonis as the second documented case of a bisexually dimorphic taxon within Eriophyoidea, suggesting that seasonal dimorphism in males may be more widespread among gall mites than previously recognized. Additionally, we documented a new instance of atypical seasonal dimorphism in A. havrylenkonis, characterized by an unstable topography of the dorsal opisthosoma, which aligns with the previously described phenomenon of “atypical deuterogeny”. Given the labor-intensive nature of biological studies on Eriophyoidea, we are only beginning to uncover the diverse adaptive strategies these mites employ. A compelling question that warrants further investigation is why different sympatric Eriophyoidea taxa, despite coexisting under similar environmental conditions, exhibit varying degrees of seasonal morphological variation within their populations. This question could be explored in future research through the application of functional genomics, transcriptomics, and proteomics, which may provide deeper insights into the evolutionary and ecological dynamics of this aberrant group of ancient microscopic phytoparasites.
Author Contributions
Conceptualization, P.E.C.; validation, L.E.P.A.; formal analysis and investigation, P.E.C. and L.E.P.A.; resources and data curation, P.E.C. and L.E.P.A.; writing—original draft preparation, P.E.C.; writing—review and editing, P.E.C. and L.E.P.A.; visualization, P.E.C.; funding acquisition, P.E.C. and L.E.P.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Zoological Institute of Russian Academy of Sciences (ZIN RAS project # 125013001089-0).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All new DNA sequences obtained in this study have been deposited in the National Center for Biotechnology Information (NCBI) GenBank database (https://www.ncbi.nlm.nih.gov/genbank) (accessed on 25 March 2025).
Acknowledgments
We are grateful to R. Petanović (Serbia) and late Vladimir Žikić (Serbia) for their help in collecting eriophyoid mites in Argentina, which was performed in the frame of the project #SASA f-195 of the Serbian Academy of Sciences and Art. PCR and sequencing were conducted with the equipment of the “Development of Molecular and Cellular Technologies”, “The Bio-Bank”, and “Microscopy and Microanalysis” Resource Centers of St. Petersburg State University (Russia). We also thank N. Kopylov (St. Petersburg State University, Russia) for his valuable assistance in light microscopy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Walter, D.E.; Lindquist, E.E.; Smith, I.M.; Cook, D.R.; Krantz, G.W. Order Trombidiformes. In A Manual of Acarology; Krantz, G.W., Walter, D.E., Eds.; Texas Tech University Press: Lubbock, TX, USA, 2009; pp. 233–420. [Google Scholar]
- Sukhareva, S.I. Family Phytoptidae Murray 1877 (Acari: Tetrapodili), its consisting, structure and suggested ways of evolution. Acarina 1994, 2, 47–72. [Google Scholar]
- Sidorchuk, E.A.; Schmidt, A.R.; Ragazzi, E.; Roghi, G.; Lindquist, E.E. Plant-feeding mite diversity in Triassic amber (Acari: Tetrapodili). J. Syst. Palaeontol. 2015, 13, 129–151. [Google Scholar] [CrossRef]
- Chetverikov, P.E.; Craemer, C.; Gankevich, V.D.; Zhuk, A.S. Integrative Taxonomy of the Gall Mite Nothopoda todeica n. sp. (Eriophyidae) from the Disjunct Afro-Australasian Fern Todea barbara: Morphology, Phylogeny, and Mitogenomics. Insects 2023, 14, 507. [Google Scholar] [CrossRef] [PubMed]
- Chetverikov, P.E.; Craemer, C.; Cvrković, T.; Klimov, P.B.; Petanović, R.U.; Romanovich, A.E.; Sukhareva, S.I.; Zukoff, S.; Bolton, S.; Amrine, J. Molecular phylogeny of the phytoparasitic mite family Phytoptidae (Acariformes: Eriophyoidea) identified the female genitalic anatomy as a major macroevolutionary factor and revealed multiple origins of gall induction. Exp. Appl. Acarol. 2021, 83, 31–68. [Google Scholar] [CrossRef] [PubMed]
- Keifer, H.H. Eriophyid studies IX. Bull. Calif. Dept. Agric. 1940, 29, 112–117. [Google Scholar]
- Ozman, S.K. Some biological and morphological differences between gall and vagrant forms of Phytoptus avellanae Nal. (Acari: Phytoptidae). Int. J. Acarol. 2000, 26, 215–219. [Google Scholar] [CrossRef]
- Cvrković, T.; Chetverikov, P.; Vidović, B.; Petanović, R. Cryptic speciation within Phytoptus avellanae s.l. (Eriophyoidea: Phytoptidae) revealed by molecular data and observations on molting Tegonotus-like nymphs. Exp. Appl. Acarol. 2016, 68, 83–96. [Google Scholar] [CrossRef]
- Sukhareva, S.I.; Chetverikov, P.E. Morphological transformations in the course of transition from protogyne to deutogene form of female in eriophyoid mites (Acari: Eriophyoidea). Vestn. St.-Peterbg. Univ. 2013, 3, 3–15. (In Russian) [Google Scholar]
- Keifer, H.H. The Eriophyoidea Nalepa. In Mites Injurious to Economic Plants; Jeppson, L.R., Keifer, H.H., Baker, E.W., Eds.; University of California Press: Berkeley, CA, USA, 1975; pp. 327–587. [Google Scholar]
- Ozman, S.K.; Toros, S. Life cycles of Phytoptus avellanae Nal. and Cecidophyopsis vermiformis Nal. (Acarina: Eriophyoidea). Acta Hortic. 1997, 445, 493–501. [Google Scholar] [CrossRef]
- Ozman, S.K.; Toros, S. Damage caused by Phytoptus avellanae Nal. and Cecidophyopsis vermiformis Nal. (Eriophyoidea: Acarina) in hazelnut. Acta Hortic. 1997, 445, 537–543. [Google Scholar] [CrossRef]
- Keifer, H.H. Eriophyid studies XIV. Bull. Calif. Dept. Agric. 1944, 28, 18–38. [Google Scholar]
- Chetverikov, P.E.; Craemer, C. Sierraphytoptines (Eriophyoidea: Phytoptidae) from relict eudicots: Reassignment of Sierraphytoptus taiwanensus to a new genus Solenoplatilobus and refinement of generic diagnosis of Austracus. Syst. Appl. Acarol. 2016, 21, 745–758. [Google Scholar] [CrossRef]
- Peralta, L. Informe Sanitario del Servicio Agrícola y Ganadero Ministerio de Agricultura Chile; Report #90601-8; Servicio Agricola y Ganadero: Santiago, Chile, 2019; pp. 1–2. [Google Scholar]
- Peralta, L. Informe Sanitario del Servicio Agrícola y Ganadero Ministerio de Agricultura Chile; Report #79962-8; Servicio Agricola y Ganadero: Santiago, Chile, 2018; pp. 1–2. [Google Scholar]
- Peralta, L. Informe Sanitario del Servicio Agrícola y Ganadero Ministerio de Agricultura Chile; Report #16659-8; Servicio Agricola y Ganadero: Santiago, Chile, 2020; pp. 1–2. [Google Scholar]
- Peralta, L. Informe Sanitario del Servicio Agrícola y Ganadero Ministerio de Agricultura Chile; Report #52694-8; Servicio Agricola y Ganadero: Santiago, Chile, 2020; pp. 1–2. [Google Scholar]
- Peralta, L. Informe Sanitario del Servicio Agrícola y Ganadero Ministerio de Agricultura Chile; Report #54517-8; Servicio Agricola y Ganadero: Santiago, Chile, 2016; pp. 1–2. [Google Scholar]
- Peralta, L. Informe Sanitario del Servicio Agrícola y Ganadero Ministerio de Agricultura Chile; Report #63839-8; Servicio Agricola y Ganadero: Santiago, Chile, 2022; pp. 1–2. [Google Scholar]
- Peralta, L. Informe Sanitario del Servicio Agrícola y Ganadero Ministerio de Agricultura Chile; Report #37512-8; Servicio Agricola y Ganadero: Santiago, Chile, 2016; pp. 1–2. [Google Scholar]
- Amrine, J.W., Jr.; Manson, D.C.M. Preparation, mounting and descriptive study of eriophyoid mites. In Eriophyoid Mites: Their Biology, Natural Enemies and Control; Lindquist, E.E., Sabelis, M.W., Bruin, J., Eds.; World Crop Pests; Elsevier Science Publishing: Amsterdam, The Netherlands, 1996; Volume 6, pp. 383–396. [Google Scholar] [CrossRef]
- Lindquist, E.E. External anatomy and notation of structures. In Eriophyoid Mites: Their Biology, Natural Enemies and Control; Lindquist, E.E., Sabelis, M.W., Bruin, J., Eds.; World Crop Pests; Elsevier Science Publishing: Amsterdam, The Netherlands, 1996; Volume 6, pp. 3–31. [Google Scholar] [CrossRef]
- Amrine, J.W., Jr.; Stasny, T.A.H.; Flechtmann, C.H.W. Revised Keys to the World Genera of the Eriophyoidea (Acari: Prostigmata); Indira Publishing House: West Bloomfield, MI, USA, 2003; pp. 1–244. [Google Scholar]
- Chetverikov, P.E. Video projector: A digital replacement for camera lucida for drawing mites and other microscopic objects. Syst. Appl. Acarol. 2016, 21, 1278–1280. [Google Scholar] [CrossRef]
- Chetverikov, P.E.; Bertone, M. First rhyncaphytoptine mite (Eriophyoidea, Diptilomiopidae) parasitizing american hazelnut (Corylus americana): Molecular identification, confocal microscopy, and phylogenetic position. Exp. Appl. Acarol. 2022, 88, 75–95. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transformation. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New Models and Efficient Methods for Phylogenetic Inference in the Genomic Era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Navia, D.; Flechtmann, C.H.; Amrine, J.W., Jr. Supposed ovoviviparity and viviparity in the coconut mite, Aceria guerreronis Keifer (Prostigmata: Eriophyidae), as a result of female senility. Int. J. Acarol. 2005, 31, 63–65. [Google Scholar] [CrossRef]
- Putman, W.L. The plum nursery mite (Phyllocoptes fockeui Nal. & Trt.). In The 70th Annual Report of the Entomological Society of Ontario; Warwick & Sons Publishing: Toronto, ON, Canada, 1939; pp. 33–40. [Google Scholar]
- Keifer, H.H. Eriophyid Studies XII. Bull. Calif. Dept. Agric. 1942, 31, 117–129. [Google Scholar]
- De-Millo, A.P. O dimorfizme samtsov u chetyrekhnogikh kleshchei (Acarina, Eriophyidae). Vestn. Leningr. Univ. 1967, 3, 26–33. (In Russian) [Google Scholar]
- Manson, D.C.M.; Oldfield, G.N. Life forms, deuterogyny, diapause and seasonal development. In Eriophyoid Mites: Their Biology, Natural Enemies and Control; Lindquist, E.E., Sabelis, M.W., Bruin, J., Eds.; World Crop Pests; Elsevier Science Publishing: Amsterdam, The Netherlands, 1996; Volume 6, pp. 173–183. [Google Scholar] [CrossRef]
- Keifer, H.H. Eriophyid Studies B-7; Bureau of Entomology of the California Department of Agriculture: Sacramento, CA, USA, 1962; pp. 1–20. [Google Scholar]
- Chetverikov, P.E. Phytoptus atherodes n. sp. (Acari: Eriophyoidea: Phytoptidae) and a supplementary description of Phytoptus hirtae Roivainen 1950 from sedges (Cyperaceae). Zootaxa 2011, 3045, 26–44. [Google Scholar] [CrossRef]
- Chetverikov, P.E.; Desnitskiy, A.G.; Klimov, P.B.; Ozman-Sullivan, S.K.; Romanovich, A.E.; Sukhareva, S.I. Deuterogyny and the association of two vagrant eriophyoid mites (Acariformes, Eriophyoidea) with the host-plant generative organs of two broad-leaved trees in North-West Russia. Zool. Stud. 2023, 62, e35. [Google Scholar] [CrossRef] [PubMed]
- Bickford, D.; Lohman, D.J.; Sodhi, N.S.; Ng, P.K.; Meier, R.; Winker, K.; Ingram, K.K.; Das, I. Cryptic species as a window on diversity and conservation. Trends Ecol. Evol. 2007, 22, 148–155. [Google Scholar] [CrossRef]
- Fišer, C.; Robinson, C.T.; Malard, F. Cryptic species as a window into the paradigm shift of the species concept. Mol. Ecol. 2018, 27, 613–635. [Google Scholar] [CrossRef]
- Dabert, M.; Witalinski, W.; Kazmierski, A.; Olszanowski, Z.; Dabert, J. Molecular phylogeny of acariform mites (Acari, Arachnida): Strong conflict between phylogenetic signal and long-branch attraction artifacts. Mol. Phylogenet. Evol. 2010, 56, 222–241. [Google Scholar] [CrossRef]
- Skoracka, A.; Magalhães, S.; Rector, B.G.; Kuczyński, L. Cryptic speciation in the Acari: A function of species lifestyles or our ability to separate species? Exp. Appl. Acarol. 2015, 67, 165–182. [Google Scholar] [CrossRef]
- Sabelis, M.W.; Bruin, J. Evolutionary ecology: Life history patterns, food plant choice and dispersal. In Eriophyoid Mites: Their Biology, Natural Enemies and Control; Lindquist, E.E., Sabelis, M.W., Bruin, J., Eds.; World Crop Pests; Elsevier Science Publishing: Amsterdam, The Netherlands, 1996; Volume 6, pp. 329–366. [Google Scholar] [CrossRef]
- Skoracka, A.; Kuczyński, L.; de Mendonça, R.S.; Dabert, M.; Szydło, W.; Knihinicki, D.; Truol, G.; Navia, D. Cryptic species within the wheat curl mite Aceria tosichella (Keifer) (Acari: Eriophyoidea), revealed by mitochondrial, nuclear and morphometric data. Invertebr. Syst. 2012, 26, 417–433. [Google Scholar] [CrossRef]
- Skoracka, A.; Kuczyński, L.; Rector, B.; Amrine, J.W., Jr. Wheat curl mite and dry bulb mite: Untangling a taxonomic conundrum through a multidisciplinary approach. Biol. J. Linn. Soc. 2014, 111, 421–436. [Google Scholar] [CrossRef]
- Miller, A.D.; Umina, P.A.; Weeks, A.R.; Hoffmann, A.A. Population genetics of the wheat curl mite (Aceria tosichella Keifer) in Australia: Implications for the management of wheat pathogens. Bull. Entomol. Res. 2012, 102, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Laska, A.; Majer, A.; Szydło, W.; Karpicka-Ignatowska, K.; Hornyák, M.; Labrzycka, A.; Skoracka, A. Cryptic diversity within grass-associated Abacarus species complex (Acariformes: Eriophyidae), with the description of a new species, Abacarus plumiger n. sp. Exp. Appl. Acarol. 2018, 76, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Knapp, M.; Stöckler, K.; Havell, D.; Delsuc, F.; Sebastiani, F.; Lockhart, P.J. Relaxed molecular clock provides evidence for long-distance dispersal of Nothofagus (southern beech). PLoS Biol. 2005, 3, e14. [Google Scholar] [CrossRef]
- Cook, L.G.; Crisp, M.D. Not so ancient: The extant crown group of Nothofagus represents a post-Gondwanan radiation. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2005, 272, 2535–2544. [Google Scholar] [CrossRef]
- Amigo, J.; Rodríguez-Guitián, M.A. Bioclimatic and phytosociological diagnosis of the species of the Nothofagus genus (Nothofagaceae) in South America. Int. J. Geobot. Res. 2011, 1, 1–20. [Google Scholar] [CrossRef]
- Marchelli, P.; Gallo, L.A. The combined role of glaciation and hybridization in shaping the distribution of genetic variation in a Patagonian southern beech. J. Biogeogr. 2004, 31, 451–460. [Google Scholar] [CrossRef]
- Guo, J.F.; Li, H.S.; Wang, B.; Xue, X.F.; Hong, X.Y. DNA barcoding reveals the protogyne and deutogyne of Tegolophus celtis sp. nov. (Acari: Eriophyidae). Exp. Appl. Acarol. 2015, 67, 393–410. [Google Scholar] [CrossRef]
- Mattera, M.G.; Pastorino, M.J.; Lantschner, M.V.; Marchelli, P.; Soliani, C. Genetic diversity and population structure in Nothofagus pumilio, a foundation species of Patagonian forests: Defining priority conservation areas and management. Sci. Rep. 2020, 10, 19231. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).