Ethogram Characteristics of Silver Carp (Hypophthalmichthys molitrix) During the Breeding Period Based on the PAE Coding System
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Experimental Fish
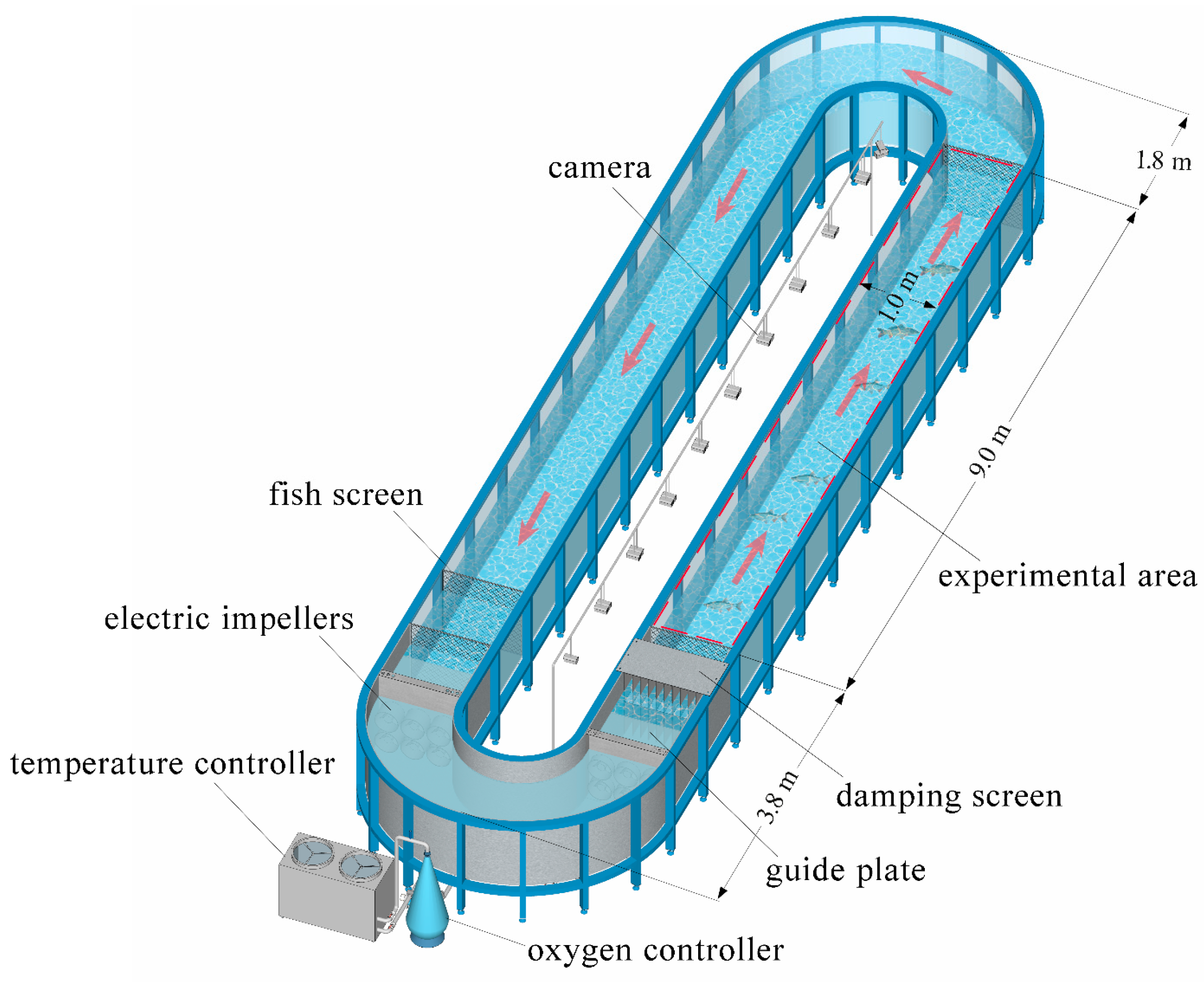
2.2. Laboratory Apparatus
2.3. Experimental Methods
2.3.1. Behavior Observation Experiment
2.3.2. Definition and Coding of Behavior
2.3.3. Classification and Temporal Analysis of Reproductive Behaviors
- (1)
- Accompanying behavior: Males and females swim together slowly underwater, either upstream or downstream (Figure 2a).
- (2)
- Guiding behavior: Within a moving school, a single male frequently assumes the leading position at the forefront (Figure 2b).
- (3)
- Chasing behavior: Mature males accelerate toward nearby females, occasionally making physical contact with the female’s body (Figure 2c).
- (4)
- Encircling behavior: Males and females engage in head-to-head contact, bending their bodies and circling in place by swaying their tails (Figure 2d).
- (5)
- (6)
- Mating behavior: The male presses against the female’s back, sometimes causing her to lie on her side in the water. Both fish contract and sway their bodies rhythmically, culminating in spawning (Videos S2 and S3) and ejaculation (Figure 2f).
2.3.4. Analysis of Reproductive Behavior Diversity
2.4. Data Processing
3. Results
3.1. Posture Coding
3.2. Action Coding
3.3. Environmental Coding
3.4. PAE Coding System and Reproductive Ethogram
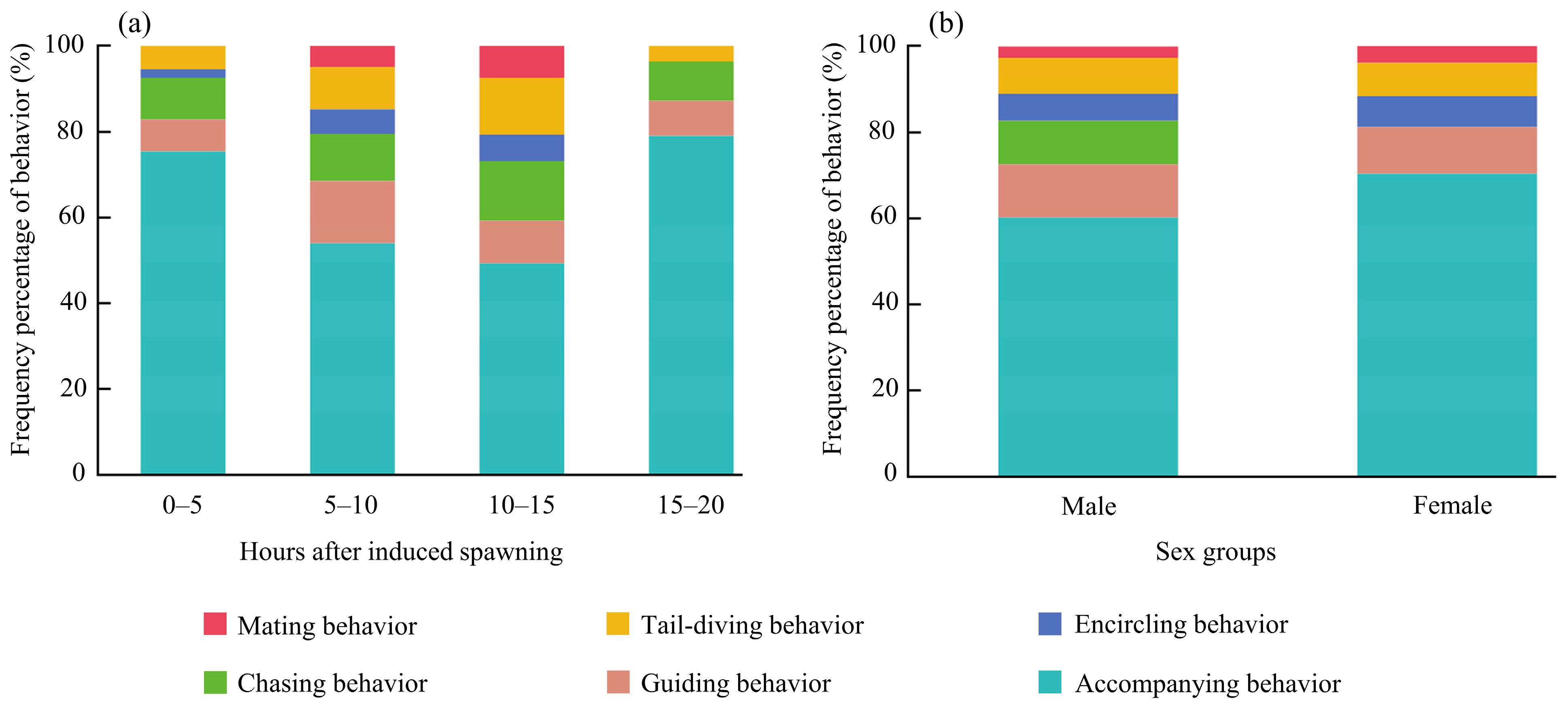
3.5. Reproductive Behavior Diversity of Silver Carp
3.5.1. Differences Among Groups with Varying Durations Following Induced Spawning
3.5.2. Gender Differences
4. Discussion
4.1. The Ethogram of Silver Carp During the Breeding Period
4.2. The Reproductive Behavior Characteristics of Silver Carp
4.3. The Significance of the PAE Coding System in the Study of Animal Behavior
4.4. Management Implications
- (1)
- Optimization of artificial breeding techniques. Priority should be given to selecting broodstock exhibiting high behavioral synchrony (e.g., complete courtship rituals) in order to enhance artificial reproduction quality. Intermittent water flow (alternating between 0.5 and 1.5 m/s) should be introduced into the aquaculture system to restore natural reproductive behavioral competence in captive populations. Additionally, wild individuals should be periodically integrated into the breeding stock to mitigate the potential degradation of reproductive behaviors that may result from artificial selection.
- (2)
- Strategies for the conservation of wild populations. In conjunction with the ecological scheduling of the Three Gorges Reservoir, the natural hydrological flood peak process should be simulated from April to June to stimulate the natural reproductive behavior of fish. Efforts should be intensified to restore the spawning grounds of the FMCCs and protect their habitats. Furthermore, the synchronization rate of sperm and egg release should be incorporated into the health assessment framework for Yangtze River spawning grounds as a quantitative indicator of reproductive success.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hong, X.Y.; Chen, H.T.; Wei, C.Q.; Chen, C.; Wang, Y.K.; Xie, M.M.; Li, W.; Zhu, X.P. Behavior coding and ethogram of juvenile domesticated asian giant softshell turtles, Pelochelys cantorii. Russ. J. Herpetol. 2023, 30, 184–190. [Google Scholar] [CrossRef]
- Banchetti, R.; Erra, F.; Ricci, N.; Dini, F. Ethogram of Aspidisca sedigita. Can. J. Zool. 2003, 81, 14–20. [Google Scholar] [CrossRef]
- Bohatová, M.; Vdacny, P. Locomotory behaviour of two phylogenetically distant predatory ciliates: Does evolutionary history matter? Ethol. Ecol. Evol. 2018, 30, 195–219. [Google Scholar] [CrossRef]
- McDonnell, S.M.; Poulin, A. Equid play ethogram. Appl. Anim. Behav. Sci. 2002, 78, 263–290. [Google Scholar] [CrossRef]
- Lehner, P.N. Handbook of Ethological Methods; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Luo, M.L.; Hu, L.J.; Shi, H.T.; Lin, L. Ethogram and PAE coding system of captive Beale’s eyed turtle (Sacalia bealei). Sichuan J. Zool. 2023, 42, 294–302. [Google Scholar]
- Hanlon, R.T.; Maxwell, M.R.; Shashar, N.; Loew, E.R.; Boyle, K.L. An ethogram of body patterning behavior in the biomedically and commercially valuable squid Loligo pealei off Cape Cod, Massachusetts. Biol. Bull. 1999, 197, 49–62. [Google Scholar] [CrossRef]
- Barbanera, F.; Erra, F.; Ricci, N. The effect of heating on the behaviour of Oxytricha bifaria (Ciliophora, Hypotrichida). Can. J. Zool. 2000, 78, 484–494. [Google Scholar] [CrossRef]
- Jiang, Z.G. Behavior coding and ethogram of the Père David’s deer. Acta Theriol. Sin. 2000, 20, 1–12. [Google Scholar]
- Qi, Y.; Li, S.; Suo, L.; Li, H.; Wang, Y. An ethogram of the toad-headed lizard Phrynocephalus vlangalii during the breeding season. Asian Herpetol. Res. 2011, 2, 110–116. [Google Scholar] [CrossRef]
- Wang, C.B.; Huang, Y.; Dong, X.; Li, J.G.; Zhou, C.Q. Ethogram and PAE coding system of Rostratula benghalensis in breeding period. Sichuan J. Zool. 2017, 36, 412–419. [Google Scholar]
- Zhang, J.H.; Zhang, M.H.; Wu, D.F.; Qiu, J. Behavior ethogram and PAE coding system of semi-free giant panda during breeding season. Chin. J. Wildl. 2020, 41, 296–302. [Google Scholar]
- Gao, R.Q.; Gao, H.Y.; Sun, S.; Wang, J.X.; Yang, J.Z.; Gu, J.Y.; Hua, Y. Construction of ethogram and PAE coding system of wild Chinese pangolin. Chin. J. Wildl. 2024, 45, 709–716. [Google Scholar]
- Chen, R.; Wei, Y.L.; Wu, L.; Zheng, B.Y.; Li, J.H. PAE coding system-based ethogram of Yangtze finless porpoise (Neophocaena phocaenoides asiaeorientalis) in a semi-natural environment. Acta Theriol. Sin. 2015, 35, 40–47. [Google Scholar]
- Zhu, T.B.; Yan, W.B.; Yang, D.G. PAE coding system-based ethogram of Schicothorax wangchiachit. J. Fish. Sci. China 2018, 25, 294–300. [Google Scholar]
- Xiang, M.; Li, L.; Xu, H.L.; Li, B.; Guo, H.X.; Yang, Z.H.; Zhu, C.H.; Wang, M.; Wang, J.; Xin, W.; et al. Reproductive Behavior in Odontobutis potamophila (Günther, 1861). Aquac. Res. 2025, 1, 8860515. [Google Scholar] [CrossRef]
- Li, M.; Chen, M.; Wu, W.; Li, J.; An, R. Differences in the natural swimming behavior of Schizothorax prenanti individual and schooling in spatially heterogeneous turbulent flows. Animals 2023, 13, 1025. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhang, J.; Zhang, X.; Li, H.; Liu, Y.; Gao, L. Effects of temperature and photoperiod on growth, physiological, and behavioral performance in steelhead trout (Oncorhynchus mykiss) under indoor aquaculture condition. Front. Mar. Sci. 2023, 10, 1114662. [Google Scholar] [CrossRef]
- Xia, Y.; Li, X.; Yang, J.; Zhu, S.; Wu, Z.; Li, J.; Li, Y. Elevated Temperatures Shorten the Spawning Period of Silver Carp (Hypophthalmichthys molitrix) in a Large Subtropical River in China. Front. Mar. Sci. 2021, 8, 708109. [Google Scholar] [CrossRef]
- Fang, D.; Sun, H.; Peng, Y.; Kuang, Z.; Zhou, Y.; Xu, D. Living status and perspective of the silver carp (Hypophthalmichthys molitrix) in the lower reach of the Yangtze River: Insights from population distribution, age structure, and habitat preference analyses. Fishes 2022, 7, 254. [Google Scholar] [CrossRef]
- Guo, W.X.; Jin, Y.G.; Zhao, R.C.; Wang, H.X. The impact of the ecohydrologic conditions of Three Gorges Reservoir on the spawning activity of four major Chinese carps in the middle of Yangtze River, China. Appl. Ecol. Environ. Res. 2021, 19, 4313–4330. [Google Scholar] [CrossRef]
- George, A.E.; Chapman, D.C. Aspects of embryonic and larval development in bighead carp Hypophthalmichthys nobilis and silver carp Hypophthalmichthys molitrix. PLoS ONE 2013, 8, e73829. [Google Scholar] [CrossRef] [PubMed]
- Harms, J.D.; Jimerson, K.R.; Schmidt, J.M.; Lucchesi, D.O.; Schall, B.J.; Coulter, A.A. Progression along the invasion curve: Silver carp growth slows temporally in two Missouri River tributaries. Aquat. Invasions 2024, 19, 109–120. [Google Scholar] [CrossRef]
- Huang, Y.F.; Liu, Y.; Yang, Y.Q.; Yu, C.; Hu, M.H.; Li, J. Comparison of age and growth of grass and silver carp above and below Changzhou Dam in the Pearl River, China. J. Freshwater Ecol. 2023, 38, 2251517. [Google Scholar] [CrossRef]
- Xia, Y.G.; Liu, Q.F.; Zhu, S.L.; Li, Y.F.; Li, X.H.; Li, J. Do changes in prey community in the environment affect the feeding selectivity of silver carp (Hypophthalmichthys molitrix) in the Pearl River, China? Sustainability 2022, 14, 11175. [Google Scholar] [CrossRef]
- Killgore, K.J.; Hoover, J.J.; Slack, W.T.; Kirk, J.P.; Lewis, B.R.; George, S.G.; Miranda, L.E. Population characteristics of silver carp from the source of their North American introduction in the Lower Mississippi River. Aquat. Invasions 2024, 19, 329–343. [Google Scholar] [CrossRef]
- Yangtze River Fisheries Research Institute. Artificial Propagation Techniques of Domestic Fish; Agricultural Press: Beijing, China, 1973.
- Lehner, P.N. Sampling methods in behavior research. Poult. Sci. 1992, 71, 643–649. [Google Scholar] [CrossRef]
- He, X.L.; Zhao, Q.C.; Feng, Y.T.; Li, Y.F.; Huang, Z.H.; Li, Y.B. PAE coding system-based ethogram of captive François’ langurs in Guangxi, China. Chin. J. Wildl. 2023, 44, 727–743. [Google Scholar]
- Liu, L.; Xiao, A.G.; Zhao, T.J.; Feng, X.M.; Shen, S.X.; Lu, X.W.; Guan, H.W.; Zhao, D.P. Behavioral ethogram and posture-act-environment coding system of wild leopard cats (Prionailurus bengalensis) based on infrared camera technology. Acta Theriol. Sin. 2023, 43, 270–279. [Google Scholar]
- Song, F.; Zhou, Y.Y.; Huang, T.F.; Yang, C.C.; Yu, G.Q.; Tian, S.R.; Xiang, Z.F. PAE coding and diversity analysis of Moschus berezovskii behavior based on infrared camera technology. Biodivers. Sci. 2024, 32, 85–95. [Google Scholar] [CrossRef]
- Stolba, A.; Baker, N.; Wood-Gush, D.G.M. The characterisation of stereotyped behaviour in stalled sows by informational redundancy. Behaviour 1983, 87, 157–182. [Google Scholar] [CrossRef]
- Cui, D.Y.; Niu, K.F.; Luen, T.C.; Yang, M.Y.; Zhang, Y.Y.; Zhang, J.G.; Yang, Y.Q. Behavior coding and ethogram of Guizhou snub-nosed monkey (Rhinopithecus brelichi). Sichuan J. Zool. 2014, 33, 815–828. [Google Scholar]
- Garwood, R.J.; Edgecombe, G.D. Early terrestrial animals, evolution, and uncertainty. Evol. Educ. Outreach 2011, 4, 489–501. [Google Scholar] [CrossRef]
- Volff, J.N. Genome evolution and biodiversity in teleost fish. Heredity 2005, 94, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Diogo, R.; Abdala, V.; Lonergan, N.; Wood, B.A. From fish to modern humans–comparative anatomy, homologies and evolution of the head and neck musculature. J. Anat. 2008, 213, 391–424. [Google Scholar] [CrossRef]
- McKinnon, A.J.; Edwards, S.A.; Stephens, D.B.; Walters, D.E. Behaviour of groups of weaner pigs in three different housing systems. Br. Vet. J. 1989, 145, 367–372. [Google Scholar] [CrossRef]
- Chen, Q.W.; Zhang, J.Y.; Chen, Y.C.; Mo, K.L.; Wang, J.; Tang, L.; Lin, Y.Q.; Chen, L.; Gao, Y.; Jiang, W.; et al. Inducing flow velocities to manage fish reproduction in regulated rivers. Engineering 2021, 7, 178–186. [Google Scholar] [CrossRef]
- Brzuska, E. Artificial spawning of herbivorous fish: Use of an LHRH-a to induce ovulation in grass carp Ctenopharyngodon idella (Valenciennes) and silver carp Hypophthalmichthys molitrix (Valenciennes). Aquac. Res. 1999, 30, 849–856. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Fostier, A.; Zanuy, S. Broodstock management and hormonal manipulations of fish reproduction. Gen. Comp. Endocrinol. 2010, 165, 516–534. [Google Scholar] [CrossRef]
- Mikołajczyk, T.; Chyb, J.; Szczerbik, P.; Sokołowska-Mikołajczyk, M.; Epler, P.; Enright, W.J.; Filipiak, M.; Breton, B. Evaluation of the potency of azagly-nafarelin (GnRH analogue), administered in combination with different formulations of pimozyde, on LH secretion, ovulation and egg quality in common carp (Cyprinus carpio L.) under laboratory, commercial hatchery and natural conditions. Aquaculture 2004, 234, 447–460. [Google Scholar]
- Hoekzema, H.; Baskir, E.; Kozlowski, C.; Elden, M.; Powell, D.M. Breeding season behaviors of captive tawny frogmouth (Podargus strigoides) pairs. Zoo Biol. 2023, 42, 616–624. [Google Scholar] [CrossRef]
- Luo, Q.H.; Tong, F.; Song, Y.J.; Wang, H.; Du, M.L.; Ji, H.B. Observation of the breeding behavior of the Chinese giant salamander (Andrias davidianus) using a digital monitoring system. Animals 2018, 8, 161. [Google Scholar] [CrossRef]
- Wemelsfelder, F.; Haskell, M.; Mendl, M.T.; Calver, S.; Lawrence, A.B. Diversity of behavior during novel object tests is reduced in pigs housed in substrate-impoverished conditions. Anim. Behav. 2000, 60, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Sih, A.; Bell, A.; Johnson, J.C. Behavioral syndromes: An ecological and evolutionary overview. Trends Ecol. Evol. 2004, 19, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Mashiko, K. Defensive capability of egg-guarding males of the goby, tridentiger kuroiwae brevispinis. Ichthyol. Res. 1998, 45, 135–139. [Google Scholar] [CrossRef]
- Wootton, R.J.; Smith, C. Reproductive Biology of Teleost Fishes; Wiley Blackwell: Chichester, UK, 2014; pp. 202–304. [Google Scholar]
- Du, H.; Ban, X.; Li, P.C.; Shih, W.; Diplas, P.; Wu, J.M.; Li, J.Y.; Cheng, P.L.; Li, P.S.; Liu, W.C.; et al. The crucial role of ecohydraulic factors in triggering sturgeon reproduction: Implications for active habitat restoration strategies in the Yangtze River. J. Appl. Ecol. 2025, 62, 1052–1062. [Google Scholar] [CrossRef]
- Moore, M.J.; Paukert, C.P.; Owens, S.P.; Moore, T.L. Habitat selection in a southern Lake sturgeon population: Implications of temporal, spatial, and ontogenetic variation for restoration. Restor. Ecol. 2021, 30, e13602. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.J.; Gessner, J.; Congiu, L.; Haxton, T.J.; Jeppesen, E.; Svenning, J.C.; Xie, P. To save sturgeons, we need river channels around hydropower dams. Proc. Natl. Acad. Sci. USA 2023, 120, e2217386120. [Google Scholar] [CrossRef]
- Skjæraasen, J.E.; Meager, J.J.; Karlsen, Ø. The expression of secondary sexual characteristics in recruit- and repeat-spawning farmed and wild Atlantic cod (Gadus morhua). Ices J. Mar. Sci. 2008, 65, 1710–1716. [Google Scholar] [CrossRef]
- Weir, L.K.; Hutchings, J.A.; Fleming, I.A.; Einum, S. Spawning behaviour and success of mature male Atlantic salmon (Salmo salar) parr of farmed and wild origin. Can. J. Fish. Aquat. Sci. 2005, 62, 1153–1160. [Google Scholar] [CrossRef]
- Brattli, M.B.; Egeland, T.B.; Nordeide, J.T.; Folstad, I. Spawning behavior of Arctic charr (Salvelinus alpinus): Spawning synchrony, vibrational communication, and mate guarding. Ecol. Evol. 2018, 8, 8076–8087. [Google Scholar] [CrossRef]
- Seki, K.; Tomiyasu, M.; Kuroda, M.; Ichimura, M.; Sato, N.; Zhu, Y.; Minami, K.; Miyashita, K. Temporal changes in behavior during the group spawning event of Pacific herring (Clupea pallasii). Sci. Rep. 2025, 15, 11337. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.H.; Yan, Q.M.; Li, W.D.; Yang, X.Y.; Zhang, S.H. Impact of dam construction on the spawning grounds of the four major Chinese carps in the Three Gorges Reservoir. J. Hydrol. 2022, 609, 127694. [Google Scholar] [CrossRef]
- Lv, Z.H.; Wang, G.S.; Zhang, P.; Ai, X.S.; Cao, X.; Zheng, W.; Mu, Z.Y.; Yu, B.W. Optimizing flow regime for the Four Major Chinese Carps by integrating habitat suitability within reservoir operation. J. Hydrol. 2023, 626, 130226. [Google Scholar] [CrossRef]
- Xiao, Y.; Deng, J.H.; Yang, S.F.; Hu, J.; Wang, L.; Li, W.J. Study on the spawning habitat suitability of four major Chinese carps in the fluctuating backwater area of the Three Gorges Reservoir. Ecol. Indic. 2022, 143, 109314. [Google Scholar] [CrossRef]
- Hou, J.; Yan, L.L.; Li, L.; Li, Y.J.; Liao, Y.S.; Zhang, J.D. Behavior coding and ethogram of the free-ranging giant pandas (Ailuropoda melanoleuca). Acta Theriol. Sin. 2020, 40, 446–457. [Google Scholar]
- Ning, Z.Y.; Dong, G.X.; Tang, H.; Lan, D.Y.; Hu, H.J. Behavioral diversity of semi-captive hamadryas baboons (Papio hamadryas) on the basis of the PAE coding system. Biodivers. Sci. 2017, 25, 1008–1018. [Google Scholar] [CrossRef]
- Meng, D.L.; Gao, W.Z.; Shi, L. Ethogram and PAE coding system of Teratoscincus roborowskii under mirror environment. Sichuan J. Zool. 2023, 42, 659–667. [Google Scholar]
- Liu, P.Z.; Chen, J.Z.; Fan, R.; He, Y.; Zhang, Y.; Lu, K.; Zhang, X.M.; Zeng, Q.; Lei, G.C. Ethogram and PAE (Posture-Act-Environment) coding system of Scaly-Sided Merganser during winter. Chin. J. Wildl. 2023, 44, 106–117. [Google Scholar]
- Luo, S.; Wang, P.; Zhang, Y.; Wang, Z.; Tian, H.; Luo, Q. Ethogram of the Chinese Giant Salamander during the Breeding Period Based on the PAE Coding System. Animals 2023, 13, 3632. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, K.H.; Li, H.L.; Xu, X.L.; Duan, W.B.; Chen, H.; Qiu, G.Q.; Chen, W.H.; Lu, J.; Ding, C.Q. Ethogram and PAE coding system of Crested Ibis in non-breeding season. Biodivers. Sci. 2024, 32, 17–29. [Google Scholar] [CrossRef]
- Qiu, J.S.; Sun, X.D.; Wang, D.; Hao, Y.J.; Zheng, J.S.; Li, W.L.; Fan, F.; Deng, X.J.; Mao, J.F.; Zeng, Q.; et al. The first case of reintroduction and behavioral adaptability of Yangtze Finless porroise. Acta Hydrob. Sin. 2023, 47, 1709–1718. [Google Scholar]
- Liu, H.; Lü, X.; Wang, X.; Kou, W.; Miu, G.; Yuan, H. Behavioral ethogram and posture-act-environment coding system of Capricornis sumatraensis. Biodivers. Sci. 2021, 29, 1650–1657. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Zhao, T.J.; Feng, X.M.; Sun, G.M.; Wang, Y.S.; Shen, S.X.; Lu, X.W.; Guan, H.W.; Zhang, W.B.; Zhao, D.P. Behavioral ethogram and PAE coding system of wild koklass pheasants based on infrared camera technology. Chin. J. Wildl. 2023, 44, 347–357. [Google Scholar]
- Sih, A.; Bell, A.M.; Johnson, J.C.; Ziemba, R.E. Behavioral syndromes: An intergrative overiew. Q. Rev. Biol. 2004, 79, 241–277. [Google Scholar] [CrossRef]

| Posture | Definition | Code |
|---|---|---|
| Swimming | The silver carp swims freely in the water by swinging its pectoral and caudal fins. | 1 |
| Rushing | The caudal fin of the silver carp rapidly undulates, enabling it to swim swiftly through the water. | 2 |
| Gliding | The silver carp maintains a fixed posture as it slows down and glides through the water. | 3 |
| Suspension | The body remains suspended in the water, with the pectoral fin moving slightly or remaining stationary. | 4 |
| Floating | The body floats at the water surface, with the pectoral fin moving slightly or remaining stationary. | 5 |
| Adhering | The silver carp adheres to the bottom of the water tank, with its body remaining motionless. | 6 |
| Jumping | The tail fin suddenly exerts force, propelling the body upwards. | 7 |
| Ovipositing | The female fish flips its body, and as its pectoral and pelvic fins quiver, it begins to spawn. | 8 |
| Turning | The trunk of the silver carp moves laterally along the dorsoventral axis. | 9 |
| Rotating | The trunk of the silver carp rotates around the longitudinal axis. | 10 |
| Inverting | The trunk of the silver carp is inverted along the dorsoventral axis, with the abdomen on top and the back on the bottom. | 11 |
| Sinking | The silver carp remains motionless and falls freely towards the bottom of the water tank. | 12 |
| Body Position | Action | Code |
|---|---|---|
| Head | Butting | 1 |
| Spitting | 2 | |
| Gaping | 3 | |
| Swallowing | 4 | |
| Breathing | 5 | |
| Heading up | 6 | |
| Heading down | 7 | |
| Shaking | 8 | |
| Trunk | Extending | 9 |
| Bending | 10 | |
| Swinging | 11 | |
| Rubbing | 12 | |
| Turning left | 13 | |
| Turning right | 14 | |
| Pectoral fin | Stretching | 15 |
| Swinging | 16 | |
| Retracting | 17 | |
| Caudal fin | Extending | 18 |
| Continuous swinging | 19 | |
| Intermittent swinging | 20 |
| Classification | Environment | Biotic (E1) | Abiotic (E2) | Codes |
|---|---|---|---|---|
| Activity location | Water surface | + | 1 | |
| Upper flume layer | + | 2 | ||
| Lower flume layer | + | 3 | ||
| Light conditions | Daytime | + | 4 | |
| Nighttime | + | 5 | ||
| Illuminated nighttime | + | 6 | ||
| Sex | Male | + | 7 | |
| Female | + | 8 | ||
| Shoaling pattern | Solitary | + | 9 | |
| Group | + | 10 |
| Behavior | Behavioral Performance Quantity | Male | Female | Number | PAE Code | ||
|---|---|---|---|---|---|---|---|
| P | A | E | |||||
| Feeding and excretion behavior | |||||||
| Swallowing | 40/42 | ++ | ++ | 1 | 1, 4, 6 | 3, 4, 5, 6, 9, 17, 20 | 2, 3, 4, 5, 6, 7, 8, 9 |
| Filtering | 42/42 | +++ | ++ | 2 | 1, 4, 6, 9 | 3, 4, 9, 13, 14, 17, 18, 20 | 2, 3, 4, 5, 6, 7, 8, 9, 10 |
| Spiting | 42/42 | ++ | ++ | 3 | 1, 3, 4, 6 | 3, 7, 9, 13, 14, 17, 20 | 2, 3, 4, 5, 6, 7, 8, 9 |
| Defecating | 42/42 | + | + | 4 | 1, 3, 4, 6, 9 | 6, 9, 13, 14, 17, 20 | 3, 4, 5, 6, 7, 8, 9 |
| Locomotion behavior | |||||||
| Observing | 42/42 | ++ | ++ | 5 | 1, 3, 6, 12 | 6, 7, 8, 9, 13, 14, 17, 18, 20 | 2, 3, 4, 6, 7, 8, 9, 10 |
| Patrolling | 41/42 | ++ | + | 6 | 1, 9 | 8, 9, 13, 14, 16, 17, 20 | 2, 4, 6, 7, 8, 9 |
| Fast-start | 32/42 | ++ | + | 7 | 1, 2, 3, 9 | 8, 9, 10, 17, 19 | 2, 3, 4, 7, 8, 9 |
| Vertical jumping | 18/42 | + | 8 | 2, 7, 9 | 6, 9, 10, 15, 17, 19 | 1, 4, 5, 6, 7, 8, 9 | |
| Goring jumping | 29/42 | ++ | + | 9 | 2, 7, 9 | 6, 9, 10, 15, 17, 19 | 1, 4, 5, 6, 7, 8, 9 |
| Sweeping water | 32/42 | ++ | ++ | 10 | 1, 2, 7, 9 | 6, 8, 9, 10, 11, 15, 19 | 1, 2, 4, 6, 7, 9 |
| Diving | 30/42 | + | ++ | 11 | 1, 12 | 5, 7, 9, 17, 18, 20 | 3, 4, 5, 6, 7, 8, 9 |
| Floating | 36/42 | ++ | ++ | 12 | 1, 5 | 5, 8, 9, 16, 17, 20 | 1, 4, 5, 6, 7, 8, 9 |
| Swimming | 42/42 | +++ | +++ | 13 | 1, 2, 7, 9 | 8, 9, 10, 13, 14, 16, 17, 19, 20 | E1, E2 |
| Slowly swimming | 42/42 | +++ | +++ | 14 | 1, 9 | 5, 8, 9, 13, 14, 17, 18, 20 | E1, E2 |
| Inverse swimming | 42/42 | +++ | +++ | 15 | 1, 2, 7, 9 | 8, 9, 10, 13, 14, 16, 17, 19 | 2, 3, 4, 5, 6, 7, 8, 9, 10 |
| Swimming downstream | 14/42 | + | 16 | 1, 3, 9, 10 | 8, 9, 13, 14, 17, 18 | 1, 2, 4, 5, 6, 7, 8, 9 | |
| Detecting object | 39/42 | + | ++ | 17 | 1, 2, 3, 6, 9 | 1, 7, 8, 9, 12, 13, 14, 17, 20 | 2, 3, 4, 5, 6, 7, 8, 9 |
| Aggregation behavior | |||||||
| Group touring | 42/42 | +++ | +++ | 18 | 1, 2, 9 | 8, 9, 13, 14, 17, 20 | 2, 3, 4, 6, 7, 8, 10 |
| Searching | 42/42 | +++ | ++ | 19 | 1, 2, 9, 10 | 6, 7, 8, 9, 13, 14, 17, 20 | 2, 3, 4, 6, 7, 8, 10 |
| Following | 42/42 | +++ | +++ | 20 | 1, 9, 10 | 6, 7, 8, 9, 10, 13, 14, 17, 20 | 2, 3, 4, 6, 7, 8, 10 |
| Clustering | 42/42 | +++ | ++ | 21 | 1, 4, 6 | 6, 7, 8, 9, 13, 14, 16, 17, 18, 20 | 2, 3, 4, 5, 6, 7, 8, 10 |
| Disperse | 40/42 | ++ | + | 22 | 1, 2, 9, 10 | 6, 7, 8, 9, 10, 13, 14, 16, 19, 20 | 2, 3, 4, 5, 6, 7, 8, 10 |
| Avoiding | 39/42 | ++ | + | 23 | 1, 9, 10 | 8, 9, 13, 14, 15, 16, 19, 20 | 2, 3, 4, 5, 6, 7, 8, 10 |
| Leaving | 37/42 | ++ | + | 24 | 1, 9 | 8, 9, 13, 14, 15, 16, 20 | 2, 3, 4, 5, 6, 7, 8, 10 |
| Frolic | 35/42 | + | ++ | 25 | 1, 2, 9 | 1, 8, 9, 11, 13, 14, 17, 18, 20 | 1, 2, 4, 6, 7, 8, 10 |
| Reproduction behavior | |||||||
| Accompanying | 42/42 | +++ | +++ | 26 | 1, 9, 10 | 8, 9, 10, 13, 14, 17, 18, 20 | 2, 3, 4, 5, 6, 7, 8, 10 |
| Guiding | 37/42 | ++ | + | 27 | 1, 9, 10 | 8, 9, 10, 13, 14, 17, 18, 20 | 2, 3, 4, 5, 6, 7, 8, 10 |
| Chasing | 23/42 | + | 28 | 1, 2, 9, 10 | 6, 8, 9, 10, 13, 14, 15, 19 | 2, 4, 5, 6, 7, 8, 10 | |
| Encircle | 28/42 | + | + | 29 | 1, 5, 9, 10 | 1, 8, 10, 12, 13, 14, 16, 19 | 1, 2, 4, 5, 6, 7, 8, 10 |
| Tail-diving | 31/42 | + | + | 30 | 1, 2, 9, 12 | 1, 6, 7, 9, 12, 15, 16, 20 | 3, 4, 5, 6, 7, 8, 10 |
| Mating | 37/42 | + | + | 31 | 1, 4, 8, 9, 10, 11, 12 | 1, 6, 9, 10, 11, 12, 16, 18, 20 | 2, 3, 4, 5, 6, 7, 8, 10 |
| Miscellaneous behavior | |||||||
| Breathing | 42/42 | +++ | +++ | 32 | 1–12 | 5 | E1, E2 |
| Sound production | 16/42 | + | + | 33 | 7 | 3, 10, 11, 17, 19 | 1, 4, 7, 9 |
| Splitting | 34/42 | ++ | ++ | 34 | 1, 4, 5, 6, 11 | 2, 3, 9, 17, 20 | 1, 2, 3, 5, 7, 9 |
| Groups | Classification | Absolute Behavioral Diversity Index (H) | Relative Behavioral Diversity Index (r) | Regulated Diversity Index (r-Variable) |
|---|---|---|---|---|
| Groups with varying durations following induced spawning | 0–5 h | 1.25 ± 0.05 c | 0.49 ± 0.02 c | 0.54 ± 0.02 c |
| 5–10 h | 2.01 ± 0.04 b | 0.78 ± 0.02 b | 0.78 ± 0.02 b | |
| 10–15 h | 2.14 s 0.01 a | 0.83 ± 0.00 a | 0.83 ± 0.00 a | |
| 15–20 h | 1.05 ± 0.04 d | 0.41 ± 0.02 d | 0.52 ± 0.02 c | |
| p-value | p < 0.001 | p < 0.001 | p < 0.001 | |
| Gender groups | Male | 1.84 ± 0.04 a | 0.71 ± 0.01 a | 0.71 ± 0.01 a |
| Female | 1.44 ± 0.03 b | 0.56 ± 0.01 b | 0.62 ± 0.01b | |
| p-value | p < 0.001 | p < 0.001 | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Zhu, F.; Yu, L.; Yang, Q.; Wang, K.; Liu, M.; Duan, X.; Chen, D. Ethogram Characteristics of Silver Carp (Hypophthalmichthys molitrix) During the Breeding Period Based on the PAE Coding System. Animals 2025, 15, 1218. https://doi.org/10.3390/ani15091218
Wang M, Zhu F, Yu L, Yang Q, Wang K, Liu M, Duan X, Chen D. Ethogram Characteristics of Silver Carp (Hypophthalmichthys molitrix) During the Breeding Period Based on the PAE Coding System. Animals. 2025; 15(9):1218. https://doi.org/10.3390/ani15091218
Chicago/Turabian StyleWang, Min, Fengyue Zhu, Lixiong Yu, Qingrui Yang, Ke Wang, Mingdian Liu, Xinbin Duan, and Daqing Chen. 2025. "Ethogram Characteristics of Silver Carp (Hypophthalmichthys molitrix) During the Breeding Period Based on the PAE Coding System" Animals 15, no. 9: 1218. https://doi.org/10.3390/ani15091218
APA StyleWang, M., Zhu, F., Yu, L., Yang, Q., Wang, K., Liu, M., Duan, X., & Chen, D. (2025). Ethogram Characteristics of Silver Carp (Hypophthalmichthys molitrix) During the Breeding Period Based on the PAE Coding System. Animals, 15(9), 1218. https://doi.org/10.3390/ani15091218







