4.1. Genetic Diversity of P. anomala
Genetic diversity is a fundamental condition for species to maintain their evolutionary potential and is crucial for the formation, development, and sustainability of biodiversity. It enables species to adapt to complex and changing environments, thereby ensuring their survival [
56,
57,
58]. Nevo [
59] proposed that genetic diversity is correlated with environmental heterogeneity across different spatial scales, highlighting the importance of diverse habitats in maintaining genetic variation. Based on mtDNA, this study analyzed the genetic diversity of populations of
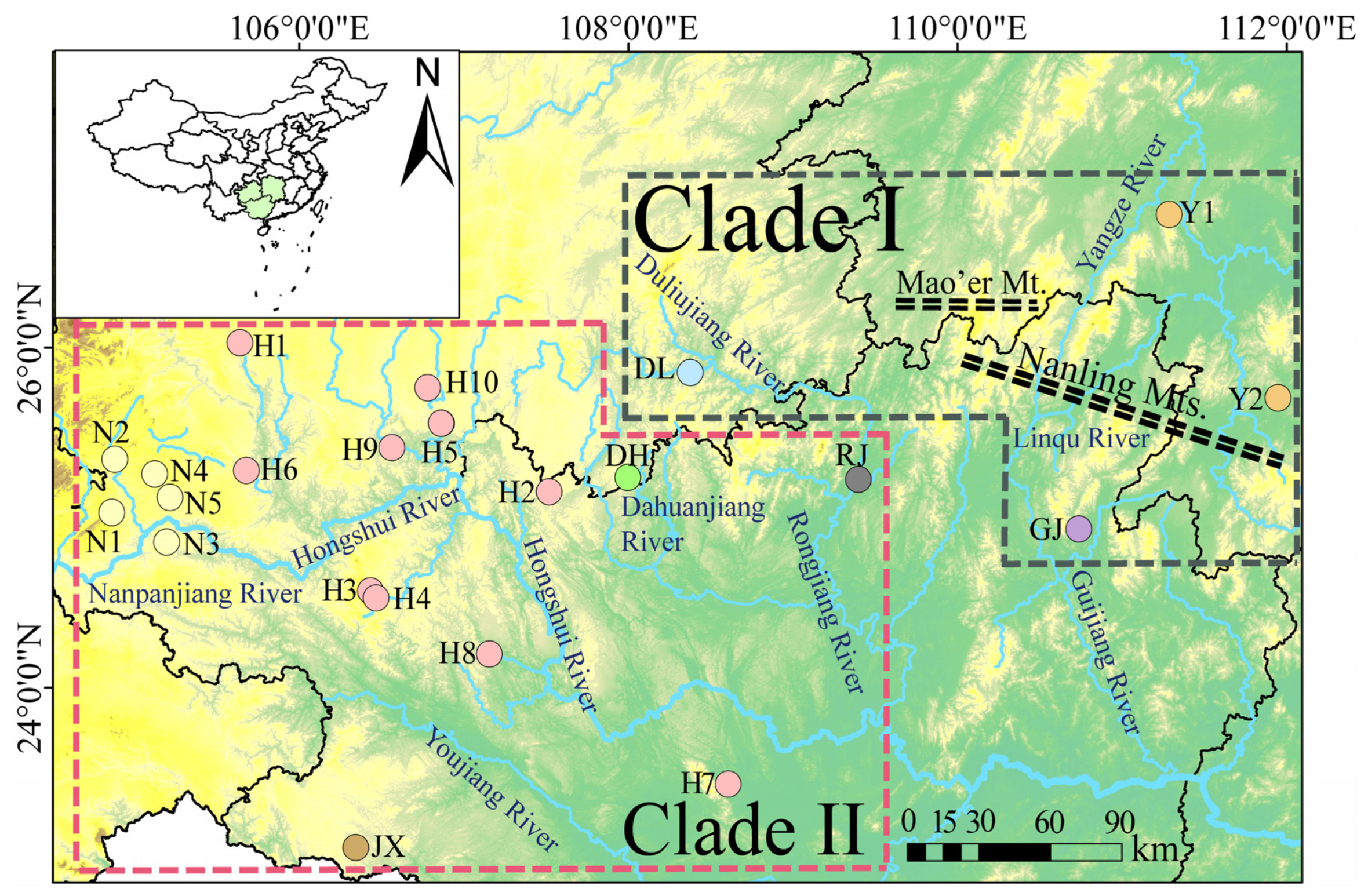
P. anomala in Clade I and Clade II. The results showed that the genetic diversity in Clade I (h = 0.790, π = 0.01030) was higher than that in Clade II (h = 0.681, π = 0.00262). This difference may be closely related to the geographical environment in which Clade II is located. Further analysis indicated significant genetic differentiation between the population in the Hongshui River and the populations in the Guijiang and Rongjiang Rivers. This differentiation may stem from the limited dispersal ability of
P. anomala itself, as well as the combined effects of geographical isolation and environmental differences. Compared with other fish species, the genetic diversity of Clade II is lower than that of the
Pelteobagrus fulvidraco (Richardson, 1846) (h = 0.848, π = 0.0480) [
60] and the
Ptychidio jordani Myers, 1930 (h = 0.768, π = 0.0023) [
61], which are also distributed in the Pearl River. This difference may be closely related to the ecological habits and habitat characteristics of
P. anomala. Once a species adapts to cave-dwelling life, reduced selective pressures and smaller population sizes can exacerbate genetic drift, thereby reducing genetic diversity [
62]. Moreover, genetic differentiation coefficients (
Fst) based on mitochondrial DNA,
RAG1, and
PLAGL2 sequences, as well as Mantel tests, showed significant genetic differentiation between populations of
P. anomala in Clade I and Clade II, revealing distinct geographical distribution patterns. These findings were also supported by phylogenetic tree and haplotype network analyses. The observed genetic differentiation may be due to the marked contrast between the tropical maritime climate of the Pearl River and the temperate monsoon climate of the Yangtze River basin. This significant north–south climatic difference may be one of the important factors leading to genetic differentiation. In addition, there were also considerable differences between the Pearl River and Yangtze River ecosystems in terms of water temperature, flow velocity, water quality, and other aspects. These factors, acting together, further exacerbate the genetic differentiation between the two populations [
63].
4.2. Genetic Structure of P. anomala
In this study, by integrating mtDNA and nuclear DNA molecular markers of
P. anomala populations, we systematically analyzed the population genetic structure characteristics of this species. Notably, we observed a significant incongruence between nuclear DNA haplotypes and mitochondrial gene haplotype networks. Comparative analysis indicated that the phylogeographical structure reconstructed based on mitochondrial gene markers exhibited a more pronounced phylogeographical differentiation pattern than nuclear gene data, while the phylogenetic topology constructed by nuclear genes failed to resolve distinct evolutionary clades. This phenomenon of incongruence between mitochondrial and nuclear DNA is widespread in many organisms [
64,
65]. Such incongruence typically implies ancient hybridization events and incomplete lineage sorting (ILS) [
66]. Mitochondrial genes, due to their faster substitution rates and smaller effective population sizes, are more susceptible to hybridization in phylogenetic analyses. In contrast, nuclear genes, with their slower substitution rates and larger effective population sizes, are more prone to ILS in phylogenetic analyses [
66,
67]. Additionally, the relatively slow mutation rate of nuclear genes somewhat limits their ability to resolve population structure, a phenomenon that has been verified in studies of the
Baryancistrus xanthellus Rapp Py-Daniel, Zuanon & de Oliveira, 2011 [
68].
The phylogenetic trees and haplotype network based on mtDNA data revealed the genetic differentiation characteristics of
P. anomala populations. The research findings indicated that
P. anomala populations could be divided into two main clades, with their distribution closely related to geographical regions (
Figure 2). These results suggested that the Yangtze River population shared a relatively close genetic relationship with the populations of the Guijiang and Duliujiang Rivers. Similar patterns had also been reported in
Hemibarbus medius Yue, 1995 and
Hemibarbus labeo (Pallas, 1776) [
69], as well as
Hypophthalmichthys nobilis (Richardson, 1845) [
70]. The close genetic relationships among subclades within Clade I and the current distribution patterns of Clade I and Clade II can be attributed to the following factors.
First, the monsoon climate impacts brought about by the uplift of the QTP, as well as the formation of the Nanling Mountains, have played significant roles. Phylogenetic analysis using BEAST estimated that these lineages diverged approximately 8.5–18.81 mya (
Figure 7), a period that was potentially influenced by the southwest monsoon [
71]. During the Miocene, as the QTP approached its present-day elevation, its uplift significantly intensified the monsoon systems and altered marine biogeochemical processes. This process not only drove the South Asian and East Asian monsoons into an enhanced phase but also fundamentally changed the evolution of the regional topography [
72,
73,
74,
75]. The mid-Miocene climatic optimum (approximately 17–14 mya) saw intensified monsoon precipitation that dramatically accelerated karst cave system development [
76]. Studies have shown that the ancestral populations of the cave-dwelling and non-cave-dwelling groups of the genus
Triplophysa initially diverged around 15.3–13 million years ago, which is inferred to be closely related to the continuous uplift of the plateau [
72]. We speculate that the differentiation of populations of the
P. anomala inhabiting the same karst environment is also likely to be significantly associated with the ongoing uplift of the QTP. Concurrently, the formation of the Nanling Mountains as a geographical barrier played a significant role in the genetic differentiation of these fish populations. During its early uplift, the Nanling Mountains had a relatively low elevation, which did not effectively block gene flow between freshwater fish populations on either side [
69]. However, during the uplift of the QTP, the Nanling Mountains experienced accelerated uplift [
77]. This rapid uplift transformed the Nanling Mountains into a geographical barrier between the northern and southern river systems [
69]. The continued uplift ultimately established a divide between the Pearl River and Yangtze River systems. This divide prevented warm air masses south of the mountains from moving northward and blocked cold air masses north of the mountains from penetrating southward. Consequently, a climatic boundary formed between the south subtropical and mid-subtropical zones, restricting gene flow and leading to genetic differentiation [
78]. In summary, the uplift of QTP and the formation of the Nanling Mountains as a geographical barrier both contributed to the genetic differentiation observed in fish populations in the region.
Second, river capture events played a role. Tectonic movements and river capture events led to connections or diversions between the Yangtze River and Pearl River systems. Early geological and ichthyological studies showed that some rivers in the Pearl River and Yangtze River were geographically very close, with a few even being directly connected [
79,
80]. For example, the Lingqu Canal, which connected the Xiangjiang (a tributary of the Yangtze River) and Guijiang Rivers [
5,
80], may also have promoted gene flow between populations of the Yangtze River and Pearl River systems.
The results of the haplotype network analysis showed that there is haplotype sharing between the Pearl River and the Yangtze River (
Figures S1 and S2). It is inferred that this phenomenon is more likely the result of the combined effects of the dispersal of the Yangtze River population and shared ancestral polymorphisms. According to the studies of Yang et al., (2022) and Li et al., (2020), if ancestral polymorphisms are shared between the Pearl River and Yangtze River, each basin should retain a subset of unique haplotypes [
70,
81]. However, the haplotype network reveals that, despite shared haplotypes, both basins exhibit distinct unique haplotypes. This indicates that genetic differentiation between populations is not solely attributable to ancestral polymorphism retention, and dispersal of the Yangtze River population likely contributed to the observed pattern. Secondly, the analysis of average genetic distances between populations (
Table 3) showed that the genetic distance between populations of Clade II and the four subclades of Clade I increased gradually from north to south. This suggested that some ancestors of the
P. anomala populations in the Pearl River likely originated from the Yangtze River. It was worth noting that, in the Pearl River and Yangtze River, genetic differentiation among different fish populations exhibited diverse characteristics. For example, the genetic distance among individuals of
P. anomala ranged from 0% to 4%, a value significantly lower than that of the sympatric species
Squaliobarbus curriculus (Richardson, 1846) (with genetic distances ranging from 0% to 7.43%) [
78] but slightly higher than the genetic distance between populations of
Hypophthalmichthys molitrix (Valenciennes, 1844) across basins (at 2.3%) [
70]. Although
P. anomala (0–4%),
H. molitrix (2.3%), and
S. curriculus (0–7.43%) all showed clear genetic differentiation between populations in the Pearl River and Yangtze River, this degree of differentiation had not reached the level of speciation. This demonstrated that there is no correspondence between genetic distance and species boundaries, and genetic differentiation between populations, even up to 7.43%, might still fall within the range of intraspecific variation. Therefore, when delimiting species, it is not sufficient to rely solely on genetic distance as a single indicator. Instead, multidimensional evidence, such as morphological differences, reproductive isolation, and ecological niche differentiation, should be taken into account [
82].
4.3. Implications for Conservation
Deciphering the genetic structure of specific species can provide robust support for species management and conservation [
83]. Given that
P. anomala’s wild resources are scarce and artificial breeding has not yet been successful, refining conservation units and implementing in situ conservation is particularly crucial. Therefore, analyzing the genetic structure of
P. anomala across different river systems through phylogeographic analysis is of great significance for accurately delineating its conservation units. In our study, we observed two clades in
P. anomala populations, which we proposed should be recognized as two molecular operational taxonomic units (MOTUs). Although
P. anomala is currently listed as a species of Least Concern on the IUCN Red List due to its wide distribution and seemingly stable population [
84], our mitochondrial DNA (mtDNA) analysis revealed relatively low haplotype and nucleotide diversity in the Youjiang, Rongjiang, and Guijiang River basins. The genetic diversity of these populations was significantly lower than that of
Pelteobagrus fulvidraco and
Hemibarbus medius in the same river basins. Moreover, the analysis of genetic diversity using nuclear gene markers also showed relatively low haplotype and nucleotide diversity in the Nanpanjiang, Duliujiang, Dahuanjiang, and Hongshui Rivers. The genetic diversity in Duliujiang and Dahuanjiang Rivers might have been limited by the small sample size. Cave-dwelling fish, due to their unique habitats, face multiple threats including habitat degradation, environmental pollution, overexploitation of resources, and invasion of non-native species. In China, most cave-dwelling fish have limited distribution ranges and small population sizes, making them highly vulnerable to even minor threats [
85]. To protect these species, effective conservation measures must be implemented, including strengthening habitat protection, reducing pollution sources, managing resource exploitation sustainably, and preventing the invasion of non-native species, to ensure their survival and reproduction.