Differentiating Canine Chronic Inflammatory Enteropathies Using Faecal Amino Acid Profiles: Potential and Limitations
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design, Animal Signalment and Diets
2.2. Measurement of Amino Acids in Faecal Samples
2.3. Statistical Analysis
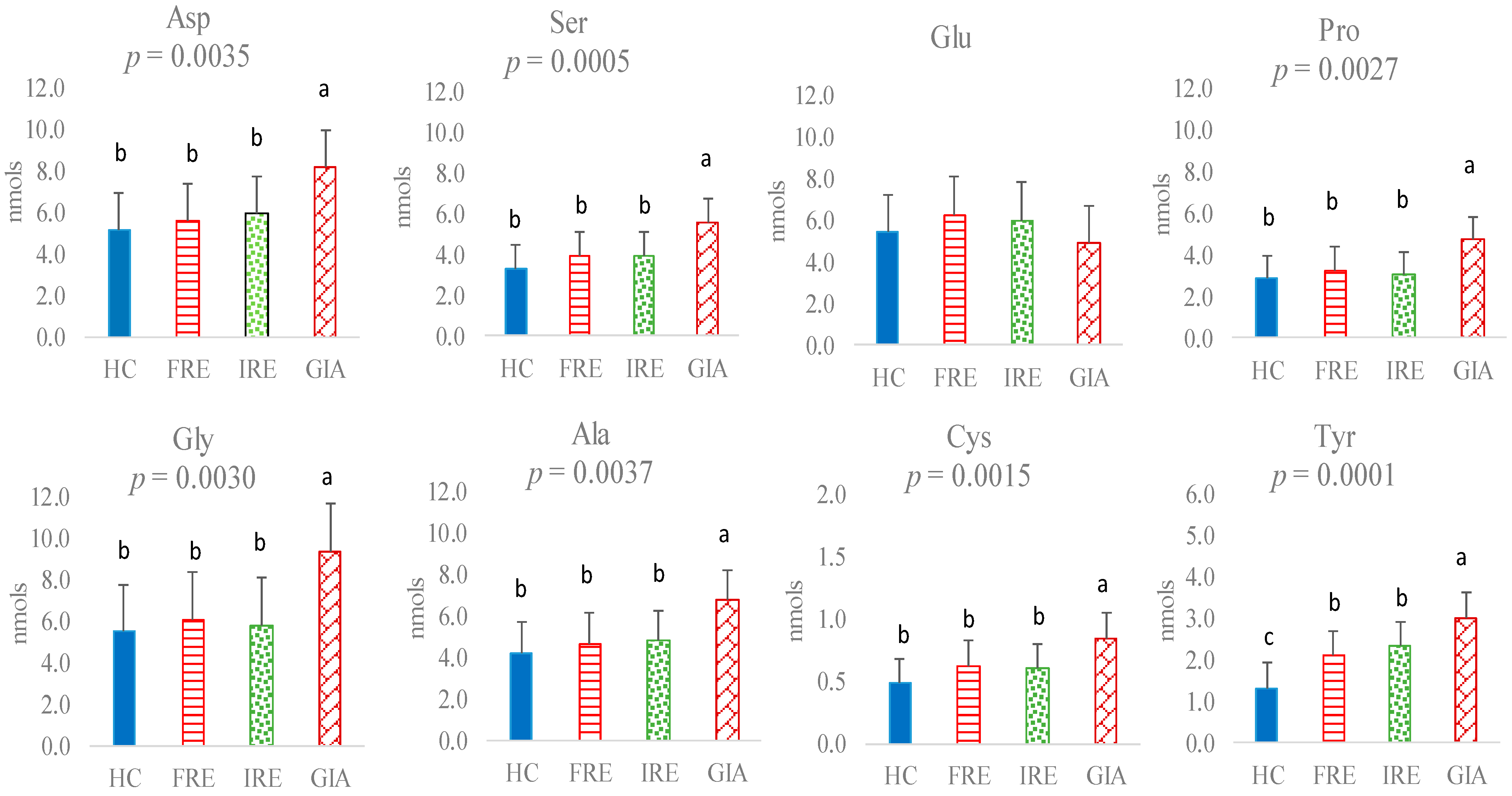
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | amino acid |
| AAA | aromatic amino acid |
| Ala | alanine |
| Arg | arginine |
| Asp | aspartic acid |
| BCAA | branched-chain amino acid |
| BCS | body condition score |
| CCECAI | canine chronic enteropathy clinical activity index |
| CIE | chronic inflammatory enteropathy |
| CIBDAI | canine inflammatory bowel disease activity index |
| CSIC | Spanish National Research Council |
| CVMTH | Complutense Veterinary Medicine Teaching Hospital |
| Cys | cysteine |
| DNA | deoxyribonucleic acid |
| FRE | food-responsive enteropathy |
| GIA | Giardia infection |
| Glu | glutamic acid |
| Gly | glycine |
| HC | healthy control |
| His | histidine |
| HCl | hydrochloric acid |
| Ile | isoleucine |
| IBD | inflammatory bowel disease |
| IRE | immunosuppressant-responsive enteropathy |
| Leu | leucine |
| Lys | lysine |
| Met | methionine |
| MCS | muscle condition score |
| NaOH | sodium hydroxide |
| Phe | phenylalanine |
| Pro | proline |
| RMSE | root mean square error |
| Ser | serine |
| Thr | threonine |
| TLI | trypsin-like immunoreactivity |
| Trp | tryptophan |
| Tyr | tyrosine |
| Val | valine |
References
- Dandrieux, J.R.S. Inflammatory bowel disease versus chronic enteropathy in dogs: Are they one and the same? J. Small Anim. Pract. 2016, 57, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Jergens, A.E.; Heilmann, R.M. Canine chronic enteropathy-Current state-of-the-art and emerging concepts. Front. Vet. Sci. 2022, 9, 923013. [Google Scholar] [CrossRef] [PubMed]
- Ruemmele, F.M. Role of diet in inflammatory bowel disease. Ann. Nutr. Metab. 2016, 68, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Tomé, S.; Hernández-Ledesma, B.; Chaparro, M.; Indiano-Romacho, P.; Bernardo, D.; Gisbert, J.P. Role of food proteins and bioactive peptides in inflammatory bowel disease. Trends Food Sci. Technol. 2019, 88, 194–206. [Google Scholar] [CrossRef]
- Forbes, A.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamin, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN guideline: Clinical nutrition in inflammatory bowel disease. Clin. Nutr. 2017, 36, 321–347. [Google Scholar] [CrossRef]
- Newsome, S.D.; Feeser, K.L.; Bradley, C.J.; Wolf, C.; Takacs-Vesbach, C.; Fogel, M.L. Isotopic and genetic methods reveal the role of the gut microbiome in mammalian host essential amino acid metabolism. Proc. R. Soc. B 2020, 287, 20192995. [Google Scholar] [CrossRef]
- Hisamatsu, T.; Okamoto, S.; Hashimoto, M.; Muramatsu, T.; Andou, A.; Uo, M.; Kitazume, M.T.; Matsuoka, K.; Yajima, T.; Inoue, N.; et al. Novel, objective, multivariate biomarkers composed of plasma amino acid profiles for the diagnosis and assessment of inflammatory bowel disease. PLoS ONE 2012, 7, e31131. [Google Scholar] [CrossRef]
- Torinsson Naluai, Å.; Saadat Vafa, L.; Gudjonsdottir, A.H.; Arnell, H.; Browaldh, L.; Nilsson, S.; Agardh, D. Altered peripheral amino acid profile indicates a systemic impact of active celiac disease and a possible role of amino acids in disease pathogenesis. PLoS ONE 2018, 13, e0193764. [Google Scholar] [CrossRef]
- Tamura, Y.; Ohta, H.; Kagawa, Y.; Osuga, T.; Morishita, K.; Sasaki, N.; Takiguchi, M. Plasma amino acid profiles in dogs with inflammatory bowel disease. J. Vet. Intern. Med. 2019, 33, 1602–1607. [Google Scholar] [CrossRef]
- Benvenuti, E.; Pierini, A.; Gori, E.; Bartoli, F.; Erba, P.; Ruggiero, P.; Marchetti, V. Serum amino acid profile in 51 dogs with immunosuppressant-responsive enteropathy (IRE): A pilot study on clinical aspects and outcomes. BMC Vet. Res. 2020, 16, 117. [Google Scholar] [CrossRef]
- Kathrani, A.; Allenspach, K.; Fascetti, A.J.; Larsen, J.A.; Hall, E.J. Alterations in serum amino acid concentrations in dogs with protein-losing enteropathy. J. Vet. Intern. Med. 2018, 32, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Maeda, S.; Yonezawa, T.; Matsuki, N. Decreased plasma amino acid concentrations in cats with chronic gastrointestinal diseases and their possible contribution in the inflammatory response. Vet. Immunol. Immunopathol. 2018, 195, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bosch, S.; El Manouni El Hasani, S.; Brizzio, M.; Ayada, I.M.; Bakkali, A.; Jansen, E.; Struys, E.A.; Benninga, M.A.; de Boer, N.K.H.; Meij, T. Fecal amino acid profiles exceed accuracy of serum amino acids in diagnosing pediatric inflammatory bowel disease. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Blake, A.M. Amino Acids and Metabolites in Dogs with Gastrointestinal Disease. Ph.D. Thesis, Texas A&M University, Texas, TX, USA, 2022. [Google Scholar]
- Higueras, C.; Escudero, R.; Rebolé, A.; García-Sancho, M.; Rodríguez-Franco, F.; Sainz, Á.; Rey, A.I. Changes in faecal and plasma amino acid profile in dogs with food-responsive enteropathy as indicators of gut homeostasis disruption: A pilot study. Vet. Sci. 2023, 10, 112. [Google Scholar] [CrossRef]
- Perrucci, S.; Berrilli, F. Giardia duodenalis infection in dogs affected by primary chronic enteropathy. Open Vet. J. 2020, 10, 74–79. [Google Scholar] [CrossRef]
- Allenspach, K.; Wieland, B.; Gröne, A.; Gaschen, F. Chronic enteropathies in dogs: Evaluation of risk factors for negative outcome. J. Vet. Intern. Med. 2007, 21, 700–708. [Google Scholar] [CrossRef]
- Higueras, C.; Sainz, Á.; García-Sancho, M.; Rodríguez-Franco, F.; Rey, A.I. Faecal short-chain, long-chain, and branched-chain fatty acids as markers of different chronic inflammatory enteropathies in dogs. Animals 2024, 14, 1825. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Nutrient Requirements of Dogs and Cats; The National Academic Press: Washington, DC, USA, 2006. [Google Scholar]
- Marchesi, J.R.; Holmes, E.; Khan, F.; Kochhar, S.; Scalan, P.; Shanahan, F.; Wilson, I.D.; Wang, Y. Rapid and noninvasive metabonomic characterization of inflammatory bowel disease. J. Proteome Res. 2007, 6, 546–551. [Google Scholar] [CrossRef]
- Jansson, J.; Willing, B.; Lucio, M.; Fekete, A.; Dicksved, J.; Halfvarson, J.; Tysk, C.; Schmitt-Kopplin, P. Metabolomics reveals metabolic biomarkers of Crohn’s disease. PLoS ONE 2009, 4, e6386. [Google Scholar] [CrossRef]
- Bjerrum, J.T.; Wang, Y.; Hao, F.; Coskun, M.; Ludwing, C.; Gunther, U.; Nielsen, O.H. Metabonomics of human fecal extracts characterize ulcerative colitis, Crohn’s disease, and healthy individuals. Metabolomics 2015, 11, 122–133. [Google Scholar] [CrossRef]
- Rui, L.; Wentian, L.; Meiyu, P.; Hong, Z. A review of the relationship between the gut microbiota and amino acid metabolism. Amino Acids 2017, 49, 2083–2090. [Google Scholar]
- Zangerle, R.; Kurz, K.; Neurauter, G.; Kitchen, M.; Sarcletti, M.; Fuchs, D. Increased blood phenylalanine to tyrosine ratio in HIV-1 infection and correction following effective antiretroviral therapy. Brain Behav. Immun. 2010, 24, 403–408. [Google Scholar] [CrossRef]
- Taciak, M.; Barszcz, M.; Święch, E.; Tuśnio, A.; Bachanek, I. Interactive effects of protein and carbohydrates on production of microbial metabolites in the large intestine of growing pigs. Arch. Anim. Nutr. 2017, 71, 192–209. [Google Scholar] [CrossRef]
- Blachier, F.; Andriamihaja, M. Effects of the L-tyrosine-derived bacterial metabolite p-cresol on colonic and peripheral cells. Amino Acids 2022, 54, 325–338. [Google Scholar] [CrossRef]
- Poesen, R.; Evenepoel, P.; de Loor, H.; Kuypers, D.; Augustijns, M.B. Metabolism, protein binding, and renal clearance of microbiota-derived p-cresol in patients with CKD. Clin. J. Am. Soc. Nephrol. 2016, 11, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Passmore, I.J.; Letertre, M.P.M.; Preston, M.D.; Bianconi, I.; Harrison, M.A.; Nasher, F.; Kaur, H.; Hong, H.A.; Baines, S.D.; Cutting, S.M.; et al. Para-cresol production by Clostridium difficile affects microbial diversity and membrane integrity of Gram-negative bacteria. PLoS Pathog. 2018, 14, e1007191. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hou, Y.; Wang, G.; Zheng, X.; Hao, H. Gut microbial metabolites of aromatic amino acids as signals in host-microbe interactions. Trends Endocrinol. Metab. 2020, 31, 11. [Google Scholar] [CrossRef]
- Crown, S.B.; Marze, N.; Antoniewicz, M.R. Catabolism of branched-chain amino acids contributes significantly to synthesis of odd-chain and even-chain fatty acids in 3T3-L1 adipocytes. PLoS ONE 2015, 10, e0145850. [Google Scholar] [CrossRef]
- Kolho, K.L.; Pessia, A.; Jaakkola, T.; de Vos, W.M.; Velagapudi, V. Faecal and serum metabolomics in paediatric inflammatory bowel disease. J. Crohns Colitis 2017, 11, 321–334. [Google Scholar] [CrossRef]
- Filimoniuk, A.; Daniluk, U.; Samczuk, P.; Wasilewska, N.; Jakimiec, P.; Kucharska, M.; Lebensztejn, D.M.; Ciborowski, M. Metabolomic profiling in children with inflammatory bowel disease. Adv. Med. Sci. 2020, 65, 65–70. [Google Scholar] [CrossRef]
- Faure, M.; Moënnoz, D.; Montigon, F.; Mettraux, C.; Breuillé, D.; Ballèvre, O. Dietary threonine restriction specifically reduces intestinal mucin synthesis in rats. J. Nutr. 2005, 135, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Xie, M.; Fan, W.; Xue, J.; Zhou, Z.; Tang, J.; Chen, G.; Hou, S. Transcriptome analysis reveals differential expression of genes regulating hepatic triglyceride metabolism in Pekin ducks during dietary threonine deficiency. Front. Genet. 2019, 10, 710. [Google Scholar] [CrossRef]
- Munasinghe, L.L.; Robinson, J.L.; Harding, S.V.; Brunton, J.A.; Bertolo, R.F. Protein synthesis in mucin-producing tissues is conserved when dietary threonine is limiting in piglets. J. Nutr. 2017, 147, 202–210. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, X.; Eicher, S.D.; Ajuwon, K.M.; Applegate, T.J. Effect of threonine on secretory immune system using a chicken intestinal ex vivo model with lipopolysaccharide challenge. Poult. Sci. 2017, 96, 3043–3051. [Google Scholar] [CrossRef] [PubMed]
- Law, G.K.; Bertolo, R.F.; Adjiri-Awere, A.; Pencharz, P.B.; Ball, R.O. Adequate oral threonine is critical for mucin production and gut function in neonatal piglets. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G1293–G1301. [Google Scholar] [CrossRef] [PubMed]
- Rémond, D.; Buffière, C.; Godin, J.P.; Mirand, P.P.; Obled, C.; Papet, I.; Dardevet, G.; Williamson, G.; Breuillé, D.; Faure, M. Intestinal inflammation increases gastrointestinal threonine uptake and mucin synthesis in enterally fed minipigs. J. Nutr. 2009, 139, 1–7. [Google Scholar] [CrossRef]
- Thompson, R.C.; Reynoldson, J.A.; Mendis, A.H. Giardia and giardiasis. Adv. Parasitol. 1993, 32, 71–160. [Google Scholar]
- Garat, B.; Musto, H. Trends of amino acid usage in the proteins from the unicellular parasite Giardia lamblia. Biochem. Biophys. Res. Commun. 2000, 279, 996–1000. [Google Scholar] [CrossRef]
- Jiménez, J.C.; Fontaine, J.; Creusy, C.; Fleurisse, L.; Grzych, J.-M.; Capron, M.; De-Cas, E. Antibody and cytokine responses to Giardia excretory/secretory proteins in Giardia intestinalis-infected BALB/c mice. Parasitol. Res. 2014, 113, 2709–2718. [Google Scholar] [CrossRef]
- Einarsson, E.; Ma’ayeh, S.; Sward, S.G. An up-date on Giardia and giardiasis. Curr. Opin. Microbiol. 2016, 34, 47–52. [Google Scholar] [CrossRef]
- Ringquist, E.; Palm, J.E.D.; Skarin, H.; Hehl, A.B.; Weiland, M.; Davids, B.J.; Reiner, D.S.; Griffiths, W.J.; Eckmann, L.; Gillin, F.D.; et al. Release of metabolic enzymes by Giardia in response to interaction with intestinal epithelial cells. Mol. Biochem. Parasitol. 2008, 159, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.M. Arginine metabolism: Boundaries of our knowledge. J. Nutr. 2007, 137, 1602S–1609S. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.R.; Quinn, L.A.; Maier, E.A.; Guedes, M.M.; Quertz, J.S.; Perry, M.; Ramprasad, C.; Lanzarini, G.M.L.; Mayneris-Perxachs, J.; Swann, J.; et al. Intervention and Mechanisms of Alanyl-glutamine for Inflammation, Nutrition, and Enteropathy: A Randomized Controlled Trial. J. Pediatr. Gastroenterol. Nutr. 2020, 71, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Meier, S.A.; Knabe, D.A. Dietary glutamine supplementation prevents jejunal atrophy in weaned pigs. J. Nutr. 1996, 126, 2578–2584. [Google Scholar] [CrossRef]
- Panigrah, P.I.; Banford, G.P.; Horvath, K. Role of glutamine in bacterial transcytosis and epithelial cell injury. J. Parenter. Enteral Nutr. 1997, 21, 75–80. [Google Scholar] [CrossRef]
- Li, J.-Y.; Guo, Y.-C.; Zhou, H.-F.; Yue, T.-T.; Wang, F.X.; Sun, F.; Wang, W.Z. Arginine metabolism regulates the pathogenesis of inflammatory bowel disease. Nutr. Rev. 2022, 81, 578–586. [Google Scholar] [CrossRef]
- McKay, D.M.; Baird, A.W. Cytokine regulation of epithelial permeability and ion transport. Gut 1999, 44, 283–289. [Google Scholar] [CrossRef]



| Variable | HC (n = 22) | FRE (n = 35) | IRE (n = 18) | GIA (n = 9) |
|---|---|---|---|---|
| Humidity | 9.50 ± 0.71 | 8.38 ± 0.48 | 8.50 ± 0.49 | 8.50 ± 0.50 |
| Crude protein | 23.05 ± 4.68 | 23.71 ± 4.50 | 23.73 ± 5.07 | 22.70 ± 4.90 |
| Crude fat | 13.78 ± 4.10 | 14.87 ± 4.30 | 13.46 ± 4.21 | 13.54 ± 4.41 |
| Crude fibre | 2.83 ± 0.78 | 2.83 ± 1.89 | 2.12 ± 1.63 | 2.57 ± 1.01 |
| Crude ash | 7.64 ± 1.20 | 6.70 ± 0.87 | 5.83 ± 1.00 | 6.23 ± 2.07 |
| Nitrogen-free extractives | 43.18 ± 7.91 | 43.49 ± 7.42 | 46.34 ± 8.36 | 46.44 ± 8.20 |
| Calcium | 1.47 ± 0.16 | 1.09 ± 0.30 | 0.91 ± 0.13 | 1.43 ± 0.06 |
| Phosphorus | 1.03 ± 0.17 | 0.77 ± 0.21 | 0.64 ± 0.09 | 0.92 ± 0.10 |
| Sodium | 0.30 ± 0.08 | 0.37 ± 0.08 | 0.35 ± 0.07 | 0.40 ± 0.08 |
| ∑n-3 | 0.67 ± 0.47 | 1.03 ± 0.89 | 2.00 ± 1.83 | 0.55 ± 0.35 |
| Metabolic energy (kcal/kg) 1 | 3583 ± 193 | 3711 ± 201 | 3742 ± 163 | 3684 ± 221 |
| FRE | GIA | IRE | HC | TOTAL (%) | |
|---|---|---|---|---|---|
| FRE | 38.24 | 2.94 | 26.47 | 32.35 | 100 |
| GIA | 28.7 | 57.14 | 14.29 | 0.00 | 100 |
| IRE | 50.00 | 14.29 | 28.57 | 7.14 | 100 |
| HC | 0.00 | 0.00 | 0.00 | 100.00 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higueras, C.; Ruiz-Capillas, C.; Herrero, A.; Sainz, A.; García-Sancho, M.; Rodríguez-Franco, F.; Larrosa, M.; Rey, A.I. Differentiating Canine Chronic Inflammatory Enteropathies Using Faecal Amino Acid Profiles: Potential and Limitations. Animals 2025, 15, 1185. https://doi.org/10.3390/ani15081185
Higueras C, Ruiz-Capillas C, Herrero A, Sainz A, García-Sancho M, Rodríguez-Franco F, Larrosa M, Rey AI. Differentiating Canine Chronic Inflammatory Enteropathies Using Faecal Amino Acid Profiles: Potential and Limitations. Animals. 2025; 15(8):1185. https://doi.org/10.3390/ani15081185
Chicago/Turabian StyleHigueras, Cristina, Claudia Ruiz-Capillas, Ana Herrero, Angel Sainz, Mercedes García-Sancho, Fernando Rodríguez-Franco, Mar Larrosa, and Ana I. Rey. 2025. "Differentiating Canine Chronic Inflammatory Enteropathies Using Faecal Amino Acid Profiles: Potential and Limitations" Animals 15, no. 8: 1185. https://doi.org/10.3390/ani15081185
APA StyleHigueras, C., Ruiz-Capillas, C., Herrero, A., Sainz, A., García-Sancho, M., Rodríguez-Franco, F., Larrosa, M., & Rey, A. I. (2025). Differentiating Canine Chronic Inflammatory Enteropathies Using Faecal Amino Acid Profiles: Potential and Limitations. Animals, 15(8), 1185. https://doi.org/10.3390/ani15081185











