The Role of Twist2 in Myoblast Proliferation, Fusion, and Its Impact on Muscle Structure During the Growth of Chinese Perch (Siniperca chuatsi)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Design and Preparation of siRNAs
2.3. Muscle Injury Model Construction, Twist2-siRNA In Vivo Inhibition Assay, and Tissue Sampling
2.4. cDNA Synthesis and Quantitative Real-Time PCR Analysis
2.5. Isolation of Single Muscle Fibers and DAPI Staining
2.6. Histological Section and Immunofluorescence Analysis
2.7. Statistical Analysis
2.8. Prediction of Transcription Factor-Target Gene Binding Sites
3. Results
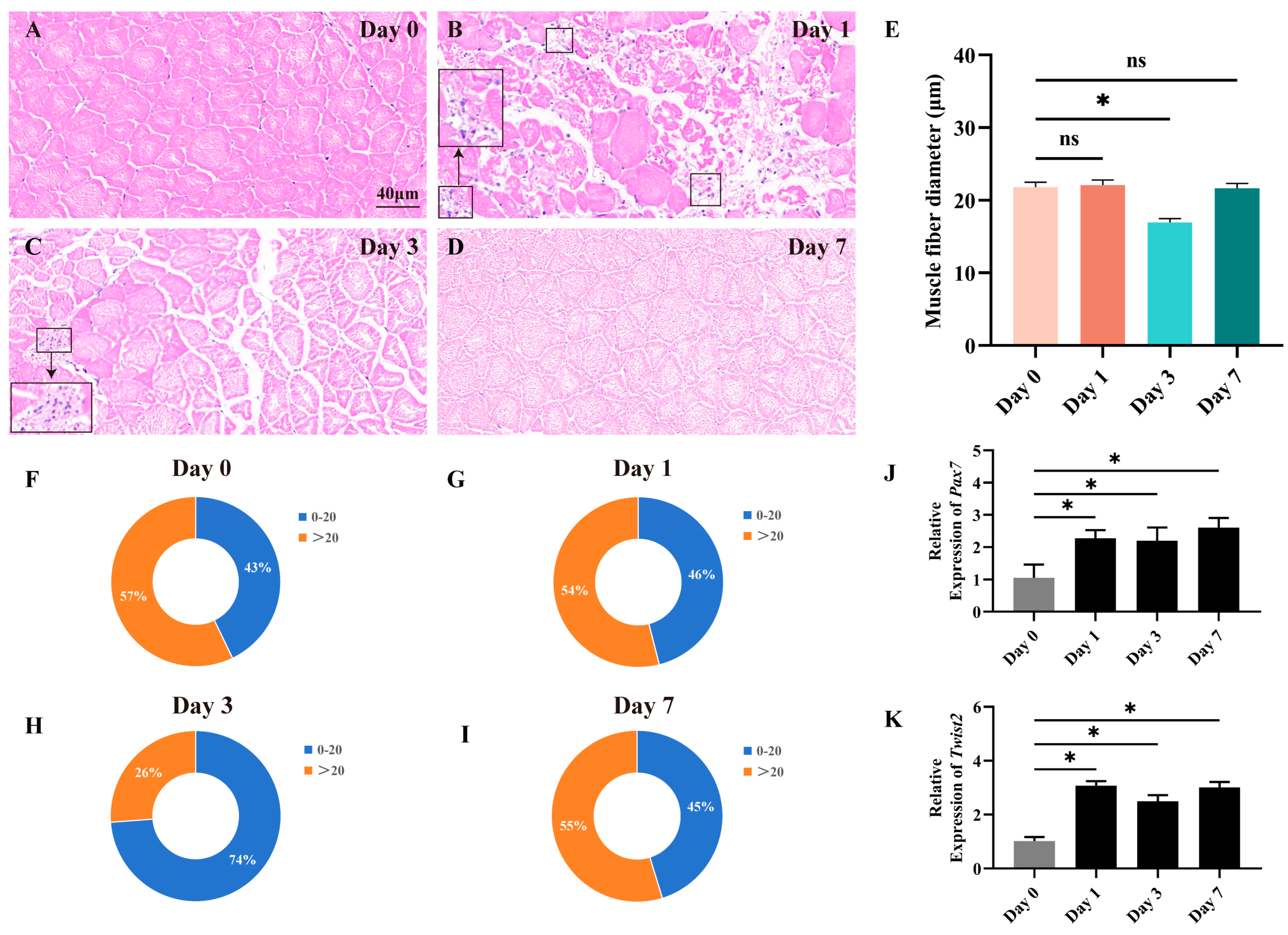
3.1. Fast Muscle Fiber Changes During Tissue Repair After Chinese Perch Muscle Injury
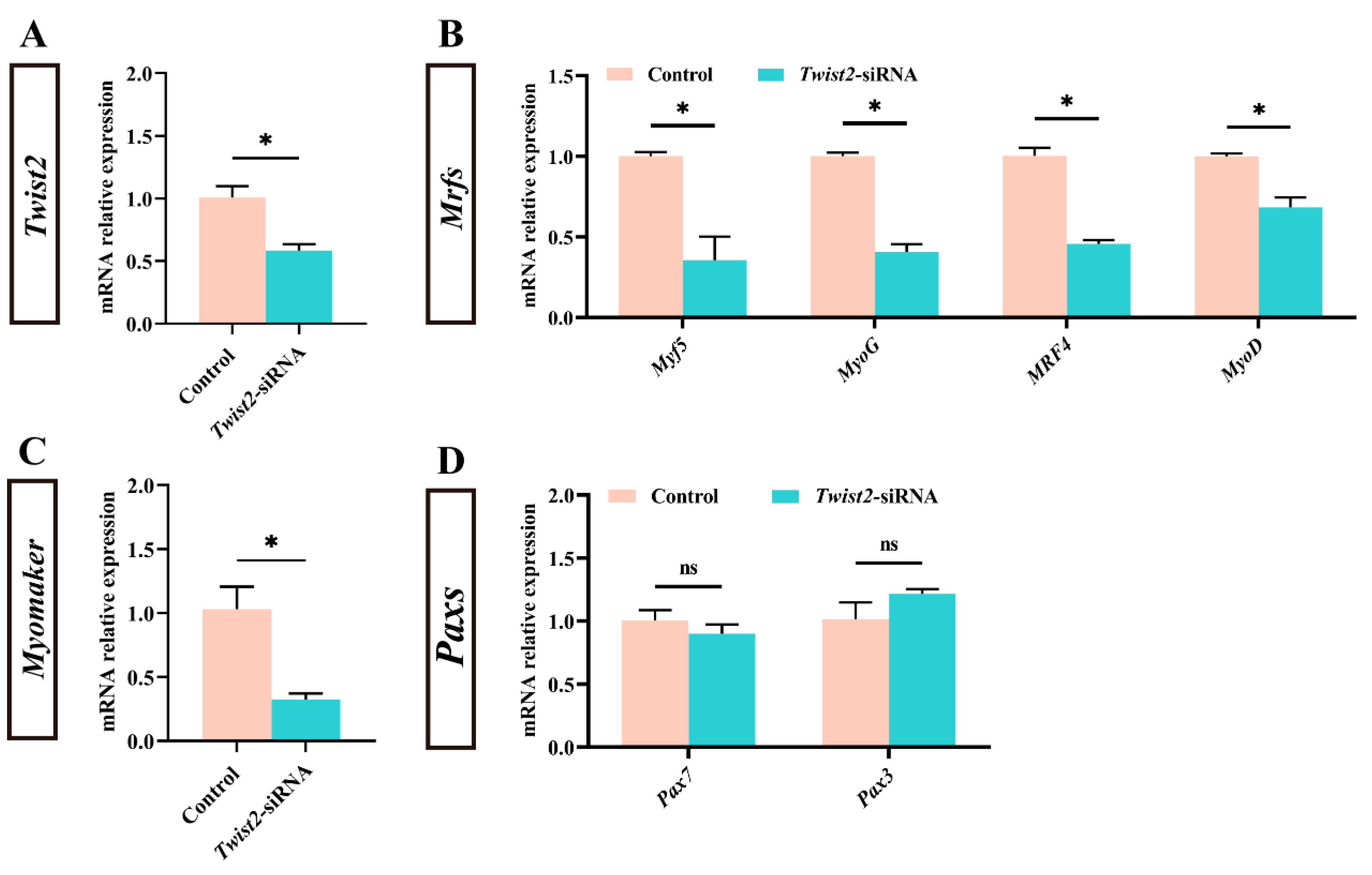
3.2. Effect of Inhibition of Twist2 on Gene Expression Related to Muscle Growth
3.3. Histological Analysis of Fast Muscle After Inhibition of Twist2
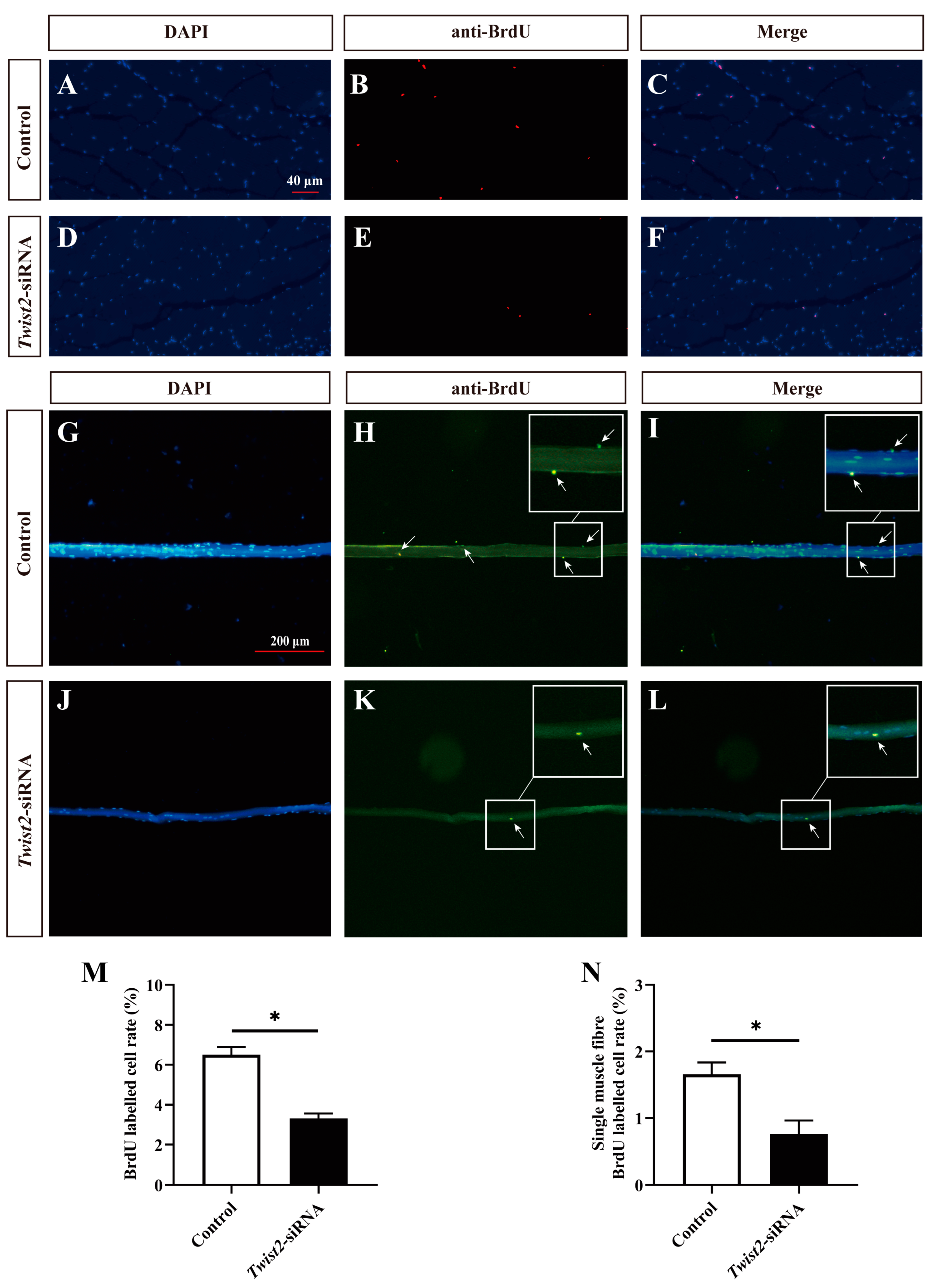
3.4. Effects of Interfering with Twist2 on the Proliferation of Myoblasts
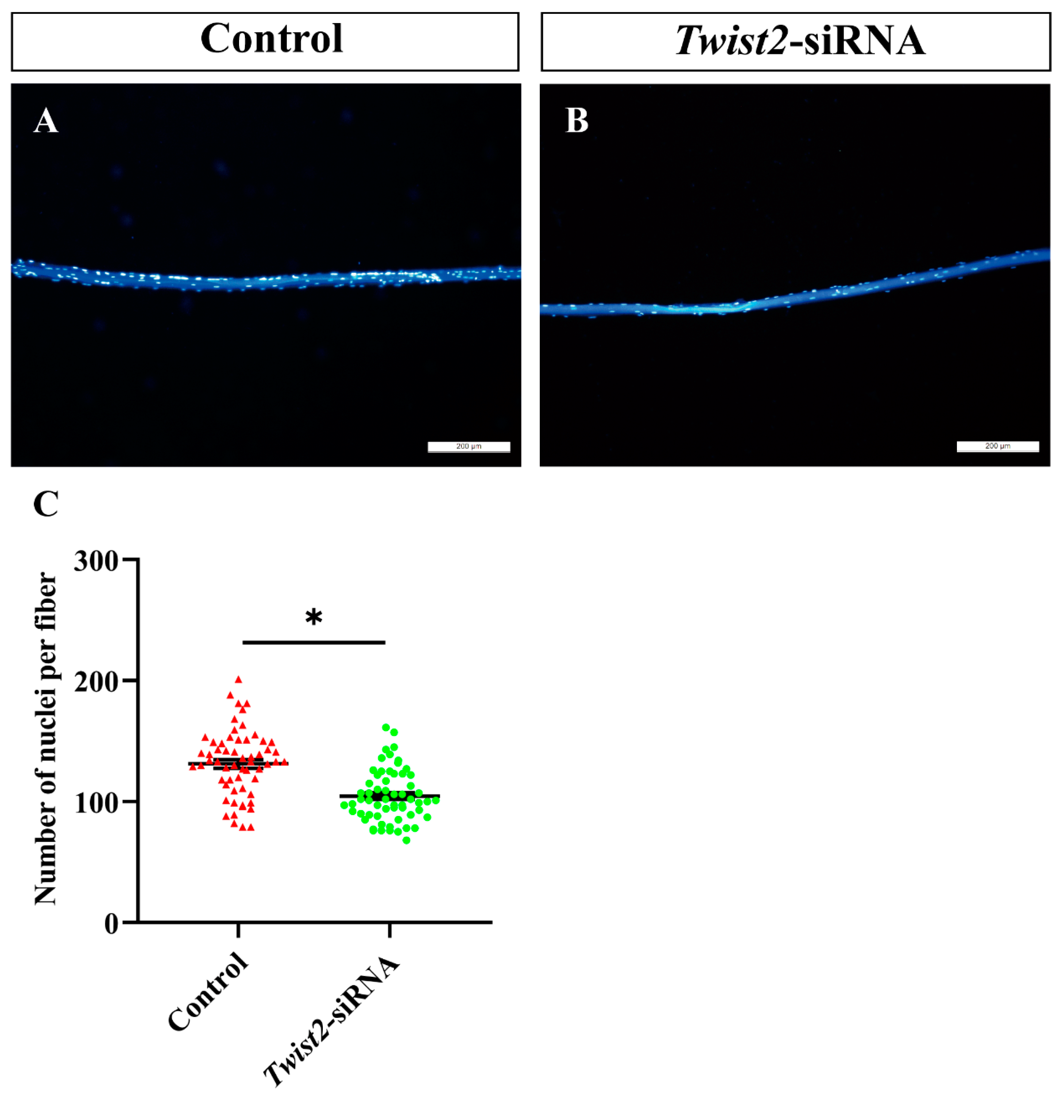
3.5. The Analysis of Nuclei Number in Single Muscle Fibers
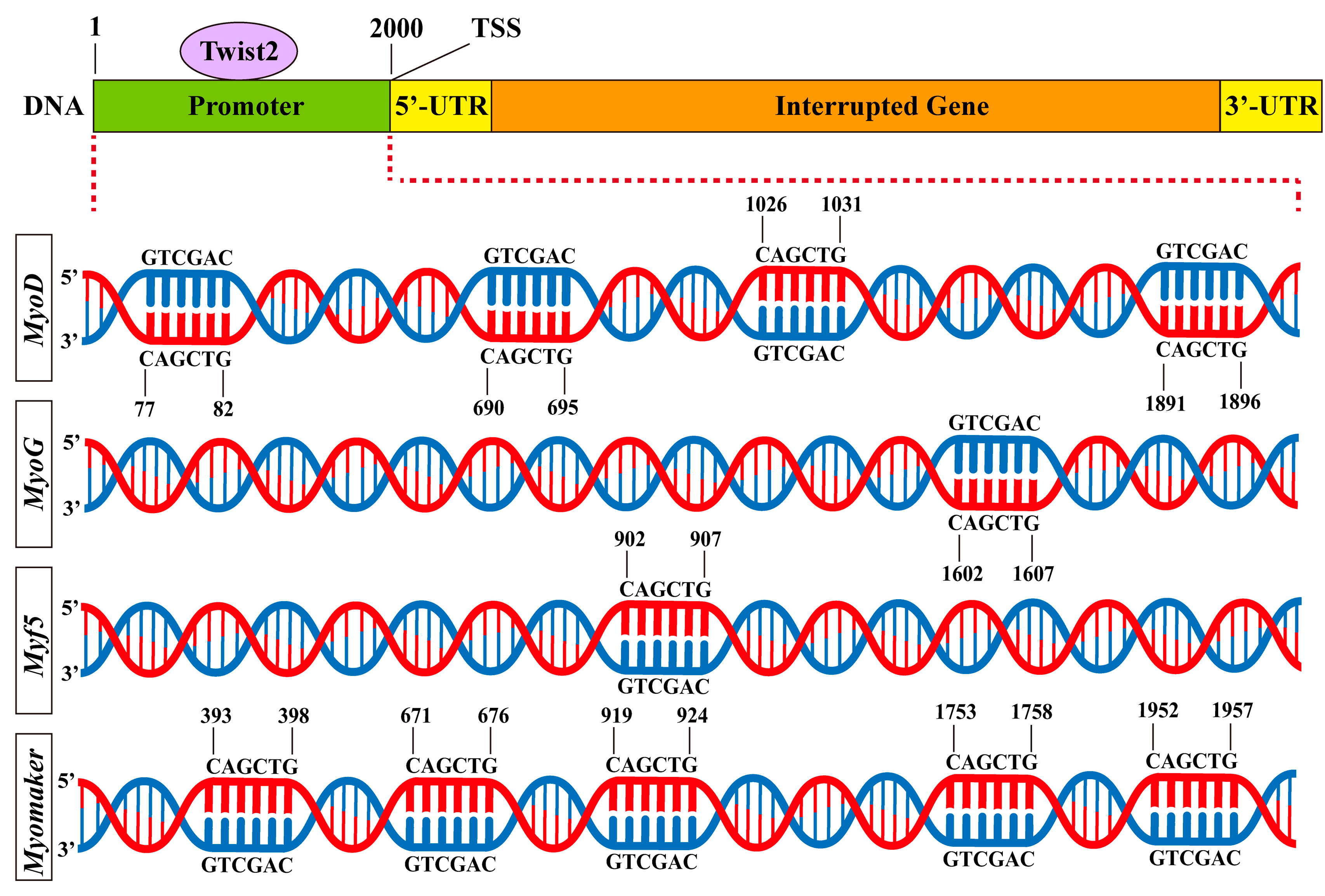
3.6. Prediction of Twist2 Interactions with Target Gene Promoters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goldberg, A.L.; Chang, T.W. Regulation and significance of amino acid metabolism in skeletal muscle. Fed. Proc. 1978, 37, 2301–2307. [Google Scholar] [CrossRef]
- Romagnoli, C.; Pampaloni, B.; Brandi, M.L. Muscle endocrinology and its relation with nutrition. Aging Clin. Exp. Res. 2019, 31, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Karagounis, L.G.; Hawley, J.A. Skeletal muscle: Increasing the size of the locomotor cell. Int. J. Biochem. Cell Biol. 2010, 42, 1376–1379. [Google Scholar] [CrossRef] [PubMed]
- Verri, T.; Terova, G.; Dabrowski, K.; Saroglia, M. Peptide transport and animal growth: The fish paradigm. Biol. Lett. 2011, 7, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Blob, R.W.; Baumann, T.; Diamond, K.M.; Young, V.K.H.; Schoenfuss, H.L. Functional correlations of axial muscle fiber type proportions in the waterfall-climbing Hawaiian stream fish Sicyopterus stimpsoni. J. Anat. 2020, 236, 1160–1166. [Google Scholar] [CrossRef]
- Palstra, A.P.; Planas, J.V. Fish under exercise. Fish Physiol. Biochem. 2011, 37, 259–272. [Google Scholar] [CrossRef]
- Sänger, A.M.; Stoiber, W. Muscle Fiber Diversity and Plasticity. Fish Physiol. 2001, 18, 187–250. [Google Scholar] [CrossRef]
- Yang, S.Y.; Liu, Z.; Yan, Z.Z.; Zhao, Z.M.; Zhang, C.Y.; Gong, Q.; Du, X.G.; Wu, J.Y.; Feng, Y.; Du, J. Improvement of skeletal muscle growth by GH/IGF growth-axis contributes to growth performance in commercial fleshy sturgeon. Aquaculture 2021, 543, 736929. [Google Scholar] [CrossRef]
- Nie, Z.L.; Zhao, N.H.; Zhao, H.; Fu, Z.Y.; Ma, Z.H.; Wei, J. Cloning, expression analysis and SNP screening of the kiss1 gene in male Schizothorax biddulphi. Genes 2023, 14, 862. [Google Scholar] [CrossRef]
- Biga, P.R.; Meyer, J. Growth hormone differentially regulates growth and growth-related gene expression in closely related fish species. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009, 154, 465–473. [Google Scholar] [CrossRef]
- Zhu, X.; Meng, Y.Y.; Zeng, W.; Pan, Y.X.; Chu, W.Y.; Zhang, J.S. miR-214 promotes the activation, proliferation and differentiation of skeletal muscle satellite cells by modulating Hedgehog signaling in Chinese perch. Aquaculture 2024, 590, 741074. [Google Scholar] [CrossRef]
- Liu, X.G.; Zeng, S.; Liu, S.; Wang, G.P.; Lai, H.; Zhao, X.P.; Bi, S.; Guo, D.L.; Chen, X.L.; Yi, H.D.; et al. Identifying the Related Genes of Muscle Growth and Exploring the Functions by Compensatory Growth in Mandarin Fish (Siniperca chuatsi). Front. Physiol. 2020, 11, 553563. [Google Scholar] [CrossRef] [PubMed]
- Bentzinger, C.F.; Wang, Y.X.; Rudnicki, M.A. Building muscle: Molecular regulation of myogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008342. [Google Scholar] [CrossRef]
- Chal, J.; Pourquié, O. Making muscle: Skeletal myogenesis in vivo and in vitro. Development 2017, 144, 2104–2122. [Google Scholar] [CrossRef]
- Shea, K.L.; Xiang, W.Y.; LaPorta, V.S.; Licht, J.D.; Keller, C.; Basson, M.A.; Brack, A.S. Sprouty1 regulates reversible quiescence of a self-renewing adult muscle stem cell pool during regeneration. Cell Stem Cell 2010, 6, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Millay, D.P.; Sutherland, L.B.; Bassel-Duby, R.; Olson, E.N. Myomaker is essential for muscle regeneration. Genes Dev. 2014, 28, 1641–1646. [Google Scholar] [CrossRef]
- Rescan, P.Y. Regulation and functions of myogenic regulatory factors in lower vertebrates. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001, 130, 1–12. [Google Scholar] [CrossRef]
- Buckingham, M.; Rigby, P.W.J. Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev. Cell 2014, 28, 225–238. [Google Scholar] [CrossRef]
- Thisse, B.; Messal, M.E.; Perrin-Schmitt, F. The twist gene: Isolation of a Drosophila zygotle gene necessary for the establishment of dorsoventral pattern. Nucleic Acids Res. 1987, 15, 3439–3453. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Meng, T.; Wang, S.Z.; Zhang, H.; Mues, G.; Qin, C.L.; Feng, J.Q.; D'Souza, R.N.; Lu, Y.B. Twist1-and Twist2-haploinsufficiency results in reduced bone formation. PLoS ONE 2014, 9, e99331. [Google Scholar] [CrossRef]
- Liu, N.; Garry, G.A.; Li, S.; Bezprozvannaya, S.; Sanchez-Ortiz, E.; Chen, B.; Shelton, J.M.; Jaichander, P.; Bassel-Duby, R.; Olson, E.N. A Twist2-dependent progenitor cell contributes to adult skeletal muscle. Nat. Cell Biol. 2017, 19, 202–213. [Google Scholar] [CrossRef]
- Zeng, W.; Meng, Y.Y.; Nie, L.T.; Cheng, C.Y.; Gao, Z.X.; Liu, L.S.; Zhu, X.; Chu, W.Y. The Regulatory Role of Myomaker in the Muscle Growth of the Chinese Perch (Siniperca chuatsi). Animals 2024, 14, 2448. [Google Scholar] [CrossRef] [PubMed]
- Millay, D.P.; O'Rourke, J.R.; Sutherland, L.B.; Bezprozvannaya, S.; Shelton, J.M.; Bassel-Duby, R.; Olson, E.N. Myomaker is a membrane activator of myoblast fusion and muscle formation. Nature 2013, 499, 301–305. [Google Scholar] [CrossRef]
- Zhu, X.; Ren, L.; Liu, J.J.; Chen, L.; Cheng, J.; Chu, W.Y.; Zhang, J.S. Transcriptome analysis provides novel insights into the function of PI3K/AKT pathway in maintaining metabolic homeostasis of Chinese perch muscle. Aquacult. Rep. 2021, 21, 100838. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.Y.; Cheng, J.; Zhu, X.; Wu, P.; Chen, L.; Wang, J.; Wu, Y.; Zeng, M.; Zhang, J.S. Identification and characterization of follistatin-related protein-1 Involved in the regulation of Chinese perch skeletal muscle hyperplasia. Curr. Mole. Med. 2016, 16, 596–604. [Google Scholar] [CrossRef]
- Mo, M.J.; Zhang, Z.; Wang, X.T.; Shen, W.J.; Zhang, L.; Lin, S.D. Molecular mechanisms underlying the impact of muscle fiber types on meat quality in livestock and poultry. Front. Vet. Sci. 2023, 10, 1284551. [Google Scholar] [CrossRef]
- Chen, L.; Pan, Y.X.; Cheng, J.; Zhu, X.; Chu, W.Y.; Meng, Y.Y.; Bin, S.Y.; Zhang, J.S. Characterization of myosin heavy chain (MYH) genes and their differential expression in white and red muscles of Chinese perch, Siniperca chuatsi. Int. J. Biol. Macromol. 2023, 250, 125907. [Google Scholar] [CrossRef]
- Miramontes, E.; Mozdziak, P.; Petitte, J.N.; Kulus, M.; Wieczorkiewicz, M.; Kempisty, B. Skeletal muscle and the effects of ammonia toxicity in fish, mammalian, and avian species: A comparative review based on molecular research. Int. J. Mol. Sci. 2020, 21, 4641. [Google Scholar] [CrossRef]
- Liu, J.; Lei, Q.X.; Li, F.W.; Zhou, Y.; Gao, J.B.; Liu, W.; Han, H.X.; Cao, D.G. Dynamic transcriptomic analysis of breast muscle development from the embryonic to post-hatching periods in chickens. Front. Genet. 2020, 10, 1308. [Google Scholar] [CrossRef]
- Wang, Z.J.; Zhang, M.; Li, K.; Chen, Y.F.; Cai, D.F.; Chen, B.; Nie, Q.H. CircMGA depresses myoblast proliferation and promotes myotube formation through miR-144-5p/FAP signal. Animals 2022, 12, 873. [Google Scholar] [CrossRef] [PubMed]
- Thornton, K.J. Impacts of nutrition on the proliferation and differentiation of satellite cells in livestock species. J. Anim. Sci. 2019, 97, 2258–2269. [Google Scholar] [CrossRef]
- Mommsen, T.P. Paradigms of growth in fish. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001, 129, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Furuichi, Y.; Yamamoto, M.; Takahashi, M.; Akimoto, Y.; Ishikawa, T.; Shimizu, T.; Fujimoto, M.; Takada-Watanabe, A.; Hayashi, A.; et al. R3hdml regulates satellite cell proliferation and differentiation. EMBO Rep. 2019, 20, e47957. [Google Scholar] [CrossRef]
- Anderson, C.M.; Hu, J.X.; Barnes, R.M.; Heidt, A.B.; Cornelissen, I.; Black, B.L. Myocyte enhancer factor 2C function in skeletal muscle is required for normal growth and glucose metabolism in mice. Skelet. Muscle 2015, 5, 7. [Google Scholar] [CrossRef]
- Bessarab, D.A.; Chong, S.W.; Srinivas, B.P.; Korzh, V. Six1a is required for the onset of fast muscle differentiation in zebrafish. Dev. Biol. 2008, 323, 216–228. [Google Scholar] [CrossRef] [PubMed]
- van der Meulen, T.; Schipper, H.; van Leeuwen, J.L.; Kranenbarg, S. Effects of decreased muscle activity on developing axial musculature in nicb107 mutant zebrafish (Danio rerio). J. Exp. Biol. 2005, 208, 3675–3687. [Google Scholar] [CrossRef]
- Shirakawa, T.; Toyono, T.; Inoue, A.; Matsubara, T.; Kawamoto, T.; Kokabu, S. Factors regulating or regulated by myogenic regulatory factors in skeletal muscle stem cells. Cells 2022, 11, 1493. [Google Scholar] [CrossRef]
- Hernández-Hernández, J.M.; García-González, E.G.; Brun, C.E.; Rudnicki, M.A. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin. Cell Dev. Biol. 2017, 72, 10–18. [Google Scholar] [CrossRef]
- Brzóska, E.; Przewoźniak, M.; Grabowska, I.; Jańczyk-Ilach, K.; Moraczewski, J. Pax3 and Pax7 expression during myoblast differentiation in vitro and fast and slow muscle regeneration in vivo. Cell Biol. Int. 2009, 33, 483–492. [Google Scholar] [CrossRef]
- Buckingham, M. Skeletal muscle progenitor cells and the role of Pax genes. C. R. Biol. 2007, 330, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Mudry, J.M.; Massart, J.; Szekeres, F.L.; Krook, A. TWIST1 and TWIST2 regulate glycogen storage and inflammatory genes in skeletal muscle. J. Endocrinol. 2015, 226, 303–313. [Google Scholar] [CrossRef]
- Cameron, A.; Wakelin, G.; Gaulton, N.; Young, L.V.; Wotherspoon, S.; Hodson, N.; Lees, M.J.; Moore, D.R.; Johnston, A.P. Identification of underexplored mesenchymal and vascular-related cell populations in human skeletal muscle. Am. J. Physiol. Cell Physiol. 2022, 323, C1586–C1600. [Google Scholar] [CrossRef]
- Li, S.; Karri, D.; Sanchez-Ortiz, E.; Jaichander, P.; Bassel-Duby, R.; Liu, N.; Olson, E.N. Sema3a-Nrp1 signaling mediates fast-twitch myofiber specificity of Tw2+ cells. Dev. Cell 2019, 51, 89–98.e4. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.C.; Rudnicki, M.A. Satellite cells: The architects of skeletal muscle. Curr. Top. Dev. Biol. 2014, 107, 161–181. [Google Scholar] [CrossRef]
- Choi, M.C.; Ryu, S.; Hao, R.; Wang, B.; Kapur, M.H.; Fan, C.M.; Yao, T.P. HDAC 4 promotes Pax7-dependent satellite cell activation and muscle regeneration. EMBO Rep. 2014, 15, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Dilworth, F.J. Differential modulation of cell cycle progression distinguishes members of the myogenic regulatory factor family of transcription factors. FEBS J. 2013, 280, 3991–4003. [Google Scholar] [CrossRef]
- Leikina, E.; Gamage, D.G.; Prasad, V.; Goykhberg, J.; Crowe, M.; Diao, J.; Kozlov, M.M.; Chernomordik, L.V.; Millay, D.P. Myomaker and Myomerger Work Independently to Control Distinct Steps of Membrane Remodeling during Myoblast Fusion. Dev. Cell 2018, 46, 767–780. [Google Scholar] [CrossRef]
- Baylies, M.K.; Bate, M. twist: A myogenic switch in Drosophila. Science 1996, 272, 1481–1484. [Google Scholar] [CrossRef]
- Nakamura, T.; Toita, H.; Yoshimoto, A.; Nishimura, D.; Takagi, T.; Ogawa, T.; Takeya, T.; Ishida-Kitagawa, N. Potential involvement of Twist2 and Erk in the regulation of osteoblastogenesis by HB-EGF-EGFR signaling. Cell Struct. Funct. 2010, 35, 53–61. [Google Scholar] [CrossRef]
- Bernard, F.; Krejci, A.; Housden, B.; Adryan, B.; Bray, S.J. Specificity of Notch pathway activation: Twist controls the transcriptional output in adult muscle progenitors. Development 2010, 137, 2633–2642. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, Y.; Tuerhanjiang, A.; Wang, W.; Wu, Z.; Yuan, M.; Maitituoheti, M.; Wang, S. Twist2 contributes to cisplatin-resistance of ovarian cancer through the AKT/GSK-3β signaling pathway. Oncol. Lett. 2014, 7, 1102–1108. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Genes | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) | Product Size (bp) | TM Value (°C) |
|---|---|---|---|---|
| Twist2 | AAGCTCGCCTCCAGATACAT | GCTGACATAGACCAAGCACC | 152 | 58.6 |
| MyoD | CAACGACGCCTTTGAGACCCTG | GTCCGAATCCCGCTGTAGTGT | 177 | 61.7 |
| MyoG | CGAGACCAACCCTTACTTCTTCCCT | GACTCCCACACAAGCCCATCAT | 137 | 61.3 |
| MRF4 | AGACCAACCCTTATCTTTCAATG | CGGTCTCGGACGGAACATTAT | 153 | 57.8 |
| Myf5 | AGGTCAACCACGCTTTCGAG | GTTTTCCACCTGCTCCCGTA | 140 | 58.5 |
| Pax3 | TGAACCCCGCCATAGGAAAC | TCAGAGGGGAGATGGCGTAG | 105 | 59 |
| Pax7 | AGCCACAACATGACTTCTCC | GTCCACCGTCTTAATGGAGG | 119 | 55 |
| Myomaker | AGTGTTTACGGCACGGCTC | CGTGGTCAACACTCCAAACAT | 105 | 57.6 |
| Rpl13 | CACAAGAAGGAGAAGGCTCGGGT | TTTGGCTCTCTTGGCACGGAT | 120 | 62.1 |
| Genes | Gene ID | Promoter Length |
|---|---|---|
| MyoD | SC7-LG05_06085 | 2 kb |
| MyoG | SC7-LG04_05388 | 2 kb |
| Myf5 | SC7-LG15_19245 | 2 kb |
| Myomaker | SC7-LG07_09935 | 2 kb |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Y.; Zeng, W.; Zhu, X.; Bao, L.; Pan, Y.; Li, H.; Zhang, J.; Liu, L.; Gao, Z.; Du, Z.; et al. The Role of Twist2 in Myoblast Proliferation, Fusion, and Its Impact on Muscle Structure During the Growth of Chinese Perch (Siniperca chuatsi). Animals 2025, 15, 1177. https://doi.org/10.3390/ani15081177
Meng Y, Zeng W, Zhu X, Bao L, Pan Y, Li H, Zhang J, Liu L, Gao Z, Du Z, et al. The Role of Twist2 in Myoblast Proliferation, Fusion, and Its Impact on Muscle Structure During the Growth of Chinese Perch (Siniperca chuatsi). Animals. 2025; 15(8):1177. https://doi.org/10.3390/ani15081177
Chicago/Turabian StyleMeng, Yangyang, Wei Zeng, Xin Zhu, Lingsheng Bao, Yaxiong Pan, Honghui Li, Jianshe Zhang, Lusha Liu, Zexia Gao, Zhenyu Du, and et al. 2025. "The Role of Twist2 in Myoblast Proliferation, Fusion, and Its Impact on Muscle Structure During the Growth of Chinese Perch (Siniperca chuatsi)" Animals 15, no. 8: 1177. https://doi.org/10.3390/ani15081177
APA StyleMeng, Y., Zeng, W., Zhu, X., Bao, L., Pan, Y., Li, H., Zhang, J., Liu, L., Gao, Z., Du, Z., & Chu, W. (2025). The Role of Twist2 in Myoblast Proliferation, Fusion, and Its Impact on Muscle Structure During the Growth of Chinese Perch (Siniperca chuatsi). Animals, 15(8), 1177. https://doi.org/10.3390/ani15081177








