Simple Summary
Twist2 is crucial for the growth of skeletal muscle in organisms. However, the role of the Twist2 gene in the growth of skeletal muscle of economically important fish during the post-embryonic stage remains unclear. This study showed that in the muscle injury model of Chinese perch, Twist2 expression increased during the rapid muscle repair phase. After in vivo knockdown of Twist2 in fast muscle, the expression of myogenic regulatory factors (MRFs) and Myomaker was significantly reduced, while there was no difference in the expression of Pax3 and Pax7. In addition, the diameter of muscle fibers, the number of nuclei in single muscle fibers, and the number of BrdU-labeled myoblasts were all significantly reduced. These findings indicate that Twist2 can promote the proliferation and fusion of myoblasts, thereby facilitating the growth of fast muscle in juvenile Chinese perch.
Abstract
Twist2 plays a pivotal regulatory role in the growth of skeletal muscle across various organisms. Nonetheless, the specific mechanism by which Twist2 governs skeletal muscle function in fish, particularly in the economically significant Chinese perch (Siniperca chuatsi), remains unclear. Within the muscle injury model in Chinese perch, we observed that Twist2 expression was upregulated during the repair phase of fast muscle tissue, exhibiting an expression pattern analogous to that of Pax7. Following the knockdown of Twist2 using Twist2-specific in vivo-siRNA in fast muscle tissues, the expression of myogenic regulatory factors (MRFs) and Myomaker was significantly reduced in the Twist2-siRNA-treated group compared with the control group, whereas no significant differences were observed for Pax3 and Pax7. Furthermore, the diameter of myofibers and the number of nuclei in single myofibers were reduced, and concurrently, the number of BrdU-positive cells (proliferating cells) was significantly reduced in the Twist2-siRNA-treated group. Taken together, this study demonstrates that Twist2 promotes myoblast proliferation and fusion, thereby regulating fast muscle growth in juvenile Chinese perch. These findings provide a clear direction for further exploration of molecular mechanisms underlying skeletal muscle growth in economic fish species.
1. Introduction
Skeletal muscle, as the main structural tissue, is not only involved in the coordination of movement but also plays an important role in regulating its own metabolism and maintaining physiological health [1,2,3]. Regarding the composition of the fish body, skeletal muscle constitutes 40–60% of the fish’s body weight [4]. Based on anatomical and physiological characteristics, skeletal muscle can be classified into glycolytic fast muscle and oxidative slow muscle [5]. Fast muscle represents the primary part of the skeletal muscle in fish. It is situated in the deep layer of the trunk and constitutes approximately 95% of the total muscle mass in fish [6]. In contrast, slow muscle is distributed in a wedge-shaped, superficial strip along the lateral line, parallel to the longitudinal axis of the trunk [7]. The growth efficiency of fish skeletal muscle and its accumulation of muscle content have a significant impact on the economic efficiency of aquaculture [8,9]. Therefore, an in-depth investigation of the molecular regulatory mechanisms of fish fast muscle growth is of crucial significance for the development of fish farming. In vertebrates, muscle growth patterns are categorized into deterministic and non-deterministic growth, whereas in fish, most of the commercially important production fish exhibit non-deterministic growth, except for zebrafish, which exhibits deterministic growth that is more similar to mammalian growth [10]. Non-deterministic growth in fish is typified by the capacity for perpetual muscle growth across the lifespan, which is manifest as an increase in the number of myofibers (hyperplasia) and enlargement of the cross-sectional area of each single myofiber (hypertrophy), occurring both pre- and post-hatching stages [11]. At present, there is limited understanding of post-hatching growth of fast muscle in fish, especially in species with non-deterministic growth patterns such as the Chinese perch. As one of China’s major economic fish species, the Chinese perch is commonly found in freshwater rivers and lakes, and it is renowned for its delicious flesh. Similar to other non-determinate growth fish species, the Chinese perch, being a fast-growing freshwater fish, undergoes throughout its life cycle muscle fiber hyperplasia and hypertrophy [12]. Therefore, investigating the regulatory mechanisms of hyperplasia and hypertrophy in fast muscle fibers of the Chinese perch can not only reveal the molecular basis of its growth patterns but also fully exploit its growth potential to enhance fishery production.
The complex process of myofiber hyperplasia and hypertrophy involves the proliferation and fusion of myoblasts, a process that is regulated by multiple muscle-specific transcription factors and protein families [13,14]. Proliferation is dependent on quiescent satellite cells that become myoblasts through activation and undergo self-renewal or differentiation, whereas Pax7 is a satellite cell marker gene that is essential for the establishment of the satellite cell pool [15]. Myofiber hypertrophy occurs through the fusion of mononuclear myoblasts to form new multinucleated myofibers, and Myomaker is one of the known muscle-specific proteins that are absolutely essential for the fusion of embryonic and adult myoblasts [16]. In addition to this, myogenic regulatory factors (MRFs), Myf5, MyoD, MyoG, and MRF4, have important functional roles in myogenic lineage determination and muscle differentiation, with distinct temporal and spatial features of activation of MRFs, while myoblast differentiation is activated by the regulatory sequence of MRFs [17,18]. However, the molecular mechanisms by which myogenesis regulators regulate muscle growth in fish during the post-hatching stage have not been identified.
Twist was first identified in Drosophila in 1987 and has been shown to have an important role in the establishment of dorsoventral patterns in the early embryo [19]. In vertebrates, the Twist family consists of two members, Twist1 and Twist2 [20], and because Twist2-expressing myogenic progenitor cells are a newly discovered fiber-type-specific stem cell, Twist2 has attracted more attention in the direction of myogenic research than Twist1 [21]. It was reported that Twist2 promotes the formation of type IIb/x fiber types in fast muscles during muscle regeneration and that Twist2+ cells can autonomously initiate myogenesis, fusing with themselves and muscle satellite cells to regenerate and repair damaged tissues. Previously, it was generally believed that muscle regeneration after injury was mainly dependent on Pax+-labeled satellite cells, and the findings of Twist2 fill the gap that there is a group of myogenic progenitor cells specialized in generating specific fiber types and are involved in regulating the regeneration process. However, Twist2 remains a gap in research on skeletal muscle growth in fish. The specific expression pattern of Twist2 in fish during the muscle regeneration process, its function in the regulation of myogenesis-related factors, and its overall impact on muscle growth remain to be elucidated.
To explore the functional role of Twist2 in the myogenesis of Chinese perch (Siniperca chuatsi), here, we revealed the expression profile of Twist2 during muscle regeneration in Chinese perch juveniles. After knocking down Twist2 using Twist2-specific in vivo siRNA, we analyzed the expression changes of genes related to muscle growth, observed the alterations in histological characteristics of muscles, and detected the proliferation and fusion of myoblasts so as to clarify the regulatory mechanism of Twist2 on the fast muscle growth of Chinese perch. This study can enrich the basic theory of skeletal muscle growth in fish during the post-hatching stage, thus providing a scientific basis for the breeding of Chinese perch.
2. Materials and Methods
2.1. Experimental Animals
The Chinese perch (2-month-old, body weight 6 ± 0.5 g) used for the muscle injury model and siRNA interference experiments were obtained from Hunan Fisheries Science Institute (Changsha, China), which all originated from the same parents and belonged to the same breeding lot. Chinese perch were maintained in a recirculating aquarium system for scientific collective rearing (water temperature of 25 ± 2 °C, dissolved oxygen level of 7.9 ± 0.3 mg/L, pH range of 7.5 ± 0.3) and fed equal amounts of Cirrhinus mrigala larvae twice a day (9:00 a.m. and 6:00 p.m.). This study was approved by the Animal Care and Use Committee of Changsha University (approval number 2024043), and the animal experimentation procedures were in accordance with the ARRIVE guidelines.
2.2. Design and Preparation of siRNAs
Obtaining the complete CDS sequence of Chinese perch Twist2 from the Chinese perch genome database (http://genomes.igb-berlin.de/Siniperca/ (accessed on 21 May 2024)). The siRNA (Twist2-specific in vivo siRNA) targeting Twist2 modified with cholesterol (sense 5′-GCUGGACAGACAGCAGAAATT-3′, antisense 5′-UUUCUGCUGUCUGUCCAGCTT-3′) and nonsense siRNA modified with cholesterol (referenced from previous studies) were used as controls [22]. Individual cholesterol molecules were linked to the 3′ end of the passenger strand. Twist2-siRNAs were designed and synthesized by Shanghai GenePharma Co., Ltd (Shanghai, China).
2.3. Muscle Injury Model Construction, Twist2-siRNA In Vivo Inhibition Assay, and Tissue Sampling
Chinese perch used for muscle injury were injected with Cardiotoxin Analog (CTX) (MedChemExpress, Shanghai, China) at the first segment of the dorsal fast muscle at a concentration of 10 μM, with a volume of 20 μL [23]. Sampling was conducted at four specific time points: 0-, 1-, 3-, and 7 days post-injection, and anesthesia was administered to Chinese perch using tricaine mesylate (MS-222, 0.15 mg/mL). Six Chinese perch were collected on ice at each time point, and the sampling site was the first dorsal fast muscle segment. Three Chinese perch from the control group and the experimental group were randomly selected for histological analysis and gene expression profiling, respectively.
In the Twist2-siRNA study, Chinese perch were allocated completely randomly to control and Twist2-siRNA interference groups, with each group consisting of six fish. The interference dose of Twist2-specific in vivo siRNA was determined according to a previous experimental report [22]. The Twist2-specific in vivo siRNA was injected once, and the same dose of nonsensical siRNA was injected into the control group. Furthermore, 7 days later, 10 mg/kg BrdU was injected into the dorsal muscle of Chinese perch at 12 h before sampling. Anesthesia was administered using tricaine mesylate (MS-222, 0.15 mg/mL), and then the Chinese perch dorsal skin was gently separated from the underlying skin at the second dorsal muscle segment using a scalpel and forceps. Regarding the processing and preservation of tissue samples, all the fast muscle samples were divided into two parts. One part was stored at −80 °C for RNA extraction, and the other part was fixed with 4% paraformaldehyde at 4 °C overnight for tissue sectioning and immunofluorescence analysis.
2.4. cDNA Synthesis and Quantitative Real-Time PCR Analysis
Total RNA from all fast muscle samples was extracted utilizing the RNAiso Plus (9109, Takara, Beijing, China), and the extracted RNA’s concentration and integrity were evaluated by ultramicro spectrophotometer (Thermo NanoDrop One, Thermo Fisher, Waltham, MA, USA), and the integrity of the RNA was detected by 1.5% agarose gel electrophoresis. Equal amounts of RNA were reverse transcribed using MonScript TM RTIII Super Mix with dsDNase (Monad, Beijing, China). The qRT-PCR was detected using SYBR Premix Ex TaqTM (TaKaRa, Beijing, China). The qPCR method was applied as previously reported [24]. The relative expression level of the target mRNA was determined by R = 2−∆∆Ct calculation [25]. The qRT-PCR primers were designed by the Primer-BLAST tool on the NCBI webpage, and the primers were synthesized by Beijing Tsingke Biotechnology Co., Ltd. (Beijing, China). The sequences are shown in Table 1.

Table 1.
The primers for RT-qPCR.
2.5. Isolation of Single Muscle Fibers and DAPI Staining
In each of the control and experimental groups, three Chinese perch were completely randomly selected, and 20 single muscle fibers at the dorsal fast muscle were taken from each fish. The isolated single muscle fibers were selected under a stereomicroscope (Stemi508, Zeiss, Jena, Germany) and placed on slides coated with poly-L-lysine. Staining was performed using DAPI (BL105A, Biosharp, Hefei, China) at a concentration of 1 µg/mL, and after 10 min of staining, single muscle fibers on each slide were triple-washed for 5 min each with PBST. Photographs were taken with a fluorescence microscope (DMI3000B, Leica, Wetzlar, Germany).
2.6. Histological Section and Immunofluorescence Analysis
Fast muscle samples were dehydrated with gradient ethanol solution and embedded in paraffin, sliced into 6 µm size using a Leica HistoCore BIOCUT slicer (Leica Biosystems, Milton Keynes, UK), placed on poly-L-lysine, dewaxed, and then stained using a Hematoxylin and Eosin Staining Kit (C0105S, Beyotime, Shanghai, China) in accordance with the manufacturer’s guidelines, with a special note of 10 min for hematoxylin staining and 30 s for eosin staining. For immunofluorescence detection, paraffin sections were deparaffinized and antigenically repaired with Antigen Repair Solution (Sangon Biotech, Shanghai, China). The muscle sections, along with the single muscle fibers, were then subjected to immunofluorescence labeling to identify proliferating cells within the muscle tissue, following a previously described protocol [26]. Tissue sections and single muscle fibers were incubated overnight with anti-BrdU antibodies: Servicebio (GB12051, Wuhan, China) for tissue sections and Abcam (ab152095, Cambridge, UK) for single muscle fibers. All samples were then incubated with fluorescence-conjugated secondary antibodies: Servicebio (GB21301, Wuhan, China) for tissue sections and Abcam (ab150077, Cambridge, UK) for single muscle fibers. Finally, nuclei in all samples were co-stained with DAPI (BL105A, Biosharp, Hefei, China). The observation of tissue sections was conducted using an inverted fluorescence microscope (DMI3000B, Leica, Germany). The acquired images were processed and analyzed with ImageJ 1.50i software.
2.7. Statistical Analysis
The data were statistically analyzed by IBM SPSS Statistics version 26. Unless otherwise specified, all data are presented as mean ± SEM. The significance of the difference between two groups was analyzed by an independent-sample t-test, and significant differences between the two groups were defined as p < 0.05.
2.8. Prediction of Transcription Factor-Target Gene Binding Sites
The promoters of the genes involved in muscle growth were obtained from the Siniperca chuatsi genome (http://genomes.igb-berlin.de/cgi-bin/hgGateway?db=sinChu7 (accessed on 21 May 2024)), and the IDs for each gene are presented in Table 2. Then, Jaspar was used to predict the binding site of Twist2 to the promoter of the target gene (Twist2 motif ID: MA0633.3), and the relative profile score threshold was set at 90%.

Table 2.
Target gene promoter information.
3. Results
3.1. Fast Muscle Fiber Changes During Tissue Repair After Chinese Perch Muscle Injury
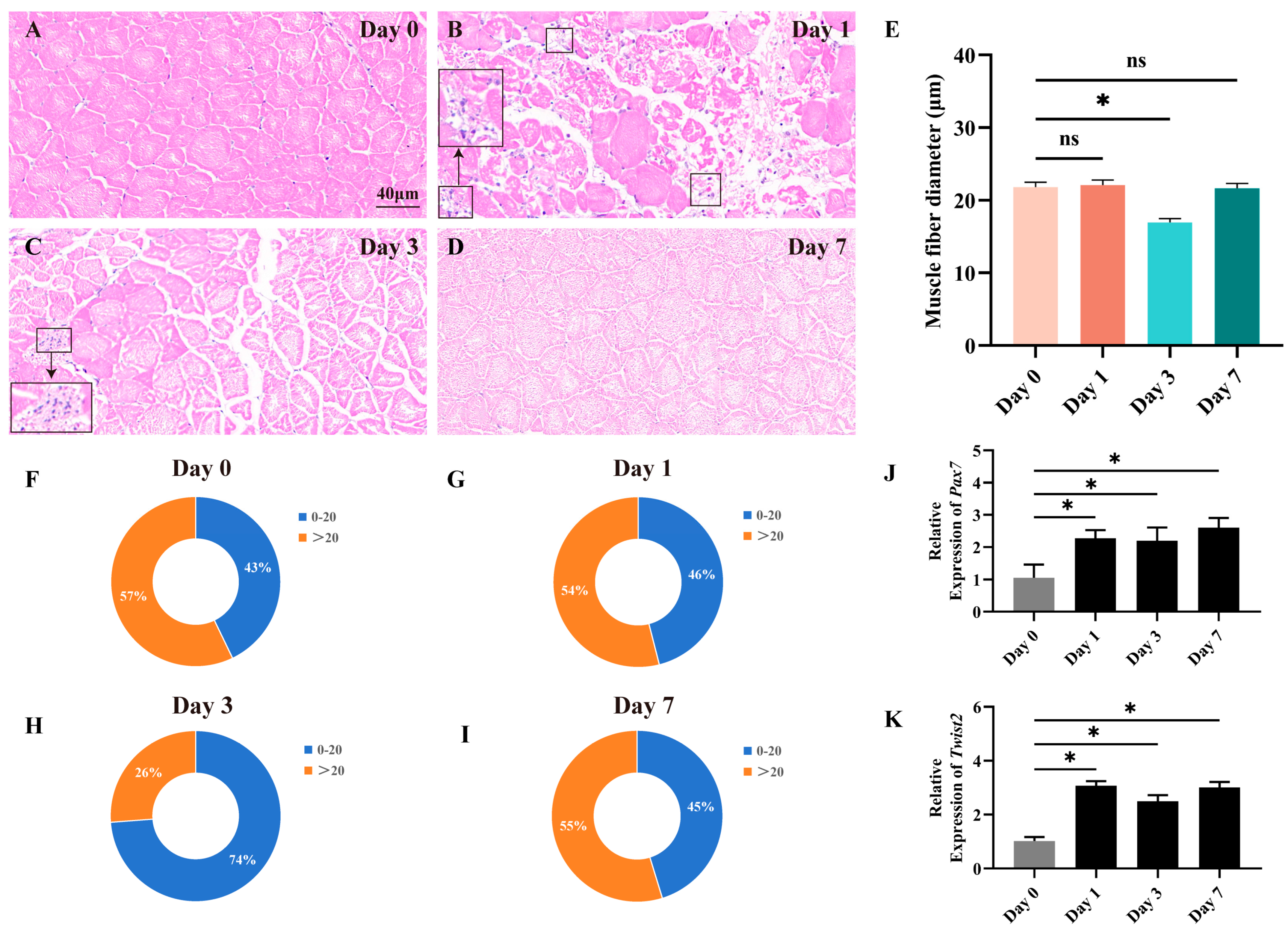
In the constructed muscle injury model, fast muscle tissue repair was assessed by histological methods on days 0, 1, 3, and 7. The results showed that injection of CTX rapidly caused muscle injury. In the progression of fast muscle injury, distinct morphological changes were observed (Figure 1A–D). On the 1st day, muscle fiber structures became disordered, with portions of the tissue fragmented. Concurrently, inflammatory cells infiltrated, marking the onset of early-stage injury. By the 3rd day, new muscle fibers began to emerge. As the injury advanced to the 7th day, muscle fibers regained regular alignment, nearing normalcy. The newly formed muscle fibers matured, leading to the restoration of muscle morphology and structure. Through the analysis of the distribution of myofiber cross-sectional area and diameter, we observed an increase in the proportion of myofibers with a diameter less than 20 μm on day 3, indicating significant changes in myofiber size following the experimental manipulation (Figure 1E–I). The findings indicate that muscle damage in Chinese perch can lead to alterations in myofiber morphology and size, which are subsequently repaired through the hyperplasia of new myofibers. The expression of Pax7 and Twist2 genes during the regeneration and repair of fast muscle tissue was assessed using RT-qPCR. The results revealed a significant upregulation of both Pax7 and Twist2 expression on the first day following CTX injection, with their high expression levels maintained thereafter (Figure 1J–K). The findings revealed that the expression pattern of Twist2 closely mirrored that of Pax7 throughout the process of fast muscle tissue regeneration and repair.
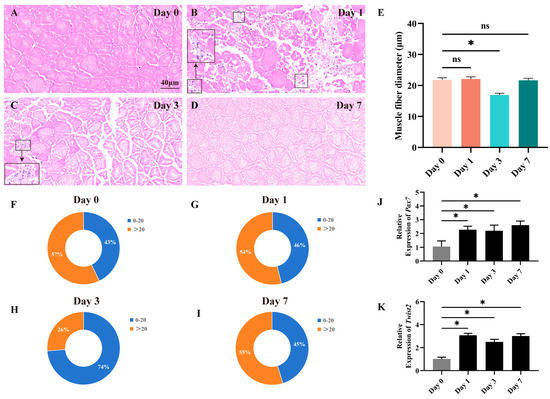
Figure 1.
Histological section analysis of the fast muscle during the damage and repair process. HE staining shows cross-sections of fast muscle on days 0, 1, 3, and 7 of muscle injury (A–D). Inflammatory cells have been marked with black square frames (B,C). Cross-sectional area distribution of muscle fibers on days 0, 1, 3, and 7 after muscle injury (E). Frequency distribution of muscle fiber diameters on days 0, 1, 3, and 7 after muscle injury (F–I). The expression of Pax7 and Twist2 after muscle injury in Chinese perch (J,K). Values in graphs are mean ± SE, n = 3. * Indicates a significant difference between groups (p < 0.05). ns stands for no significant difference (p > 0.05).
3.2. Effect of Inhibition of Twist2 on Gene Expression Related to Muscle Growth
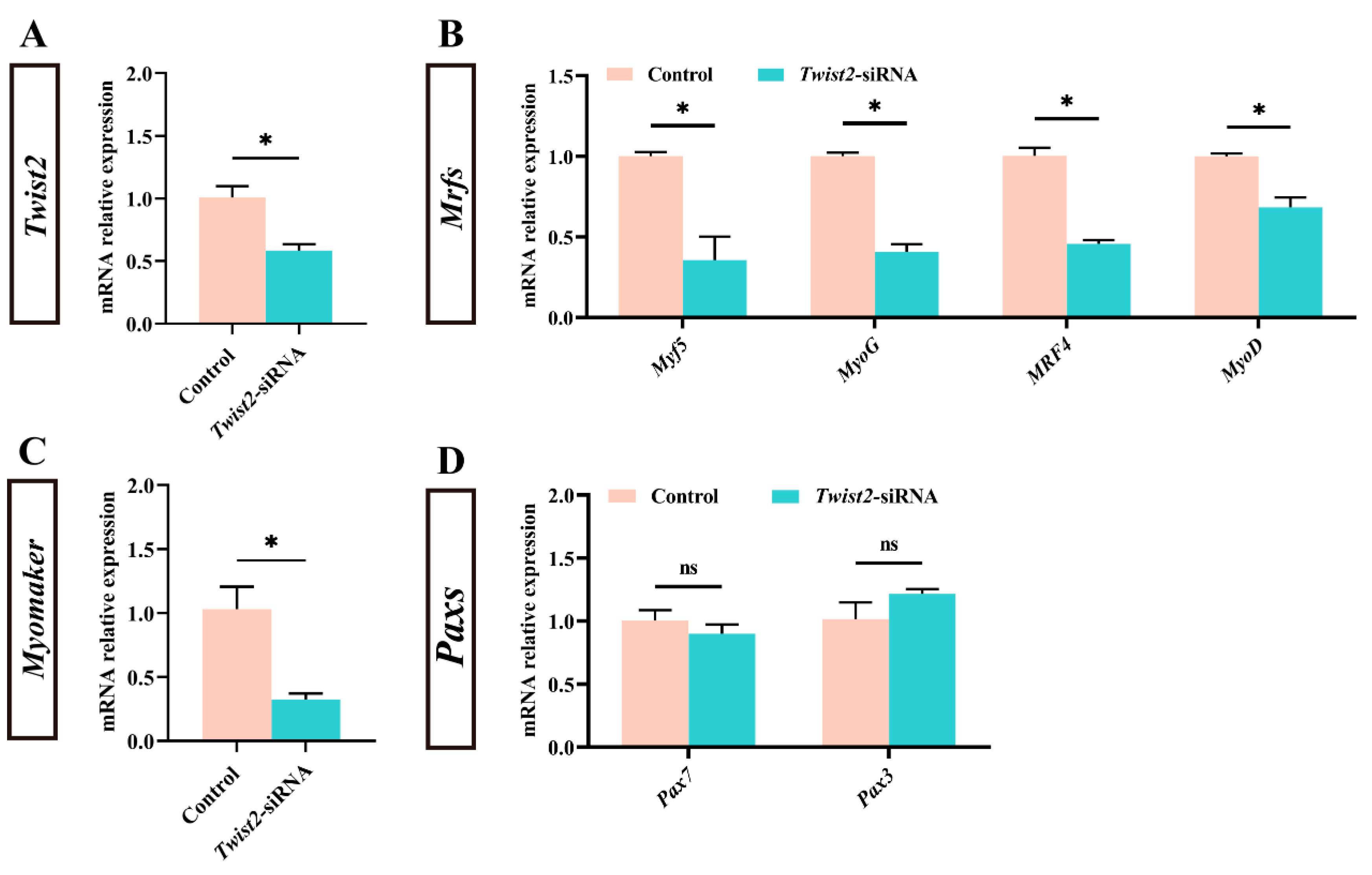
To investigate the effect of Twist2 on the expression of genes related to muscle growth of Chinese perch, the expression of myogenic regulator factors and Pax family genes in muscle was analyzed following the interference with the Twist2 gene. The expression level of Twist2 was significantly reduced in the muscle of the Twist2-siRNA group (p < 0.05) (Figure 2A). In addition, the expression levels of the myogenic regulatory factors MyoD, MyoG, MRF4, and Myf5 also showed a significant decrease in the muscle of Chinese perch, while the expression levels of Pax3 and Pax7 were not significantly changed (Figure 2B–D). The results suggested that interference with Twist2 affected the expression of myogenic regulator factors in Chinese perch muscle.
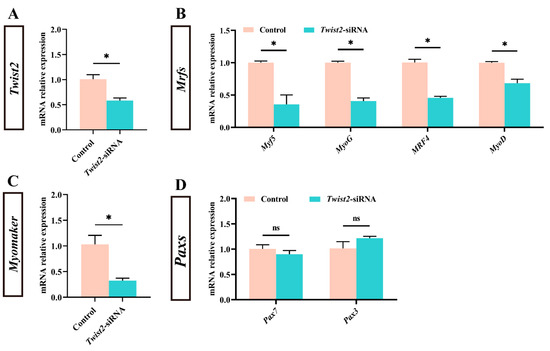
Figure 2.
Effect of Twist2-siRNA injection on the expression of genes related to muscle growth. The relative expression of Twist2 (A). The relative expression of Myf5, MyoG, MRF4 and MyoD (B). The relative expression of Myomaker (C). The relative expression of Pax7 and Pax3 (D). Values are plotted as mean ± SE, n = 6. * Indicates the significant difference between the control and the Twist2-siRNA groups (p < 0.05). ns stands for no significant difference (p > 0.05).
3.3. Histological Analysis of Fast Muscle After Inhibition of Twist2
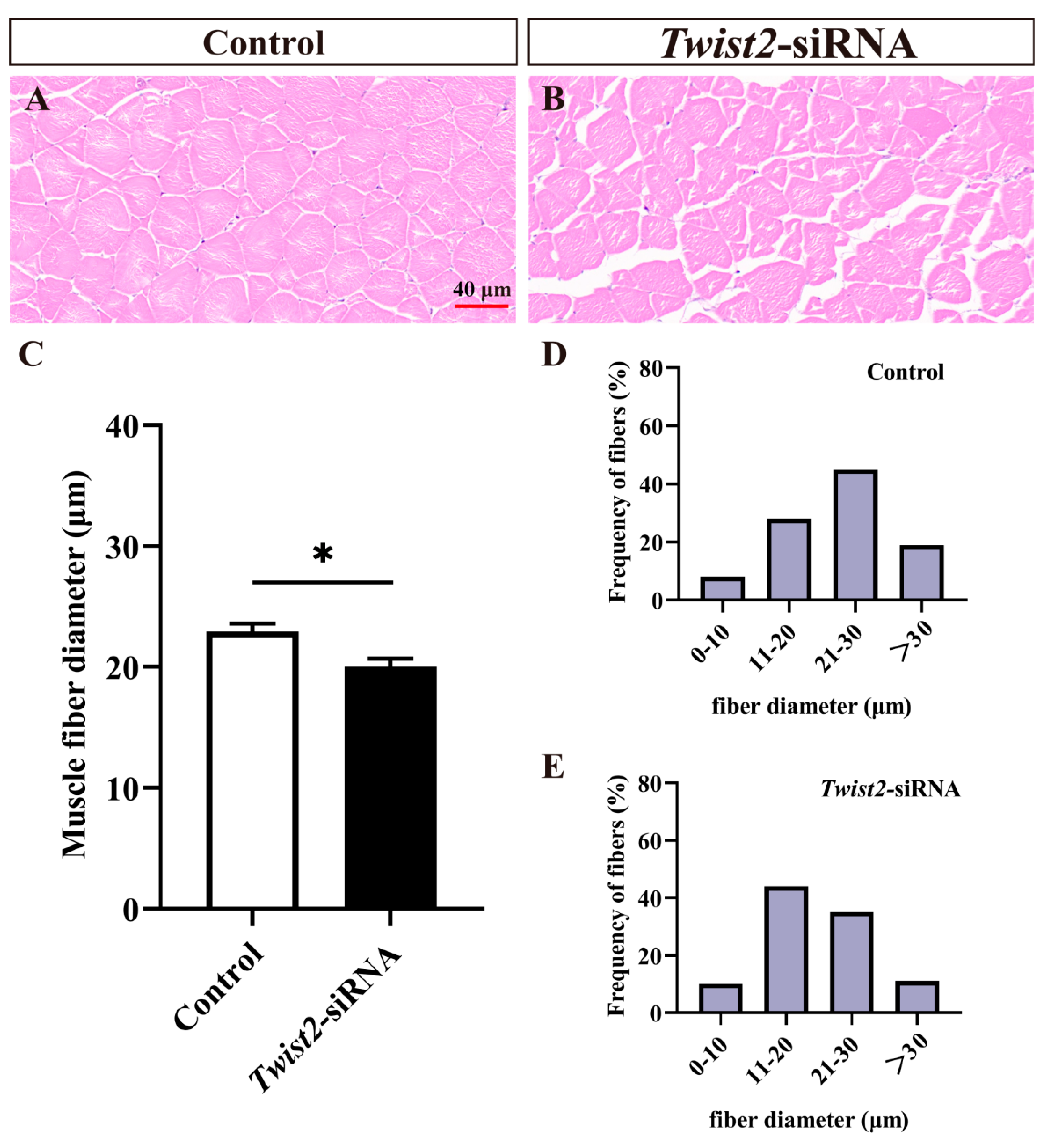
Fast muscle section analysis showed that muscle fiber diameters were significantly reduced in the Twist2-siRNA group in comparison with the control group (Figure 3A–C). The muscle fiber diameters in the control group were mainly distributed in 20–30 µm (Figure 3D), while those of muscle fibers in the Twist2-siRNA group were mainly distributed in 10–20 µm (Figure 3E). The results indicated that Twist2 played an important role in the process of muscle fiber thickening in Chinese perch.
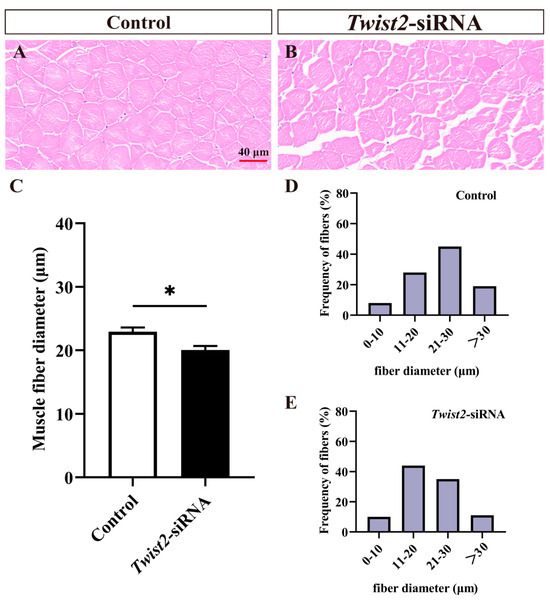
Figure 3.
Histological section analysis of fast muscle in the control and Twist2-siRNA groups. HE staining showed cross-sections of fast muscles in the control and Twist2-siRNA groups (A,B). The muscle fiber diameters in the control and Twist2-siRNA groups (C). Frequency distribution of muscle fiber diameters in the control and Twist2-siRNA groups (D,E). Values in the figures are the mean ± SEM, n = 6. * Indicates the significant difference in expression between the control and Twist2-siRNA groups (p < 0.05).
3.4. Effects of Interfering with Twist2 on the Proliferation of Myoblasts
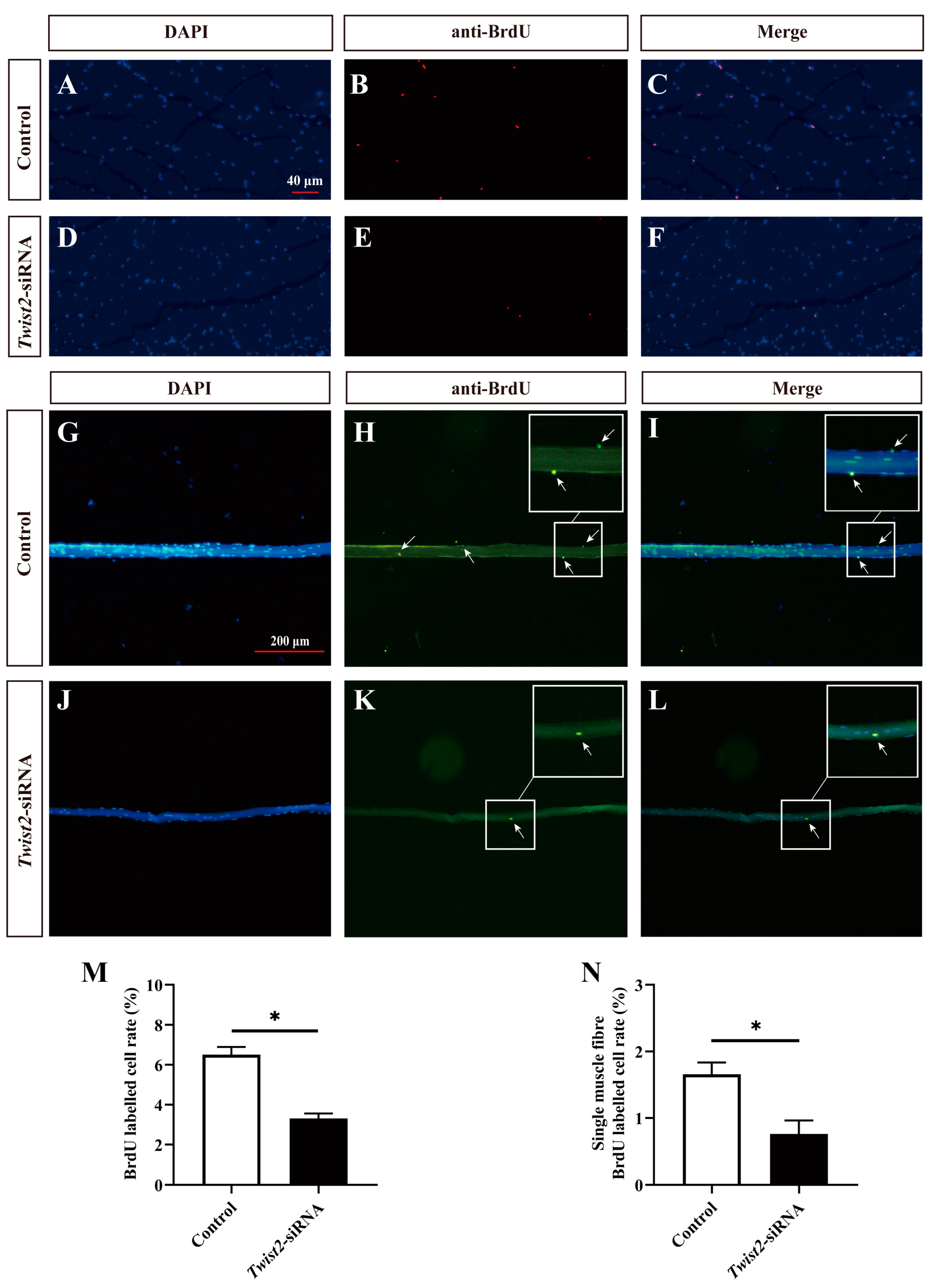
The proliferation of myoblasts was evaluated by immunofluorescence labeling on the cross-section of muscle fibers and single muscle fibers. BrdU-stained positive cells were regarded as proliferating cells. The findings indicated that the number of proliferating myoblasts was significantly decreased in the Twist2-siRNA group after inhibition of Twist2 expression compared with the control group (Figure 4). The results indicated that Twist2 plays an important role in the proliferation of myoblasts in Chinese perch.
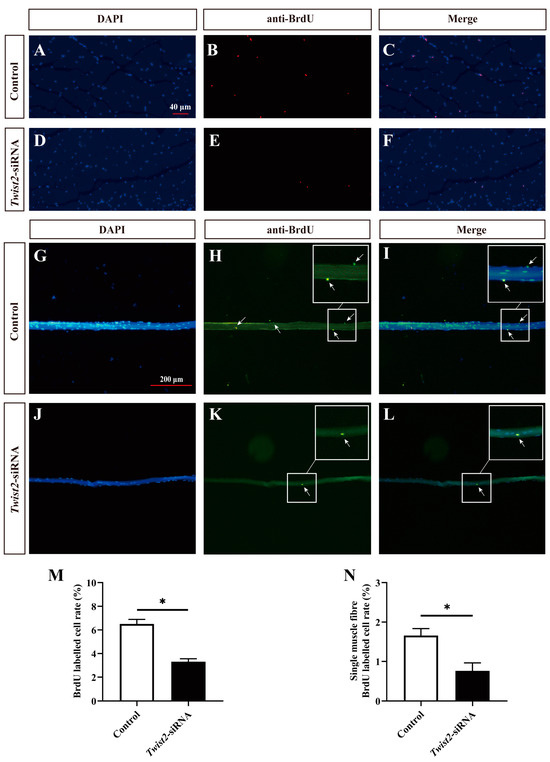
Figure 4.
Effects of interfering with Twist2 expression on myoblast proliferation. Immunofluorescence labeling of the transverse section of muscle fibers in the control and Twist2-siRNA groups (A–F). Immunofluorescent labeling of single muscle fibers in the control and Twist2-siRNA groups (G–L). Nuclei were labeled with DAPI (blue), proliferating cells in cross-sections of muscle fibers were labeled with BrdU antibody (red), and proliferating cells of single muscle fibers were labeled with BrdU antibody (green), the arrows indicate BrdU positive cells. (M) Comparison of proliferating cell frequencies in muscle fiber cross-sections between the control and Twist2-siRNA groups. (N) Comparison of proliferating cell frequencies of single muscle fibers in the control and Twist2-siRNA groups. The scale bars for muscle fiber cross-sections and single muscle fibers were 40 µm and 200 µm, respectively. * Indicates the significant difference in expression between the control and Twist2-siRNA groups (p < 0.05).
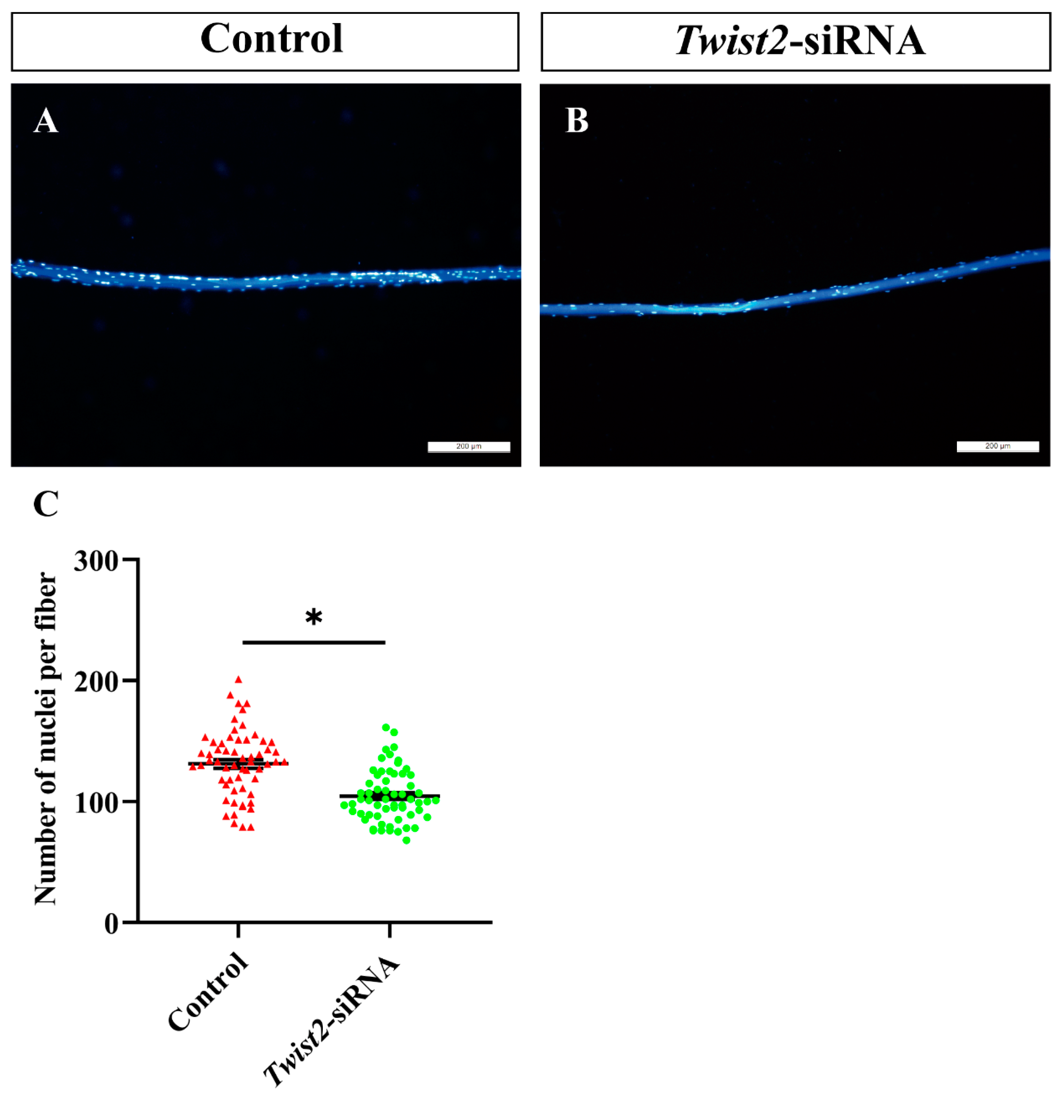
3.5. The Analysis of Nuclei Number in Single Muscle Fibers
To further explore the role of Twist2 in myoblast fusion, the nuclei on single muscle fibers were stained with DAPI. The findings indicated a significant decrease in the number of nuclei on single muscle fibers in the Twist2-siRNA group compared with the control group (Figure 5). This study demonstrated that the inhibition of Twist2 expression impedes myoblast fusion, consequently resulting in a decrease in myofiber diameter.
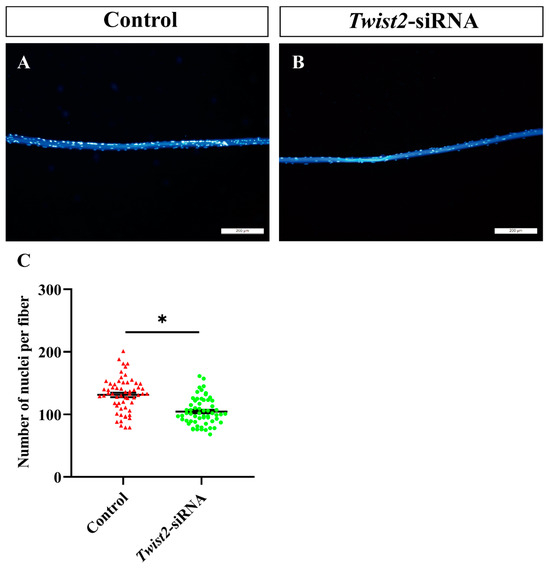
Figure 5.
Analysis of the number of nuclei in single myofiber cells in the control and Twist2-siRNA groups. Single muscle fibers from the control (A) and Twist2-siRNA (B) groups were isolated and stained with DAPI (blue). Statistical analysis of nuclear numbers in muscle fibers from the control and Twist2-siRNA groups (C). Values in the figures are the mean ± SEM, n = 60. Each red triangle and each green circle represent the number of individual nuclei in the control and Twist2-siRNA groups, respectively. * Indicates the significant difference between the control and the Twist2-siRNA groups (p < 0.05).
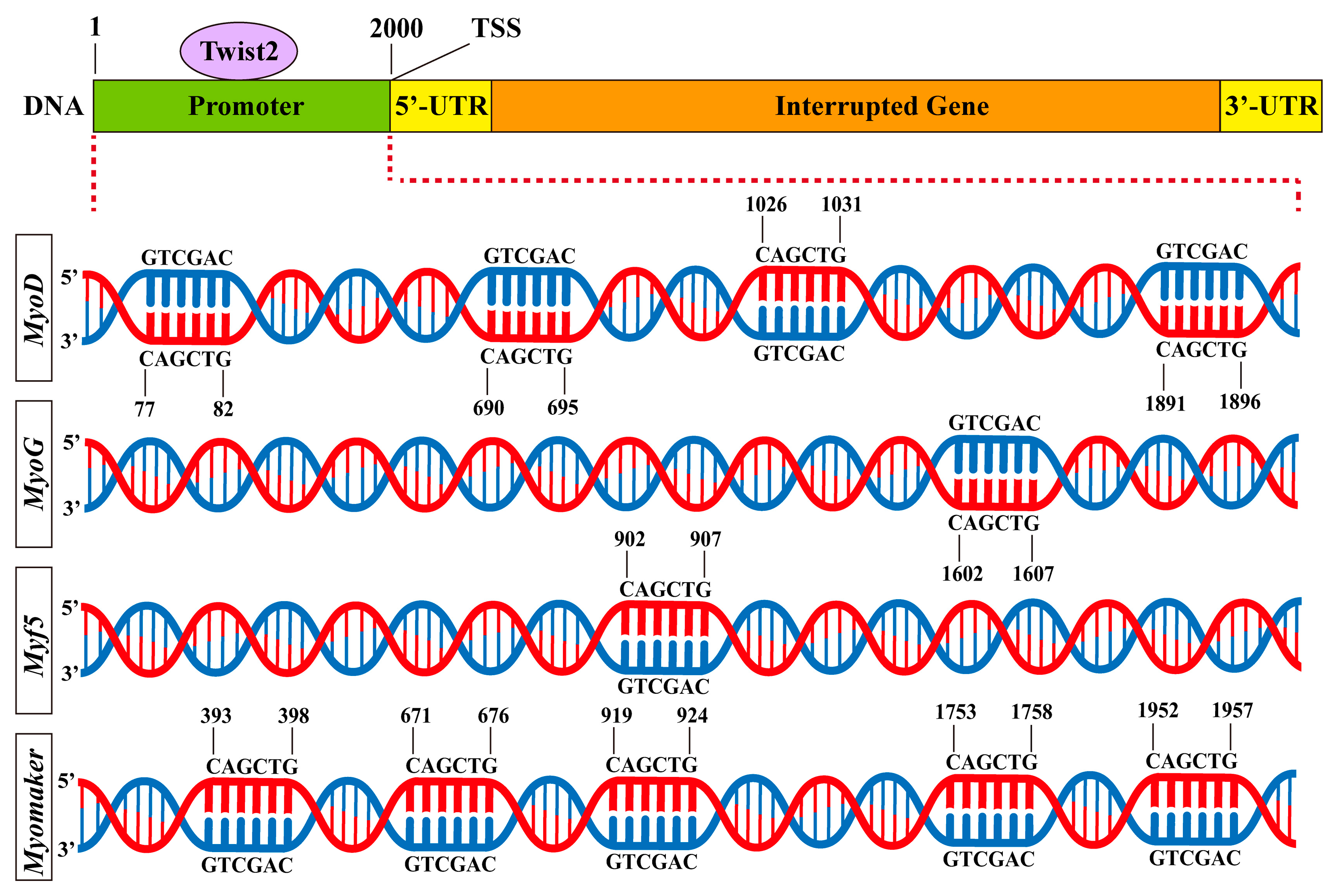
3.6. Prediction of Twist2 Interactions with Target Gene Promoters
To examine the interaction of the transcription factor Twist2 with various muscle growth-related target genes, Jaspar was used to predict the binding of Twist2 to the promoter sites of each target gene. The results showed that Twist2 had different numbers of binding sites with MyoD, Myf5, MyoG, and Myomaker genes (Figure 6). This suggests that Twist2 may regulate the transcription of MyoD, Myf5, MyoG, and Myomaker genes.
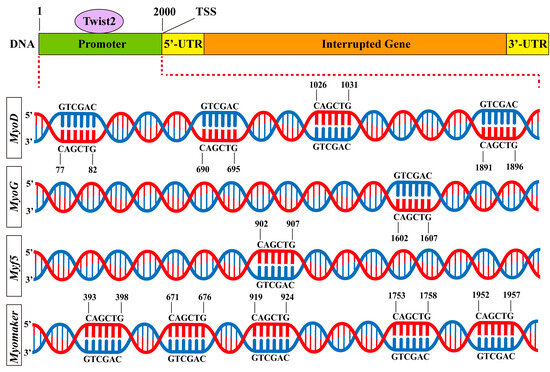
Figure 6.
Predicted binding sites for muscle growth-related genes to Twist2.
4. Discussion
Investigating the regulatory mechanisms behind skeletal muscle growth is crucial for offering essential scientific and technological foundations, as well as theoretical insights, which are vital for genetically enhancing economic animal breeds, improving muscle quality, and boosting overall production efficiency [27,28]. Fish, as one of the economically important animals, exhibit a distinct trajectory of skeletal muscle growth compared to mammals and avians [29]. Unlike mammals and avian, where postnatal muscle growth relies solely on the hypertrophy of pre-existing muscle fibers [30,31,32], numerous fish species experience a combination of myofiber hyperplasia and hypertrophy for muscle growth after hatching [33]. Currently, muscle growth-related studies are mainly focused on model animals such as mice (Mus musculus) and zebrafish (Danio rerio); however, the molecular regulatory mechanisms of muscle growth in economic fish have not been fully identified [34,35,36,37]. Focusing on economically valuable fish species as the primary subject of cultivation and enhancing their muscle growth can lead to accelerated growth rates and improved productivity in aquaculture. In this study, we found that inhibition of Twist2 leads to a decrease in the proliferation and fusion of myoblasts in the fast muscle of juvenile Chinese perch, which suggests that Twist2 is crucial for promoting the proliferation and fusion of myoblasts during the post-embryonic stage of the Chinese perch.
It has been demonstrated that a variety of transcription factors are involved in the regulation of myogenesis, for example, myogenic regulatory factors (MRFs) and myogenic transcription factors (Pax3, Pax7) [38,39,40,41]. Additionally, multiple studies have revealed the role of Twist2 in the regulation of muscle growth [42,43,44]. Previous studies have shown that satellite cells located beneath the muscle basal lamina and expressing Pax7 are considered to be the only muscle-resident cell population with innate myogenic potential [45]. However, Liu et al. in 2017 challenged this view by describing a second population of myogenic progenitor cells in a mouse study that are located within the myofiber mesenchyme and express the transcription factor Twist2 [21]. They found that cells expressing Twist2 specialize in the repair and maintenance of type IIx/b muscle fibers in vivo, and the Twist2+ progenitor cells are highly myogenic in vitro, fusing with both their own and satellite cells [21]. In the present study, we assessed the effect of the regenerative repair phase of muscle-damaged tissues in Chinese perch and found that the proportion of newly proliferated myoblasts increased and the regenerative capacity of muscle fibers was enhanced at 24 h after CTX injection. In addition, the expression pattern of Twist2 showed a high degree of consistency with that of Pax7 in this study. Pax7, a signature gene of satellite cells, stimulates the differentiation of satellite cells to the muscle lineage during muscle regeneration [46]. Although Twist2 labeled a whole new class of myogenic progenitor cells molecularly and anatomically distinct from satellite cells, Twist2+ cells were found to be equally capable of autonomously initiating myogenesis during regeneration [21]. In the mouse model, during muscle regeneration, the population of Twist2+ cells initially decreased promptly; however, the count of Twist2+ cells rebounded swiftly by day 7 following CTX treatment [21]. The above results suggest that in mice, Twist2-positive cells can be seen to play a role in the regenerative repair process, with a reduced number in the repair phase, possibly differentiating into other cells, such as myoblasts, which are then involved in the fusion. In our study, Twist2 and Pax7 expression showed a significant up-regulation on the first day after CTX administration and remained at a high level up to seven days. Based on the above experimental results, it suggests that Twist2 may be involved in the process of muscle tissue regeneration and repair in Chinese perch. In this process, Twist2 may play a similar biological function to that of Pax7. The specific mechanism of the role of Twist2 in the muscle growth of Chinese perch after hatching still needs to be verified by further experiments.
In mice, lineage tracing using tamoxifen-induced insertion of the CreERT2 transgene into the Twist2 locus revealed that Twist2+ mesenchymal stromal cells are able to fuse into myofibers, and ablation of Twist2+ cells throughout the body results in significant atrophy of type IIb fibers [21]. This result suggests that Twist2+ mesenchymal cells are important for the maintenance of type IIb fibers. In this study, in order to investigate the role of Twist2 in fast muscle in juvenile Chinese perch, we employed Twist2-siRNA to interfere with the expression of the Twist2 gene in the fast muscle of juvenile Chinese perch. The results showed that the expression of myogenic regulatory factor family genes (MyoD, MyoG, MRF4, and Myf5) and muscle fusion Myomaker, which are associated with the regulation of muscle growth, were also significantly down-regulated compared to the control group, except for Pax3 and Pax7. This suggests that interfering with Twist2 during the juvenile stage of Chinese perch affects the normal expression pattern of fast muscle growth-regulated genes. MRFs are able to drive the expression of muscle genes in the process of myogenesis and are considered to be major transcription factors up-regulated during myogenesis, which can affect the differentiation of stem cells into myogenic lineage cells [38,47]. Moreover, inhibition of Twist2 leads to a decrease in the number of proliferating myoblasts. Therefore, the inhibition of Twist2 might prevent the differentiation of myogenic stem cells and inhibit the proliferation of myoblasts. Whether the new class of Twist2-labeled myogenic progenitor cells differentiated into myoblasts requires further validation. Myomaker was reported to directly regulate myoblast fusion and muscle formation [23,48]. Predictions of the interaction of Twist2 with the promoters of the target genes indicate that Twist2 can directly regulate MyoD, MyoG, Myf5, and Myomaker. The results suggest that Twist2 may directly regulate the expression of MyoD, MyoG, Myf5, and Myomaker and affect the proliferation, differentiation, and fusion of myoblasts, thereby contributing to the growth of fast muscle fibers in the post-hatching stage of Chinese perch.
We have preliminarily investigated the functional role of Twist2 in the fast muscle growth of Chinese perch during the post-embryonic stage. Compared with common model animals such as mice and Drosophila, we found that Twist2 has both similarities and differences in its regulatory role in certain processes of muscle growth. For example, in mice, in addition to the aforementioned functions of Twist2 in muscle growth, its expression can maintain myogenic progenitor cells in an undifferentiated state, preparing for the initiation of muscle growth [21]. In Drosophila, Twist2 promotes myogenesis, activates muscle development-related genes, and drives mesodermal cells to differentiate into muscle cells [49]. We speculate that the differences in the role of Twist2 in muscle growth among different organisms may be due to variations in growth patterns and species. Moreover, the regulation of Twist2 on cell growth signaling pathways forms a complex network, with its regulatory targets including pathways such as HB-EGF-EGFR, Notch, and AKT/GSK-3β, which collectively influence cell growth [50,51,52]. In summary, our findings provide a theoretical basis for the efficient aquaculture of Chinese perch. Further research will be conducted on the regulatory mechanisms of Twist2 in Chinese perch to support the sustainable development of the aquaculture industry.
5. Conclusions
Overall, our findings suggest that Twist2 plays a crucial role in stimulating the proliferation and fusion of myoblasts and promoting the hypertrophy of myofibers during the juvenile phase of Chinese perch. This research may offer novel insights into uncovering the molecular mechanisms underlying muscle growth in fish and assist in the cultivation of new fish species with superior growth traits.
Author Contributions
Y.M.: investigation, data curation, writing—original draft. W.Z.: investigation, writing—original draft. X.Z.: methodology, writing—review and editing, funding acquisition. L.B.: funding acquisition. Y.P.: validation. H.L.: data curation. J.Z.: methodology, resources. L.L.: formal analysis. Z.G.: resources. Z.D.: resources. W.C.: writing—review and editing, funding acquisition, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the National Natural Science Foundation of China (U21A20263), the Scientific Research Foundation of Hunan Provincial Education Department (24B0801), and the Natural Science Foundation of Hunan Province (2022JJ30633).
Institutional Review Board Statement
This study was approved by the Animal Care and Use Committee of Changsha University (approval number: 2024043) in May 2024.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article. Additional data related to this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
All authors state that they have no relevant competing interests with any individuals or organizations.
References
- Goldberg, A.L.; Chang, T.W. Regulation and significance of amino acid metabolism in skeletal muscle. Fed. Proc. 1978, 37, 2301–2307. [Google Scholar] [CrossRef]
- Romagnoli, C.; Pampaloni, B.; Brandi, M.L. Muscle endocrinology and its relation with nutrition. Aging Clin. Exp. Res. 2019, 31, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Karagounis, L.G.; Hawley, J.A. Skeletal muscle: Increasing the size of the locomotor cell. Int. J. Biochem. Cell Biol. 2010, 42, 1376–1379. [Google Scholar] [CrossRef] [PubMed]
- Verri, T.; Terova, G.; Dabrowski, K.; Saroglia, M. Peptide transport and animal growth: The fish paradigm. Biol. Lett. 2011, 7, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Blob, R.W.; Baumann, T.; Diamond, K.M.; Young, V.K.H.; Schoenfuss, H.L. Functional correlations of axial muscle fiber type proportions in the waterfall-climbing Hawaiian stream fish Sicyopterus stimpsoni. J. Anat. 2020, 236, 1160–1166. [Google Scholar] [CrossRef]
- Palstra, A.P.; Planas, J.V. Fish under exercise. Fish Physiol. Biochem. 2011, 37, 259–272. [Google Scholar] [CrossRef]
- Sänger, A.M.; Stoiber, W. Muscle Fiber Diversity and Plasticity. Fish Physiol. 2001, 18, 187–250. [Google Scholar] [CrossRef]
- Yang, S.Y.; Liu, Z.; Yan, Z.Z.; Zhao, Z.M.; Zhang, C.Y.; Gong, Q.; Du, X.G.; Wu, J.Y.; Feng, Y.; Du, J. Improvement of skeletal muscle growth by GH/IGF growth-axis contributes to growth performance in commercial fleshy sturgeon. Aquaculture 2021, 543, 736929. [Google Scholar] [CrossRef]
- Nie, Z.L.; Zhao, N.H.; Zhao, H.; Fu, Z.Y.; Ma, Z.H.; Wei, J. Cloning, expression analysis and SNP screening of the kiss1 gene in male Schizothorax biddulphi. Genes 2023, 14, 862. [Google Scholar] [CrossRef]
- Biga, P.R.; Meyer, J. Growth hormone differentially regulates growth and growth-related gene expression in closely related fish species. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009, 154, 465–473. [Google Scholar] [CrossRef]
- Zhu, X.; Meng, Y.Y.; Zeng, W.; Pan, Y.X.; Chu, W.Y.; Zhang, J.S. miR-214 promotes the activation, proliferation and differentiation of skeletal muscle satellite cells by modulating Hedgehog signaling in Chinese perch. Aquaculture 2024, 590, 741074. [Google Scholar] [CrossRef]
- Liu, X.G.; Zeng, S.; Liu, S.; Wang, G.P.; Lai, H.; Zhao, X.P.; Bi, S.; Guo, D.L.; Chen, X.L.; Yi, H.D.; et al. Identifying the Related Genes of Muscle Growth and Exploring the Functions by Compensatory Growth in Mandarin Fish (Siniperca chuatsi). Front. Physiol. 2020, 11, 553563. [Google Scholar] [CrossRef] [PubMed]
- Bentzinger, C.F.; Wang, Y.X.; Rudnicki, M.A. Building muscle: Molecular regulation of myogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008342. [Google Scholar] [CrossRef]
- Chal, J.; Pourquié, O. Making muscle: Skeletal myogenesis in vivo and in vitro. Development 2017, 144, 2104–2122. [Google Scholar] [CrossRef]
- Shea, K.L.; Xiang, W.Y.; LaPorta, V.S.; Licht, J.D.; Keller, C.; Basson, M.A.; Brack, A.S. Sprouty1 regulates reversible quiescence of a self-renewing adult muscle stem cell pool during regeneration. Cell Stem Cell 2010, 6, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Millay, D.P.; Sutherland, L.B.; Bassel-Duby, R.; Olson, E.N. Myomaker is essential for muscle regeneration. Genes Dev. 2014, 28, 1641–1646. [Google Scholar] [CrossRef]
- Rescan, P.Y. Regulation and functions of myogenic regulatory factors in lower vertebrates. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001, 130, 1–12. [Google Scholar] [CrossRef]
- Buckingham, M.; Rigby, P.W.J. Gene regulatory networks and transcriptional mechanisms that control myogenesis. Dev. Cell 2014, 28, 225–238. [Google Scholar] [CrossRef]
- Thisse, B.; Messal, M.E.; Perrin-Schmitt, F. The twist gene: Isolation of a Drosophila zygotle gene necessary for the establishment of dorsoventral pattern. Nucleic Acids Res. 1987, 15, 3439–3453. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Meng, T.; Wang, S.Z.; Zhang, H.; Mues, G.; Qin, C.L.; Feng, J.Q.; D'Souza, R.N.; Lu, Y.B. Twist1-and Twist2-haploinsufficiency results in reduced bone formation. PLoS ONE 2014, 9, e99331. [Google Scholar] [CrossRef]
- Liu, N.; Garry, G.A.; Li, S.; Bezprozvannaya, S.; Sanchez-Ortiz, E.; Chen, B.; Shelton, J.M.; Jaichander, P.; Bassel-Duby, R.; Olson, E.N. A Twist2-dependent progenitor cell contributes to adult skeletal muscle. Nat. Cell Biol. 2017, 19, 202–213. [Google Scholar] [CrossRef]
- Zeng, W.; Meng, Y.Y.; Nie, L.T.; Cheng, C.Y.; Gao, Z.X.; Liu, L.S.; Zhu, X.; Chu, W.Y. The Regulatory Role of Myomaker in the Muscle Growth of the Chinese Perch (Siniperca chuatsi). Animals 2024, 14, 2448. [Google Scholar] [CrossRef] [PubMed]
- Millay, D.P.; O'Rourke, J.R.; Sutherland, L.B.; Bezprozvannaya, S.; Shelton, J.M.; Bassel-Duby, R.; Olson, E.N. Myomaker is a membrane activator of myoblast fusion and muscle formation. Nature 2013, 499, 301–305. [Google Scholar] [CrossRef]
- Zhu, X.; Ren, L.; Liu, J.J.; Chen, L.; Cheng, J.; Chu, W.Y.; Zhang, J.S. Transcriptome analysis provides novel insights into the function of PI3K/AKT pathway in maintaining metabolic homeostasis of Chinese perch muscle. Aquacult. Rep. 2021, 21, 100838. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.Y.; Cheng, J.; Zhu, X.; Wu, P.; Chen, L.; Wang, J.; Wu, Y.; Zeng, M.; Zhang, J.S. Identification and characterization of follistatin-related protein-1 Involved in the regulation of Chinese perch skeletal muscle hyperplasia. Curr. Mole. Med. 2016, 16, 596–604. [Google Scholar] [CrossRef]
- Mo, M.J.; Zhang, Z.; Wang, X.T.; Shen, W.J.; Zhang, L.; Lin, S.D. Molecular mechanisms underlying the impact of muscle fiber types on meat quality in livestock and poultry. Front. Vet. Sci. 2023, 10, 1284551. [Google Scholar] [CrossRef]
- Chen, L.; Pan, Y.X.; Cheng, J.; Zhu, X.; Chu, W.Y.; Meng, Y.Y.; Bin, S.Y.; Zhang, J.S. Characterization of myosin heavy chain (MYH) genes and their differential expression in white and red muscles of Chinese perch, Siniperca chuatsi. Int. J. Biol. Macromol. 2023, 250, 125907. [Google Scholar] [CrossRef]
- Miramontes, E.; Mozdziak, P.; Petitte, J.N.; Kulus, M.; Wieczorkiewicz, M.; Kempisty, B. Skeletal muscle and the effects of ammonia toxicity in fish, mammalian, and avian species: A comparative review based on molecular research. Int. J. Mol. Sci. 2020, 21, 4641. [Google Scholar] [CrossRef]
- Liu, J.; Lei, Q.X.; Li, F.W.; Zhou, Y.; Gao, J.B.; Liu, W.; Han, H.X.; Cao, D.G. Dynamic transcriptomic analysis of breast muscle development from the embryonic to post-hatching periods in chickens. Front. Genet. 2020, 10, 1308. [Google Scholar] [CrossRef]
- Wang, Z.J.; Zhang, M.; Li, K.; Chen, Y.F.; Cai, D.F.; Chen, B.; Nie, Q.H. CircMGA depresses myoblast proliferation and promotes myotube formation through miR-144-5p/FAP signal. Animals 2022, 12, 873. [Google Scholar] [CrossRef] [PubMed]
- Thornton, K.J. Impacts of nutrition on the proliferation and differentiation of satellite cells in livestock species. J. Anim. Sci. 2019, 97, 2258–2269. [Google Scholar] [CrossRef]
- Mommsen, T.P. Paradigms of growth in fish. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001, 129, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Furuichi, Y.; Yamamoto, M.; Takahashi, M.; Akimoto, Y.; Ishikawa, T.; Shimizu, T.; Fujimoto, M.; Takada-Watanabe, A.; Hayashi, A.; et al. R3hdml regulates satellite cell proliferation and differentiation. EMBO Rep. 2019, 20, e47957. [Google Scholar] [CrossRef]
- Anderson, C.M.; Hu, J.X.; Barnes, R.M.; Heidt, A.B.; Cornelissen, I.; Black, B.L. Myocyte enhancer factor 2C function in skeletal muscle is required for normal growth and glucose metabolism in mice. Skelet. Muscle 2015, 5, 7. [Google Scholar] [CrossRef]
- Bessarab, D.A.; Chong, S.W.; Srinivas, B.P.; Korzh, V. Six1a is required for the onset of fast muscle differentiation in zebrafish. Dev. Biol. 2008, 323, 216–228. [Google Scholar] [CrossRef] [PubMed]
- van der Meulen, T.; Schipper, H.; van Leeuwen, J.L.; Kranenbarg, S. Effects of decreased muscle activity on developing axial musculature in nicb107 mutant zebrafish (Danio rerio). J. Exp. Biol. 2005, 208, 3675–3687. [Google Scholar] [CrossRef]
- Shirakawa, T.; Toyono, T.; Inoue, A.; Matsubara, T.; Kawamoto, T.; Kokabu, S. Factors regulating or regulated by myogenic regulatory factors in skeletal muscle stem cells. Cells 2022, 11, 1493. [Google Scholar] [CrossRef]
- Hernández-Hernández, J.M.; García-González, E.G.; Brun, C.E.; Rudnicki, M.A. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin. Cell Dev. Biol. 2017, 72, 10–18. [Google Scholar] [CrossRef]
- Brzóska, E.; Przewoźniak, M.; Grabowska, I.; Jańczyk-Ilach, K.; Moraczewski, J. Pax3 and Pax7 expression during myoblast differentiation in vitro and fast and slow muscle regeneration in vivo. Cell Biol. Int. 2009, 33, 483–492. [Google Scholar] [CrossRef]
- Buckingham, M. Skeletal muscle progenitor cells and the role of Pax genes. C. R. Biol. 2007, 330, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Mudry, J.M.; Massart, J.; Szekeres, F.L.; Krook, A. TWIST1 and TWIST2 regulate glycogen storage and inflammatory genes in skeletal muscle. J. Endocrinol. 2015, 226, 303–313. [Google Scholar] [CrossRef]
- Cameron, A.; Wakelin, G.; Gaulton, N.; Young, L.V.; Wotherspoon, S.; Hodson, N.; Lees, M.J.; Moore, D.R.; Johnston, A.P. Identification of underexplored mesenchymal and vascular-related cell populations in human skeletal muscle. Am. J. Physiol. Cell Physiol. 2022, 323, C1586–C1600. [Google Scholar] [CrossRef]
- Li, S.; Karri, D.; Sanchez-Ortiz, E.; Jaichander, P.; Bassel-Duby, R.; Liu, N.; Olson, E.N. Sema3a-Nrp1 signaling mediates fast-twitch myofiber specificity of Tw2+ cells. Dev. Cell 2019, 51, 89–98.e4. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.C.; Rudnicki, M.A. Satellite cells: The architects of skeletal muscle. Curr. Top. Dev. Biol. 2014, 107, 161–181. [Google Scholar] [CrossRef]
- Choi, M.C.; Ryu, S.; Hao, R.; Wang, B.; Kapur, M.H.; Fan, C.M.; Yao, T.P. HDAC 4 promotes Pax7-dependent satellite cell activation and muscle regeneration. EMBO Rep. 2014, 15, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Dilworth, F.J. Differential modulation of cell cycle progression distinguishes members of the myogenic regulatory factor family of transcription factors. FEBS J. 2013, 280, 3991–4003. [Google Scholar] [CrossRef]
- Leikina, E.; Gamage, D.G.; Prasad, V.; Goykhberg, J.; Crowe, M.; Diao, J.; Kozlov, M.M.; Chernomordik, L.V.; Millay, D.P. Myomaker and Myomerger Work Independently to Control Distinct Steps of Membrane Remodeling during Myoblast Fusion. Dev. Cell 2018, 46, 767–780. [Google Scholar] [CrossRef]
- Baylies, M.K.; Bate, M. twist: A myogenic switch in Drosophila. Science 1996, 272, 1481–1484. [Google Scholar] [CrossRef]
- Nakamura, T.; Toita, H.; Yoshimoto, A.; Nishimura, D.; Takagi, T.; Ogawa, T.; Takeya, T.; Ishida-Kitagawa, N. Potential involvement of Twist2 and Erk in the regulation of osteoblastogenesis by HB-EGF-EGFR signaling. Cell Struct. Funct. 2010, 35, 53–61. [Google Scholar] [CrossRef]
- Bernard, F.; Krejci, A.; Housden, B.; Adryan, B.; Bray, S.J. Specificity of Notch pathway activation: Twist controls the transcriptional output in adult muscle progenitors. Development 2010, 137, 2633–2642. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, Y.; Tuerhanjiang, A.; Wang, W.; Wu, Z.; Yuan, M.; Maitituoheti, M.; Wang, S. Twist2 contributes to cisplatin-resistance of ovarian cancer through the AKT/GSK-3β signaling pathway. Oncol. Lett. 2014, 7, 1102–1108. [Google Scholar] [CrossRef] [PubMed][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).