The Role of Energy-Dispersive X-Ray Fluorescence to Predict Mineral Content in Untreated Bovine Plasma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasma Samples
2.2. ED-XRF Analysis
2.3. Statistical Analysis
3. Results and Discussion
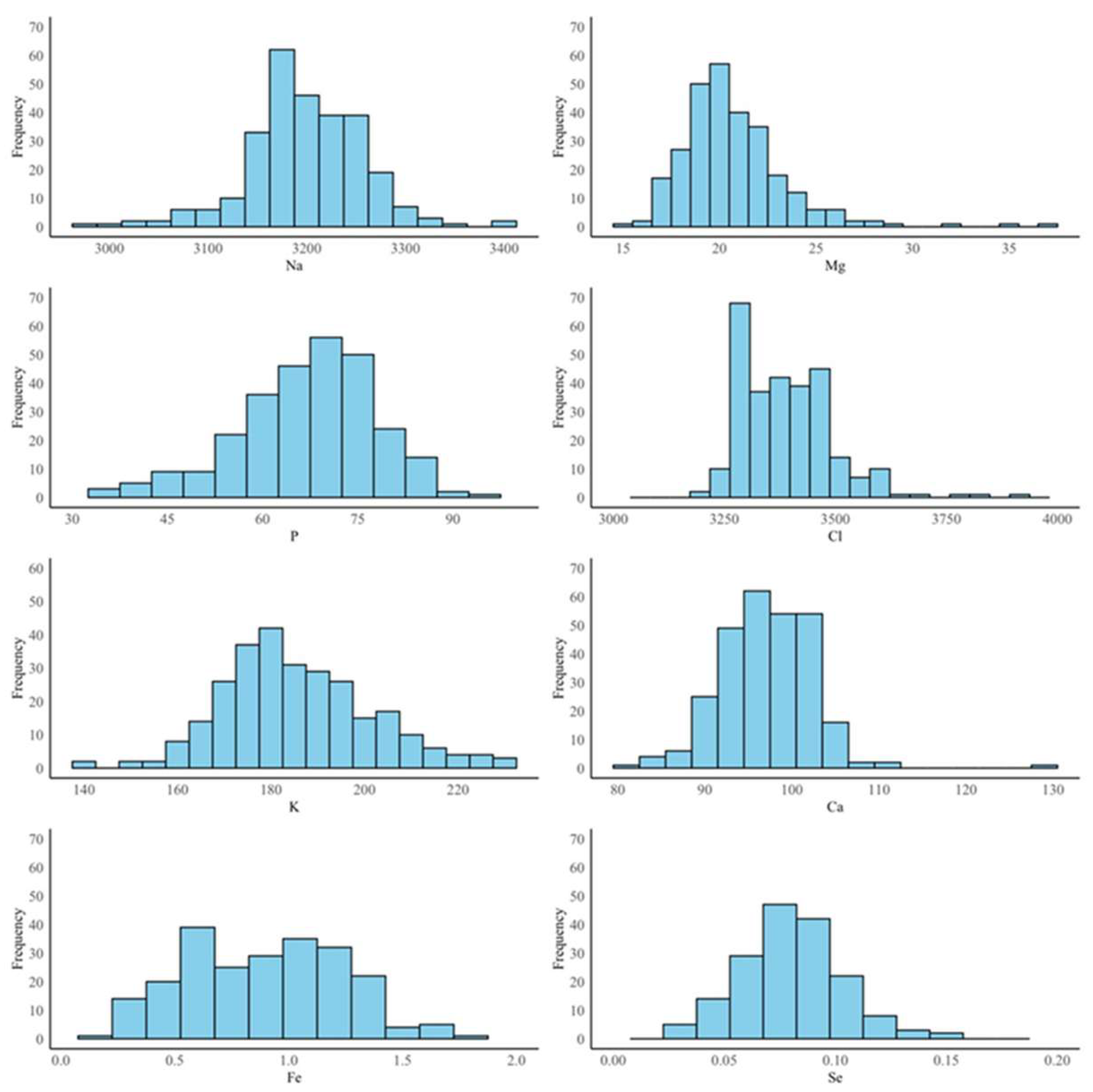
3.1. Descriptive Statistics
3.2. Comparison Between Reference Methods and ED-XRF
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ICP-MS | Inductively Coupled Plasma Mass Spectrometry |
| ED-XRF | Energy-Dispersed X-ray Fluorescence |
| Na | Sodium |
| Mg | Magnesium |
| P | Phosphorus |
| S | Sulfur |
| Cl | Chloride |
| K | Potassium |
| Ca | Calcium |
| Fe | Iron |
| Se | Selenium |
| ISE | Ion-Selective Electrode |
| UV | Ultraviolet |
| R2 | Coefficient of Determination |
| IVDR | In Vitro Diagnostics Regulation |
| CV | Coefficient of Variation |
References
- Galyean, M.L.; Perino, L.J.; Duff, G.C. Interaction of Cattle Health/Immunity and Nutrition. J. Anim. Sci. 1999, 77, 1120–1134. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, L.; Klandorf, H.; Yancey, P. Animal Physiology: From Genes to Organisms; Cengage Learning: Singapore, 2012; ISBN 978-1-133-70951-0. [Google Scholar]
- Harrington, J.M.; Young, D.J.; Essader, A.S.; Sumner, S.J.; Levine, K.E. Analysis of Human Serum and Whole Blood for Mineral Content by ICP-MS and ICP-OES: Development of a Mineralomics Method. Biol. Trace Elem. Res. 2014, 160, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Rawat, K.; Sharma, N.; Singh, V.K. X-Ray Fluorescence and Comparison with Other Analytical Methods (AAS, ICP-AES, LA-ICP-MS, IC, LIBS, SEM-EDS, and XRD). In X-Ray Fluorescence in Biological Sciences; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2022; pp. 1–20. ISBN 978-1-119-64571-9. [Google Scholar]
- Nielsen, S.; Ismail, P. Traditional Methods for MIneral Analysis. In Nielsen’s Food Analysis; Food science text series; Springer Nature: Berlin/Heidelberg, Germany, 2024; pp. 350–351. ISBN 978-3-031-50642-0. [Google Scholar]
- Vogt, C.; Wondergem, C.S.; Weckhuysen, B.M. Ultraviolet-Visible (UV-Vis) Spectroscopy. In Springer Handbook of Advanced Catalyst Characterization; Springer: Berlin/Heidelberg, Germany, 2023; pp. 237–264. [Google Scholar]
- Pozza, M.; De Marchi, M.; Visentin, E.; Niero, G. Effectiveness of Energy Dispersive X-Ray Fluorescence for the Quantification of Mineral Elements in Skim Milk and Whey Powders. J. Dairy Sci. 2024, 107, 10352–10360. [Google Scholar] [CrossRef] [PubMed]
- Visentin, E.; Niero, G.; Cassandro, M.; Penasa, M.; De Marchi, M. Assessment of the ED-XRF Technique to Quantify Mineral Elements in Nonlyophilised Milk and Cheese. Int. J. Dairy Technol. 2023, 76, 102–110. [Google Scholar] [CrossRef]
- Rastegar, F.; Maier, E.A.; Heimburger, R.; Christophe, C.; Ruch, C.; Leroy, M.J. Simultaneous Determination of Trace Elements in Serum by Energy-Dispersive X-Ray Fluorescence Spectrometry. Clin. Chem. 1984, 30, 1300–1303. [Google Scholar] [CrossRef] [PubMed]
- Rautray, T.R.; Vijayan, V.; Hota, P.K. Elemental Analysis of Blood in Oral Cancer. Int. J. PIXE 2002, 12, 41–46. [Google Scholar] [CrossRef]
- Viksna, A.; Selin Lindgren, E.; Kjellmer, I.; Bursa, J. Analysis of Whole Blood and Placenta—A Case Study of Mothers and Their Babies. J. Trace Microprobe Tech. 2002, 20, 553–564. [Google Scholar] [CrossRef]
- Custódio, P.J.; Carvalho, M.L.; Nunes, F.; Pedroso, S.; Campos, A. Direct Analysis of Human Blood (Mothers and Newborns) by Energy Dispersive X-Ray Fluorescence. J. Trace Elem. Med. Biol. 2005, 19, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Magro, S.; Costa, A.; Cavallini, D.; Chiarin, E.; De Marchi, M. Phenotypic Variation of Dairy Cows’ Hematic Metabolites and Feasibility of Non-Invasive Monitoring of the Metabolic Status in the Transition Period. Front. Vet. Sci. 2024, 11, 1437352. [Google Scholar] [CrossRef] [PubMed]
- Santinello, M.; Lora, I.; Villot, C.; Cozzi, G.; Penasa, M.; Chevaux, E.; Martin, B.; Guerra, A.; Righi, F.; De Marchi, M. Metabolic Profile of Charolais Young Bulls Transported over Long-Distance. Prev. Vet. Med. 2024, 231, 106296. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, R.M. Corrections for Matrix Effects in X-Ray Fluorescence Analysis—A Tutorial. Spectrochim. Acta Part B At. Spectrosc. 2006, 61, 759–777. [Google Scholar] [CrossRef]
- Benedet, A.; Franzoi, M.; Penasa, M.; Pellattiero, E.; De Marchi, M. Prediction of Blood Metabolites from Milk Mid-Infrared Spectra in Early-Lactation Cows. J. Dairy Sci. 2019, 102, 11298–11307. [Google Scholar] [CrossRef] [PubMed]
- Goi, A.; Costa, A.; Visentin, G.; De Marchi, M. Mid-Infrared Spectroscopy for Large-Scale Phenotyping of Bovine Colostrum Gross Composition and Immunoglobulin Concentration. J. Dairy Sci. 2023, 106, 6388–6401. [Google Scholar] [CrossRef] [PubMed]
- McAdam, P.A.; O’Dell, G.D. Mineral Profile of Blood Plasma of Lactating Dairy Cows1. J. Dairy Sci. 1982, 65, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Luna, D.; López-Alonso, M.; Cedeño, Y.; Rigueira, L.; Pereira, V.; Miranda, M. Determination of Essential and Toxic Elements in Cattle Blood: Serum vs. Plasma. Animals 2019, 9, 465. [Google Scholar] [CrossRef] [PubMed]
- Herdt, T.H.; Rumbeiha, W.; Braselton, W.E. The Use of Blood Analyses to Evaluate Mineral Status in Livestock. Vet. Clin. N. Am. Food Anim. Pract. 2000, 16, 423–444. [Google Scholar] [CrossRef] [PubMed]
- Herdt, T.H.; Hoff, B. The Use of Blood Analysis to Evaluate Trace Mineral Status in Ruminant Livestock. Vet. Clin. Food Anim. Pract. 2011, 27, 255–283. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, Y.; Dufrasne, I. Selenium in Cattle: A Review. Molecules 2016, 21, 545. [Google Scholar] [CrossRef] [PubMed]
- Byers, H.L.; McHenry, L.J.; Grundl, T.J. XRF Techniques to Quantify Heavy Metals in Vegetables at Low Detection Limits. Food Chem. X 2019, 1, 100001. [Google Scholar] [CrossRef]


| Mineral 2 | Analytical Technique | Sensitivity (mmol/L) | Intra-Assay CV (%) | Inter-Assay CV (%) |
|---|---|---|---|---|
| Na | ISE | 80 | 0.3 | 0.5 |
| Mg | Colorimetric (xylidine Blue) | 0.1 | 1.1 | 1.3 |
| P | UV Complexometric (phosphomolybdate without reduction) | 0.1 | 0.7 | 1.4 |
| K | ISE | 1.5 | 0.5 | 0.7 |
| Cl | ISE | 60 | 0.3 | 0.6 |
| Ca | Colorimetric (o-Cresolphthalein) | 0.2 | 1.0 | 1.6 |
| Fe | Colorimetric (ferrozine without deproteinization) | 0.9 | 1.3 | 1.8 |
| Mineral 3 | Reference | Reference vs. ED-XRF 4 | ||||
|---|---|---|---|---|---|---|
| Mean | SD | CV, % | Range | R2 | SE | |
| Na | 3195.36 | 56.06 | 1.75 | 2965.71–3402.71 | 0.01 | 56.33 |
| Mg | 20.71 | 2.71 | 13.09 | 15.07–37.19 | 0.00 | 2.72 |
| P | 66.93 | 11.61 | 17.35 | 32.21–93.53 | 0.01 | 10.75 |
| Cl | 3390.21 | 102.72 | 3.03 | 3203.85–3934.95 | 0.21 | 83.97 |
| K | 185.76 | 19.39 | 10.44 | 140.76–230.69 | 0.64 | 9.52 |
| Ca | 97.05 | 5.29 | 5.45 | 81.76–128.65 | 0.06 | 5.13 |
| Fe | 0.90 | 0.47 | 52.23 | 0.06–1.90 | 0.03 | 0.35 |
| Se | 0.09 | 0.07 | 80.12 | 0.03–0.15 | 0.09 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martini, D.; Magro, S.; Pozza, M.; Penasa, M.; De Marchi, M. The Role of Energy-Dispersive X-Ray Fluorescence to Predict Mineral Content in Untreated Bovine Plasma. Animals 2025, 15, 1133. https://doi.org/10.3390/ani15081133
Martini D, Magro S, Pozza M, Penasa M, De Marchi M. The Role of Energy-Dispersive X-Ray Fluorescence to Predict Mineral Content in Untreated Bovine Plasma. Animals. 2025; 15(8):1133. https://doi.org/10.3390/ani15081133
Chicago/Turabian StyleMartini, Davide, Silvia Magro, Marta Pozza, Mauro Penasa, and Massimo De Marchi. 2025. "The Role of Energy-Dispersive X-Ray Fluorescence to Predict Mineral Content in Untreated Bovine Plasma" Animals 15, no. 8: 1133. https://doi.org/10.3390/ani15081133
APA StyleMartini, D., Magro, S., Pozza, M., Penasa, M., & De Marchi, M. (2025). The Role of Energy-Dispersive X-Ray Fluorescence to Predict Mineral Content in Untreated Bovine Plasma. Animals, 15(8), 1133. https://doi.org/10.3390/ani15081133







