Bottom Temperature Effect on Growth of Multiple Demersal Fish Species in Flemish Cap, Northwest Atlantic
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Fish Sampling and Aging
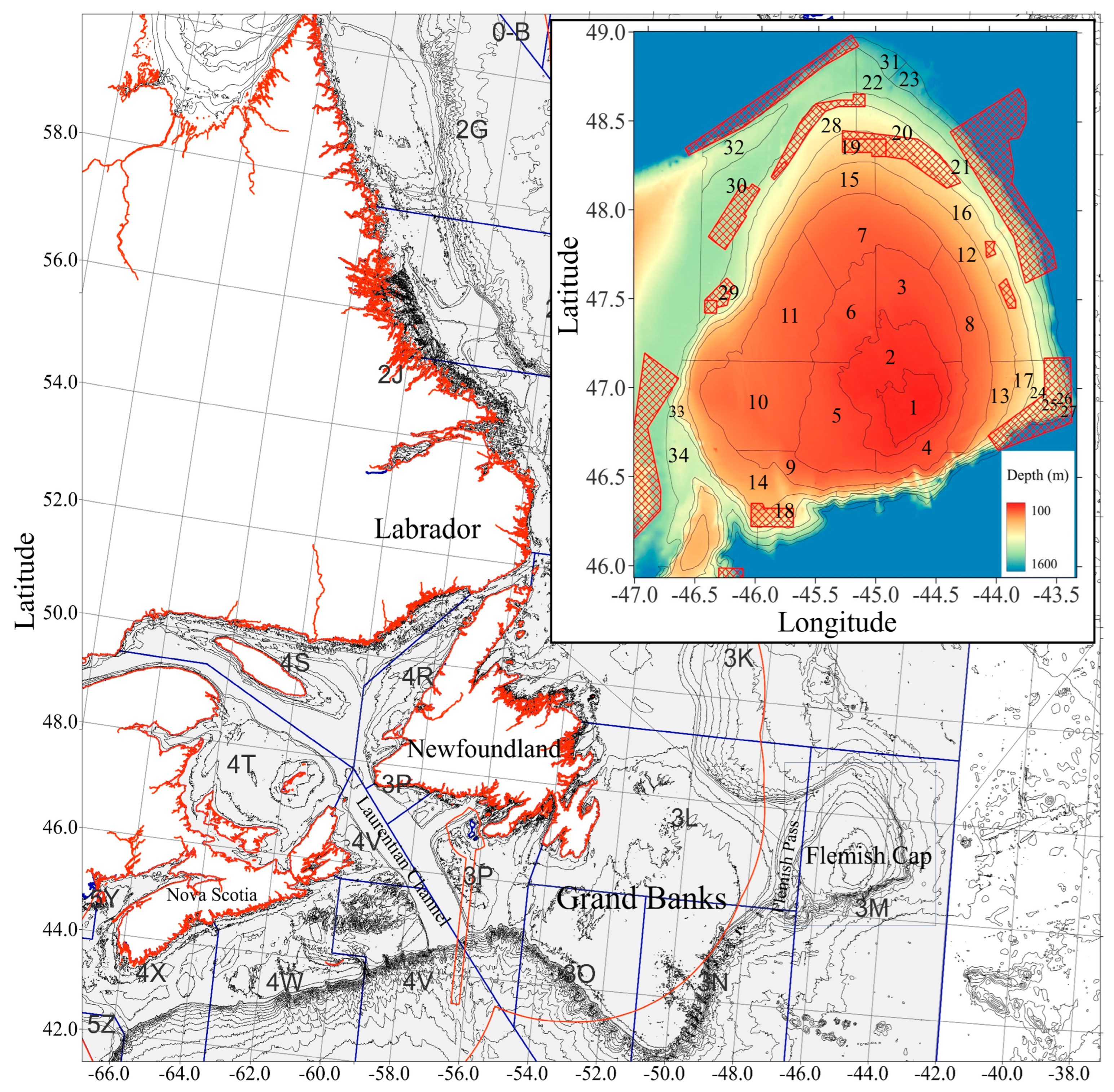
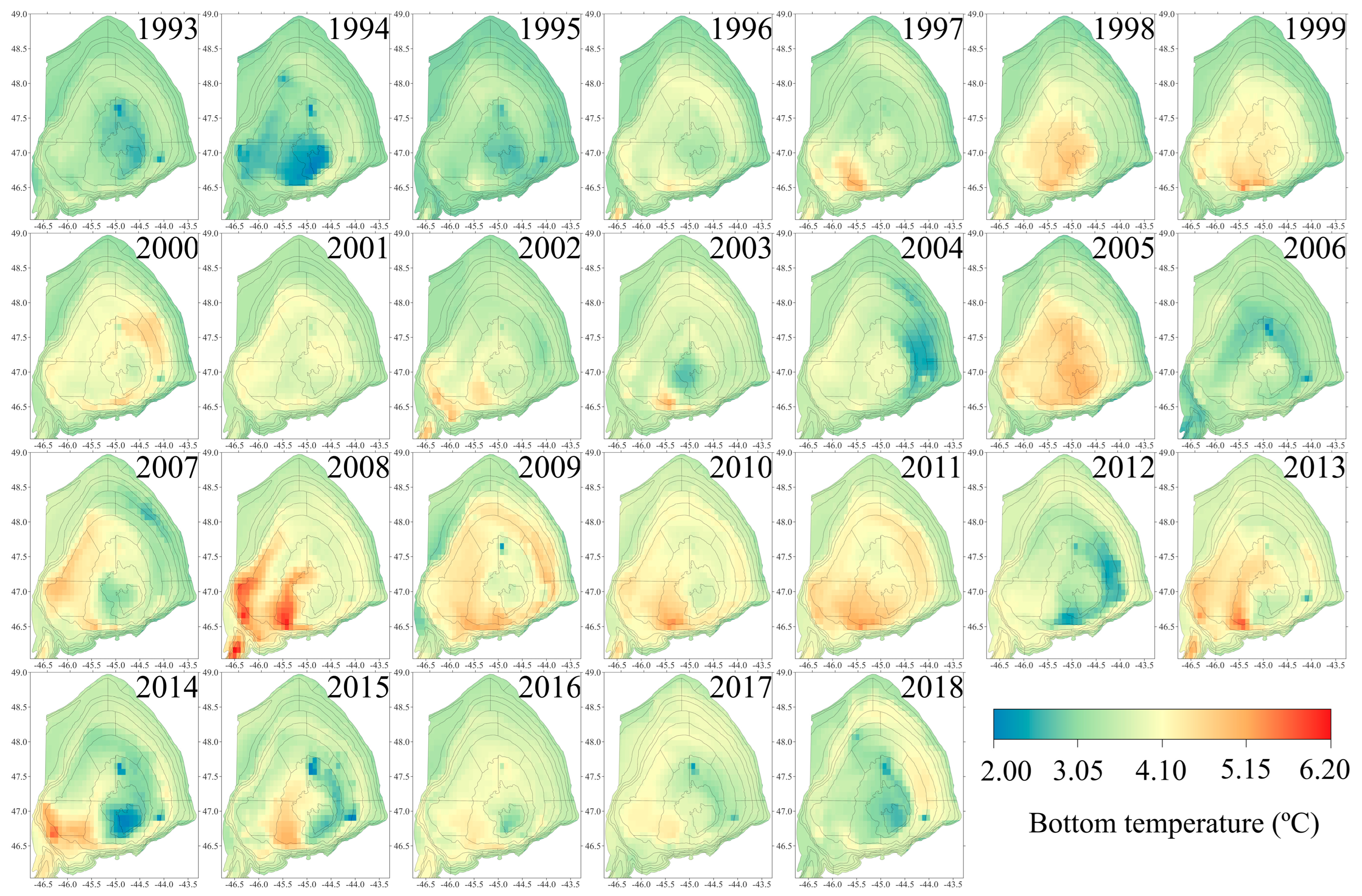
2.2. Temperature Data
2.3. Length-At-Age Models
3. Results
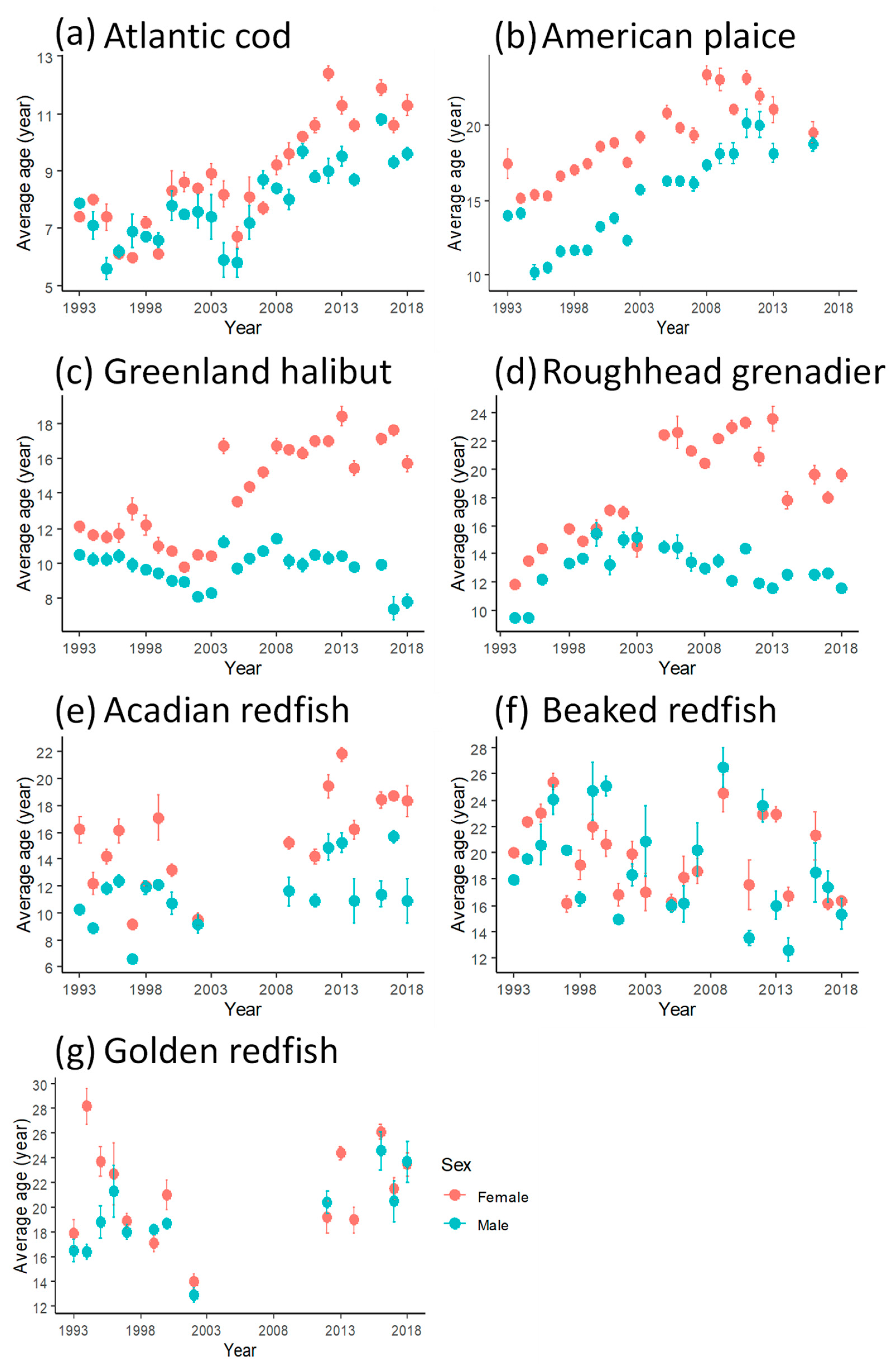
3.1. Maximum Ages
3.2. Temperature Coverage
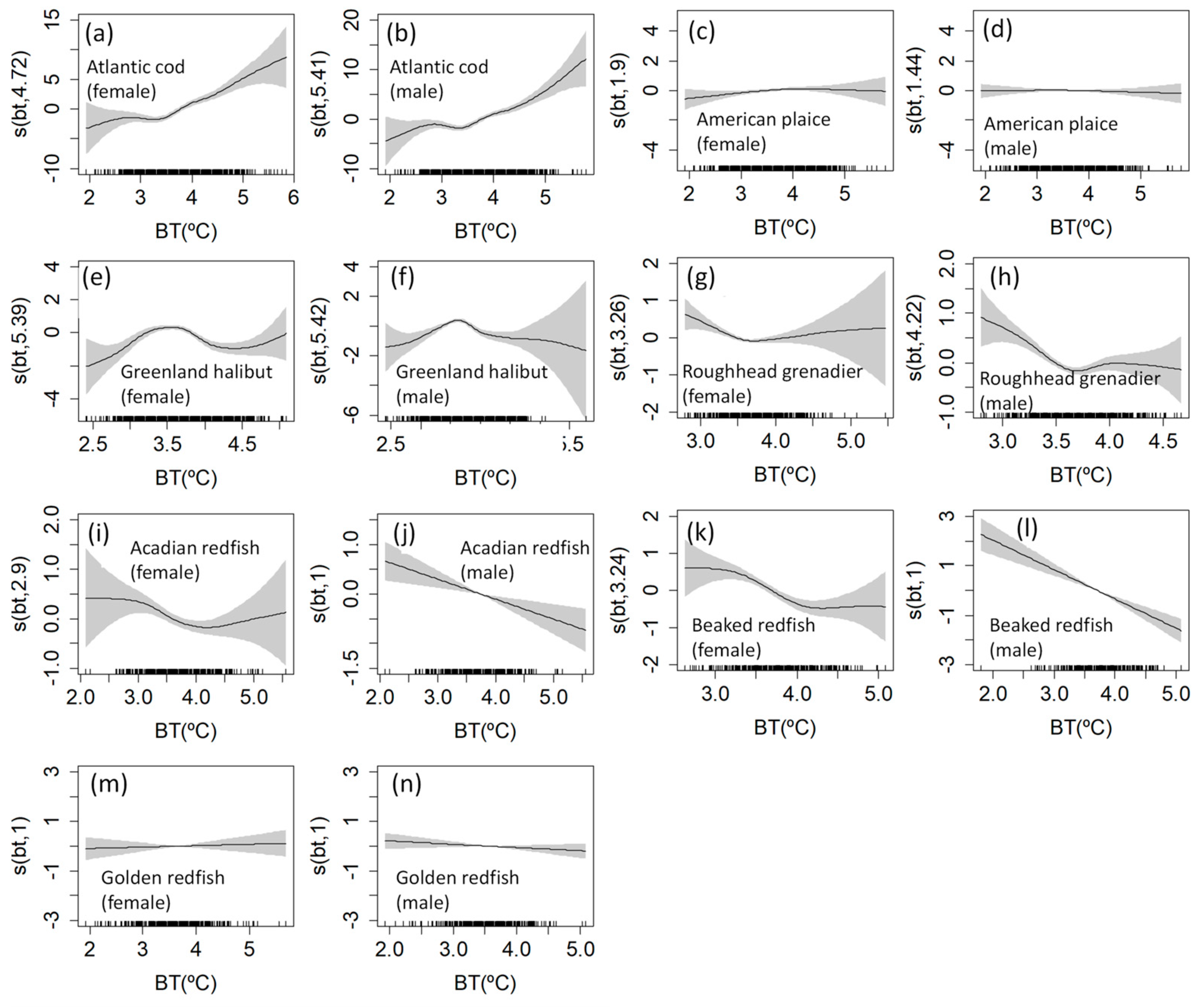
3.3. Length-at-Age GAMM
3.4. VBGF Parameterization
4. Discussion
4.1. Modelling Growth
4.2. Maximum Age and Size Variation
4.3. Temperature Effects on Fish
4.4. Ecological and Fishery Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abraham, J.P.; Baringer, M.; Bindoff, N.L.; Boyer, T.; Cheng, L.J.; Church, J.A.; Conroy, J.L.; Domingues, C.M.; Fasullo, J.T.; Gilson, J.; et al. A Review of Global Ocean Temperature Observations: Implications for Ocean Heat Content Estimates and Climate Change. Rev. Geophys. 2013, 51, 450–483. [Google Scholar] [CrossRef]
- Saba, V.S.; Griffies, S.M.; Anderson, W.G.; Winton, M.; Alexander, M.A.; Delworth, T.L.; Hare, J.A.; Harrison, M.J.; Rosati, A.; Vecchi, G.A.; et al. Enhanced Warming of the Northwest Atlantic Ocean under Climate Change. J. Geophys. Res. Ocean. 2016, 121, 118–132. [Google Scholar] [CrossRef]
- Alexander, M.A.; Shin, S.I.; Scott, J.D.; Curchitser, E.; Stock, C. The Response of the Northwest Atlantic Ocean to Climate Change. J. Clim. 2020, 33, 405–428. [Google Scholar] [CrossRef]
- Swain, D.P.; Benoît, H.P. Extreme Increases in Natural Mortality Prevent Recovery of Collapsed Fish Populations in a Northwest Atlantic Ecosystem. Mar. Ecol. Prog. Ser. 2015, 519, 165–182. [Google Scholar] [CrossRef]
- Ruiz-Díaz, R.; Dominguez-Petit, R.; Saborido-Rey, F. Atlantic Cod Growth History in Flemish Cap Between 1981 and 2016: The Impact of Fishing and Climate on Growth Performance. Front. Mar. Sci. 2022, 9, 876488. [Google Scholar] [CrossRef]
- Gislason, H.; Daan, N.; Rice, J.C.; Pope, J.G. Size, Growth, Temperature and the Natural Mortality of Marine Fish. Fish Fish. 2010, 11, 149–158. [Google Scholar] [CrossRef]
- Killen, S.S. Growth Trajectory Influences Temperature Preference in Fish through an Effect on Metabolic Rate. J. Anim. Ecol. 2014, 83, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Schulte, P.M. The Effects of Temperature on Aerobic Metabolism: Towards a Mechanistic Understanding of the Responses of Ectotherms to a Changing Environment. J. Exp. Biol. 2015, 218, 1856–1866. [Google Scholar] [CrossRef]
- Crozier, L.G.; Hutchings, J.A. Plastic and Evolutionary Responses to Climate Change in Fish. Evol. Appl. 2014, 7, 68–87. [Google Scholar] [CrossRef]
- Tanner, S.E.; Giacomello, E.; Menezes, G.M.; Mirasole, A.; Neves, J.; Sequeira, V.; Vasconcelos, R.P.; Vieira, A.R.; Morrongiello, J.R. Marine Regime Shifts Impact Synchrony of Deep-Sea Fish Growth in the Northeast Atlantic. Oikos 2020, 129, 1781–1794. [Google Scholar] [CrossRef]
- Pörtner, H.O.; Peck, M.A. Climate Change Effects on Fishes and Fisheries: Towards a Cause-and-Effect Understanding. J. Fish Biol. 2010, 77, 1745–1779. [Google Scholar] [CrossRef] [PubMed]
- Doney, S.C.; Ruckelshaus, M.; Emmett Duffy, J.; Barry, J.P.; Chan, F.; English, C.A.; Galindo, H.M.; Grebmeier, J.M.; Hollowed, A.B.; Knowlton, N.; et al. Climate Change Impacts on Marine Ecosystems. Ann. Rev. Mar. Sci. 2012, 4, 11–37. [Google Scholar] [CrossRef] [PubMed]
- Cheung, W.W.L.; Frölicher, T.L.; Lam, V.W.Y.; Oyinlola, M.A.; Reygondeau, G.; Rashid Sumaila, U.; Tai, T.C.; Teh, L.C.L.; Wabnitz, C.C.C. Marine High Temperature Extremes Amplify the Impacts of Climate Change on Fish and Fisheries. Sci. Adv. 2021, 7, eabh0895. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Bellwood, D.R.; Folke, C.; Steneck, R.S.; Wilson, J. New Paradigms for Supporting the Resilience of Marine Ecosystems. Trends Ecol. Evol. 2005, 20, 380–386. [Google Scholar] [CrossRef]
- Huang, M.; Ding, L.; Wang, J.; Ding, C.; Tao, J. The Impacts of Climate Change on Fish Growth: A Summary of Conducted Studies and Current Knowledge. Ecol. Indic. 2021, 121, 106976. [Google Scholar] [CrossRef]
- Sparholt, H.; Bogstad, B.; Christensen, V.; Collie, J.; Van Gemert, R.; Hilborn, R.; Horbowy, J.; Howell, D.; Melnychuk, M.C.; Pedersen, S.A.; et al. Estimating Fmsy from an Ensemble of Data Sources to Account for Density Dependence in Northeast Atlantic Fish Stocks. ICES J. Mar. Sci. 2021, 78, 55–69. [Google Scholar] [CrossRef]
- Lee, W.S.; Monaghan, P.; Metcalfe, N.B. Experimental Demonstration of the Growth Rate-Lifespan Trade-Off. Proc. R. Soc. B Biol. Sci. 2013, 280, 20122370. [Google Scholar] [CrossRef]
- Folkvord, A.; Jørgensen, C.; Korsbrekke, K.; Nash, R.D.M.; Skjæraasen, J.E.; Nilsen, T. Trade-Offs between Growth and Reproduction in Wild Atlantic Cod. Can. J. Fish. Aquat. Sci. 2014, 71, 1106–1112. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, A.; Morgan, J.; Koen-Alonso, M.; Saborido-Rey, F. Disentangling Genetic Change from Phenotypic Response in Reproductive Parameters of Flemish Cap Cod Gadus morhua. Fish. Res. 2013, 138, 62–70. [Google Scholar] [CrossRef]
- Devine, B.M.; Baker, K.D.; Edinger, E.N.; Fisher, J.A.D. Habitat Associations and Assemblage Structure of Demersal Deep-Sea Fishes on the Eastern Flemish Cap and Orphan Seamount. Deep. Sea Res. 1 Oceanogr. Res. Pap. 2020, 157, 103210. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, A.; Koen-Alonso, M.; González-Iglesias, C.; Saborido-Rey, F. Analysis of Common Trends in the Feeding Habits of Main Demersal Fish Species on the Flemish Cap; NAFO: Halifax, NS, Canada, 2011; 30p, Available online: https://www.nafo.int/Portals/0/PDFs/sc/2011/scr11-077.pdf (accessed on 21 November 2023).
- Nogueira, A.; Paz, X.; González-Troncoso, D. Demersal Groundfish Assemblages and Depth-Related Trends on Flemish Cap (NAFO Division 3M): 2004–2013. Fish. Res. 2017, 186, 192–204. [Google Scholar] [CrossRef]
- Höffle, H.; Planque, B. Natural Mortality Estimations for Beaked Redfish (Sebastes Mentella)—A Long-Lived Ovoviviparous Species of the Northeast Arctic. Fish. Res. 2023, 260, 106581. [Google Scholar] [CrossRef]
- Colbourne, E.; Perez-Rodriguez, A.; Cabrero, A.; Gonzalez_Nuevo, G. Ocean Climate Variability on the Flemish Cap in NAFO Subdivision 3M During 2017; NAFO: Halifax, NS, Canada, 2018; 29p, Available online: https://www.nafo.int/Portals/0/PDFs/sc/2018/scr18-010.pdf (accessed on 1 December 2023).
- Drévillon, M.; Lellouche, J.-M.; Régnier, C.; Garric, G.; Bricaud, C.; Hernandez, O.; Bourdallé-Badie, R. Quality Information Document for Global Ocean Reanalysis Products: GLOBAL_REANALYSIS_PHY_001_030; EU Copernicus—Marine Environment Monitoring Service: Prague, Czech Republic, 2021; 52p. [Google Scholar] [CrossRef]
- Saborido-Rey, F.; Garabana, D.; Cerviño, S. Age and Growth of Redfish (Sebastes Marinus, S. Mentella, and S. Fasciatus) on the Flemish Cap (Northwest Atlantic). ICES J. Mar. Sci. 2004, 61, 231–242. [Google Scholar] [CrossRef]
- Murua, H.; Gónzalez, F. A Review on Roughhead Grenadier (Macrourus Berglax) Biology and Population Structure on Flemish Cap (NAFO Division 3M) 1991-2006 Based upon EU Flemish Cap Bottom Survey Data; NAFO: Halifax, NS, Canada, 2007; 21p, Available online: https://www.nafo.int/Portals/0/PDFs/sc/2007/scr07-025.pdf (accessed on 11 April 2024).
- González-Troncoso, D.; Paz, X. Growth Features of American Plaice Hippoglossoides Platessoides in Northwest Atlantic; NAFO: Halifax, NS, Canada, 2008; 21p, Available online: https://www.nafo.int/Portals/0/PDFs/sc/2008/scr08-053.pdf (accessed on 26 February 2024).
- Dwyer, K.S.; Treble, M.A.; Campana, S.E. Age and Growth of Greenland Halibut (Reinhardtius Hippoglossoides) in the Northwest Atlantic: A Changing Perception Based on Bomb Radiocarbon Analyses. Fish. Res. 2016, 179, 342–350. [Google Scholar] [CrossRef]
- Vázquez, A.; Miguel Casas, J.; Alpoim, R. Protocols of the EU Bottom Trawl Survey of Flemish Cap. NAFO Sci. Counc. Stud. 2014, 46, 1–51. [Google Scholar] [CrossRef]
- NAFO. Northwest Atlantic Fisheries Organization Conservation and Enforcement Measures 2023; NAFO: Halifax, NS, Canada, 2023; 204p, Available online: https://www.nafo.int/Portals/0/PDFs/COM/2023/comdoc23-01.pdf (accessed on 21 November 2023).
- González-Troncoso, D.; Rábade, S.; Casas Sánchez, J.M.; Garrido, I.; Fabeiro, M.; Román, E.; Alpoim, R. Results from Bottom Trawl Survey on Flemish Cap of June–July 2022; NAFO SCR Doc.: Halifax, NS, Canada, 2023; 90p, Available online: https://www.nafo.int/Portals/0/PDFs/COM/2023/comdoc23-01REV.pdf (accessed on 19 August 2024).
- Songin, K.; Pierce, G.; Saborido-Rey, F. Spatiotemporal Changes in the Atlantic Cod (Gadus morhua) Stock at Flemish Cap (1993–2019) and Their Relationships with Demersal Communities. J. Northwest Atl. Fish. Sci. 2024, 55, 59–78. [Google Scholar] [CrossRef]
- Wood, S.N. Mgcv: Mixed GAM Computation Vehicle with Automatic Smoothness Estimation. In Generalized Additive Models: An Introduction with R, 2nd ed.; CRC Press: New York, NY, USA, 2023; pp. 1–476. [Google Scholar]
- Chen, Y.; Jackson, D.A.; Harvey, H.H. A Comparison of von Bertalanffy and Polynomial Functions in Modelling Fish Growth Data. Can. J. Fish. Aquat. Sci. 1992, 49, 1228–1235. [Google Scholar] [CrossRef]
- Murua, H. Population Structure, Growth and Reproduction of Roughhead Grenadier on the Flemish Cap and Flemish Pass. J. Fish Biol. 2003, 63, 356–373. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, A.; Howell, D.; Casas, M.; Saborido-Rey, F.; Ávila-De Melo, A. Dynamic of the Flemish Cap Commercial Stocks: Use of a Gadget Multispecies Model to Determine the Relevance and Synergies among Predation, Recruitment, and Fishing. Can. J. Fish. Aquat. Sci. 2017, 74, 582–597. [Google Scholar] [CrossRef]
- Kimura, D.K. Extending the von Bertalanffy Growth Model Using Explanatory Variables. Can. J. Fish. Aquat. Sci. 2008, 65, 1879–1891. [Google Scholar] [CrossRef]
- Brunel, T. Investigation of a Growth Model Incorporating Density-Dependence for the Cod 3M Management Plan Simulations; NAFO: Halifax, NS, Canada, 2019; 12p, Available online: https://www.nafo.int/Portals/0/PDFs/sc/2019/scr19-003.pdf (accessed on 20 December 2023).
- Varkey, D.; Gullage, N.; Regular, P.M.; Kumar, R. Review and Update of the State-Space Management Strategy Evaluation for Greenland Halibut in NAFO Subarea 2 and Divisions 3KLMNO with MseSurv; NAFO: Halifax, NS, Canada, 2023; 67p, Available online: https://www.nafo.int/Portals/0/PDFs/sc/2023/scr23-035.pdf (accessed on 26 August 2024).
- Fraser, H.M.; Greenstreet, S.P.R.; Piet, G.J. Taking Account of Catchability in Groundfish Survey Trawls: Implications for Estimating Demersal Fish Biomass. ICES J. Mar. Sci. 2007, 64, 1800–1819. [Google Scholar] [CrossRef]
- Garrido, I.; González-Troncoso, D.; González-Costas, F. Assessment of the Cod Stock in NAFO Division 3M.; NAFO: Halifax, NS, Canada, 2023; 51p, Available online: https://www.nafo.int/Portals/0/PDFs/sc/2023/scr23-009.pdf (accessed on 21 November 2023).
- Ohlberger, J.; Langangen, Ø.; Stige, L.C. Age Structure Affects Population Productivity in an Exploited Fish Species. Ecol. Appl. 2022, 32, e2614. [Google Scholar] [CrossRef] [PubMed]
- Kritzer, J.P.; Davies, C.R.; Mapstone, B.D. Characterizing Fish Populations: Effects of Sample Size and Population Structure on the Precision of Demographic Parameter Estimates. Can. J. Fish. Aquat. Sci. 2001, 58, 1557–1568. [Google Scholar] [CrossRef]
- NAFO. Annual Fisheries Statistics Databases STATLANT 21; NAFO: Halifax, NS, Canada, 2024; Available online: https://www.nafo.int/Data/Catch-Statistics-STATLANT (accessed on 29 November 2024).
- Alpoim, R. An Assessment of American Plaice (Hippoglossoides Platessoides) in NAFO Division 3M.; NAFO: Halifax, NS, Canada, 2023; 20p, Available online: https://www.nafo.int/Portals/0/PDFs/sc/2023/scr23-024.pdf (accessed on 23 December 2023).
- Gonçalves, P.; Alpoim, R.; Ávila de Melo, A. Redfish Div. 3M Biological Reference Points and Advice under the PA Alternative Framework; NAFO: Halifax, NS, Canada, 2023; Volume 23. [Google Scholar]
- Iglesias, G.; González-Costas, F.; González-Troncoso, D. Atlantic Cod Predation on Redfish in Flemish Cap; NAFO: Halifax, NS, Canada, 2012; 29p, Available online: https://www.nafo.int/Portals/0/PDFs/sc/2012/scr12-027.pdf (accessed on 21 November 2023).
- Meehl, G.A.; Arblaster, J.M.; Fasullo, J.T.; Hu, A.; Trenberth, K.E. Model-Based Evidence of Deep-Ocean Heat Uptake during Surface-Temperature Hiatus Periods. Nat. Clim. Chang. 2011, 1, 360–364. [Google Scholar] [CrossRef]
- Roberts, E.M.; Mienis, F.; Rapp, H.T.; Hanz, U.; Meyer, H.K.; Davies, A.J. Oceanographic Setting and Short-Timescale Environmental Variability at an Arctic Seamount Sponge Ground. Deep. Sea Res. 1 Oceanogr. Res. Pap. 2018, 138, 98–113. [Google Scholar] [CrossRef]
- Claireaux, G.; Webber, D.M.; Lagardère, J.P.; Kerr, S.R. Influence of Water Temperature and Oxygenation on the Aerobic Metabolic Scope of Atlantic Cod (Gadus morhua). J. Sea Res. 2000, 44, 257–265. [Google Scholar] [CrossRef]
- Tirsgaard, B.; Behrens, J.W.; Steffensen, J.F. The Effect of Temperature and Body Size on Metabolic Scope of Activity in Juvenile Atlantic Cod Gadus morhua L. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 179, 89–94. [Google Scholar] [CrossRef]
- Sünksen, K.; Stenberg, C.; Grønkjær, P. Temperature Effects on Growth of Juvenile Greenland Halibut (Reinhardtius Hippoglossoides Walbaum) in West Greenland Waters. J. Sea Res. 2010, 64, 125–132. [Google Scholar] [CrossRef]
- Baudron, A.R.; Needle, C.L.; Rijnsdorp, A.D.; Tara Marshall, C. Warming Temperatures and Smaller Body Sizes: Synchronous Changes in Growth of North Sea Fishes. Glob. Chang. Biol. 2014, 20, 1023–1031. [Google Scholar] [CrossRef]
- Tirsgaard, B.; Svendsen, J.C.; Steffensen, J.F. Effects of Temperature on Specific Dynamic Action in Atlantic Cod Gadus morhua. Fish Physiol. Biochem. 2015, 41, 41–50. [Google Scholar] [CrossRef]
- van Rijn, I.; Buba, Y.; DeLong, J.; Kiflawi, M.; Belmaker, J. Large but Uneven Reduction in Fish Size across Species in Relation to Changing Sea Temperatures. Glob. Chang. Biol. 2017, 23, 3667–3674. [Google Scholar] [CrossRef] [PubMed]
- Ghinter, L.; Lambert, Y.; Audet, C. Juvenile Greenland Halibut (Reinhardtius Hippoglossoides) Growth in the Context of Rising Temperature in the Estuary and Gulf of St. Lawrence. Fish. Res. 2021, 233, 105766. [Google Scholar] [CrossRef]
- Martínez-Silva, M.A.; Vagner, M.; Senay, C.; Audet, C. Using Gene Expression to Identify the Most Suitable Environmental Conditions for Growth and Metabolism of Juvenile Deepwater Redfish (Sebastes Mentella) in the Estuary and the Gulf of St. Lawrence. ICES J. Mar. Sci. 2022, 79, 382–393. [Google Scholar] [CrossRef]
- Richter, A.; Kolmes, S.A. Maximum Temperature Limits for Chinook, Coho, and Chum Salmon, and Steelhead Trout in the Pacific Northwest. Rev. Fish. Sci. 2005, 13, 23–49. [Google Scholar] [CrossRef]
- Neuheimer, A.B.; Thresher, R.E.; Lyle, J.M.; Semmens, J.M. Tolerance Limit for Fish Growth Exceeded by Warming Waters. Nat. Clim. Chang. 2011, 1, 110–113. [Google Scholar] [CrossRef]
- O’Brien, C.L.; Spooner, P.T.; Wharton, J.H.; Papachristopoulou, E.; Dutton, N.; Fairman, D.; Garratt, R.; Li, T.; Pallottino, F.; Stringer, F.; et al. Exceptional 20th Century Shifts in Deep-Sea Ecosystems Are Spatially Heterogeneous and Associated with Local Surface Ocean Variability. Front. Mar. Sci. 2021, 8, 663009. [Google Scholar] [CrossRef]
- Metcalfe, N.B.; Monaghan, P. Growth versus Lifespan: Perspectives from Evolutionary Ecology. Exp. Gerontol. 2003, 38, 935–940. [Google Scholar] [CrossRef]
- Rountrey, A.N.; Coulson, P.G.; Meeuwig, J.J.; Meekan, M. Water Temperature and Fish Growth: Otoliths Predict Growth Patterns of a Marine Fish in a Changing Climate. Glob. Chang. Biol. 2014, 20, 2450–2458. [Google Scholar] [CrossRef]
- Lilly, G.R.; Nakken, O.; Brattey, J. A Review of the Contributions of Fisheries and Climate Variability to Contrasting Dynamics in Two Arcto-Boreal Atlantic Cod (Gadus morhua) Stocks: Persistent High Productivity in the Barents Sea and Collapse on the Newfoundland and Labrador Shelf. Prog. Oceanogr. 2013, 114, 106–125. [Google Scholar] [CrossRef]
- Carozza, D.A.; Bianchi, D.; Galbraith, E.D. Metabolic Impacts of Climate Change on Marine Ecosystems: Implications for Fish Communities and Fisheries. Glob. Ecol. Biogeogr. 2019, 28, 158–169. [Google Scholar] [CrossRef]
- Ávila De Melo, A.; Saborido-Rey, F.; Troncoso, D.G.; Pochtar, M.; Alpoim, R. An Assessment of Beaked Redfish (S. mentella and S. fasciatus) in NAFO Division 3M (With an Approach to the Likely Impact of Recent 3M Cod Growth on Redfish Natural Mortality); NAFO: Halifax, NS, Canada, 2011; 65p, Available online: https://www.nafo.int/Portals/0/PDFs/sc/2011/scr11-026.pdf (accessed on 21 November 2023).
- Urban, M.C.; Holt, R.D.; Gilman, S.E.; Tewksbury, J. Heating up Relations between Cold Fish: Competition Modifies Responses to Climate Change. J. Anim. Ecol. 2011, 80, 505–507. [Google Scholar] [CrossRef]
- Barneche, D.R.; Robertson, D.R.; White, C.R.; Marshall, D.J. Fish Reproductive-Energy Output Increases Disproportionately with Body Size. Science 2018, 360, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Devine, J.A.; Haedrich, R.L. The Role of Environmental Conditions and Exploitation in Determining Dynamics of Redfish (Sebastes Species) in the Northwest Atlantic. Fish. Oceanogr. 2011, 20, 66–81. [Google Scholar] [CrossRef]
- Barbeaux, S.J.; Holsman, K.; Zador, S. Marine Heatwave Stress Test of Ecosystem-Based Fisheries Management in the Gulf of Alaska Pacific Cod Fishery. Front. Mar. Sci. 2020, 7, 703. [Google Scholar] [CrossRef]
- Sogn-Grundvåg, G.; Zhang, D.; Henriksen, E.; Joensen, S.; Bendiksen, B.I.; Hermansen, Ø. Fish Quality and Market Performance: The Case of the Coastal Fishery for Atlantic Cod in Norway. Mar. Policy 2021, 127, 104449. [Google Scholar] [CrossRef]
- Szuwalski, C.S.; Hollowed, A.B. Climate Change and Non-Stationary Population Processes in Fisheries Management. ICES J. Mar. Sci. 2016, 73, 1297–1395. [Google Scholar] [CrossRef]
- Sydeman, W.J.; Poloczanska, E.; Reed, T.E.; Thompson, S.A. Climate Change and Marine Vertebrates. Science 2015, 350, 772–777. [Google Scholar] [CrossRef]
- Fulton, E.A.; Link, J.S.; Kaplan, I.C.; Savina-Rolland, M.; Johnson, P.; Ainsworth, C.; Horne, P.; Gorton, R.; Gamble, R.J.; Smith, A.D.M.; et al. Lessons in Modelling and Management of Marine Ecosystems: The Atlantis Experience. Fish Fish. 2011, 12, 171–188. [Google Scholar] [CrossRef]
- Travers-Trolet, M.; Shin, Y.J.; Field, J.G. An End-to-End Coupled Model ROMS-N2P2Z2D2-OSMOSE of the Southern Benguela Foodweb: Parameterisation, Calibration and Pattern-Oriented Validation. Afr. J. Mar. Sci. 2014, 36, 11–29. [Google Scholar] [CrossRef]
- Fulton, E.A. Approaches to End-to-End Ecosystem Models. J. Mar. Syst. 2010, 81, 171–183. [Google Scholar] [CrossRef]
- Moullec, F.; Barrier, N.; Drira, S.; Guilhaumon, F.; Marsaleix, P.; Somot, S.; Ulses, C.; Velez, L.; Shin, Y.J. An End-to-End Model Reveals Losers and Winners in a Warming Mediterranean Sea. Front. Mar. Sci. 2019, 6, 345. [Google Scholar] [CrossRef]
- Travers-Trolet, M.; Bourdaud, P.; Genu, M.; Velez, L.; Vermard, Y. The Risky Decrease of Fishing Reference Points Under Climate Change. Front. Mar. Sci. 2020, 7, 568232. [Google Scholar] [CrossRef]
- Christensen, V.; Walters, C.J.; Pauly, D. Ecopath with Ecosim: A User’s Guide; Fisheries Centre, University of British Columbia: Vancouver, BC, Canada, 2005; Available online: www.ecopath.org (accessed on 31 January 2025).
- Pérez-Rodríguez, A.; Umar, I.; Goto, D.; Howell, D.; Mosqueira, I.; González-Troncoso, D. Evaluation of Harvest Control Rules for a Group of Interacting Commercial Stocks Using a Multispecies MSE Framework. Can. J. Fish. Aquat. Sci. 2022, 79, 1302–1320. [Google Scholar] [CrossRef]


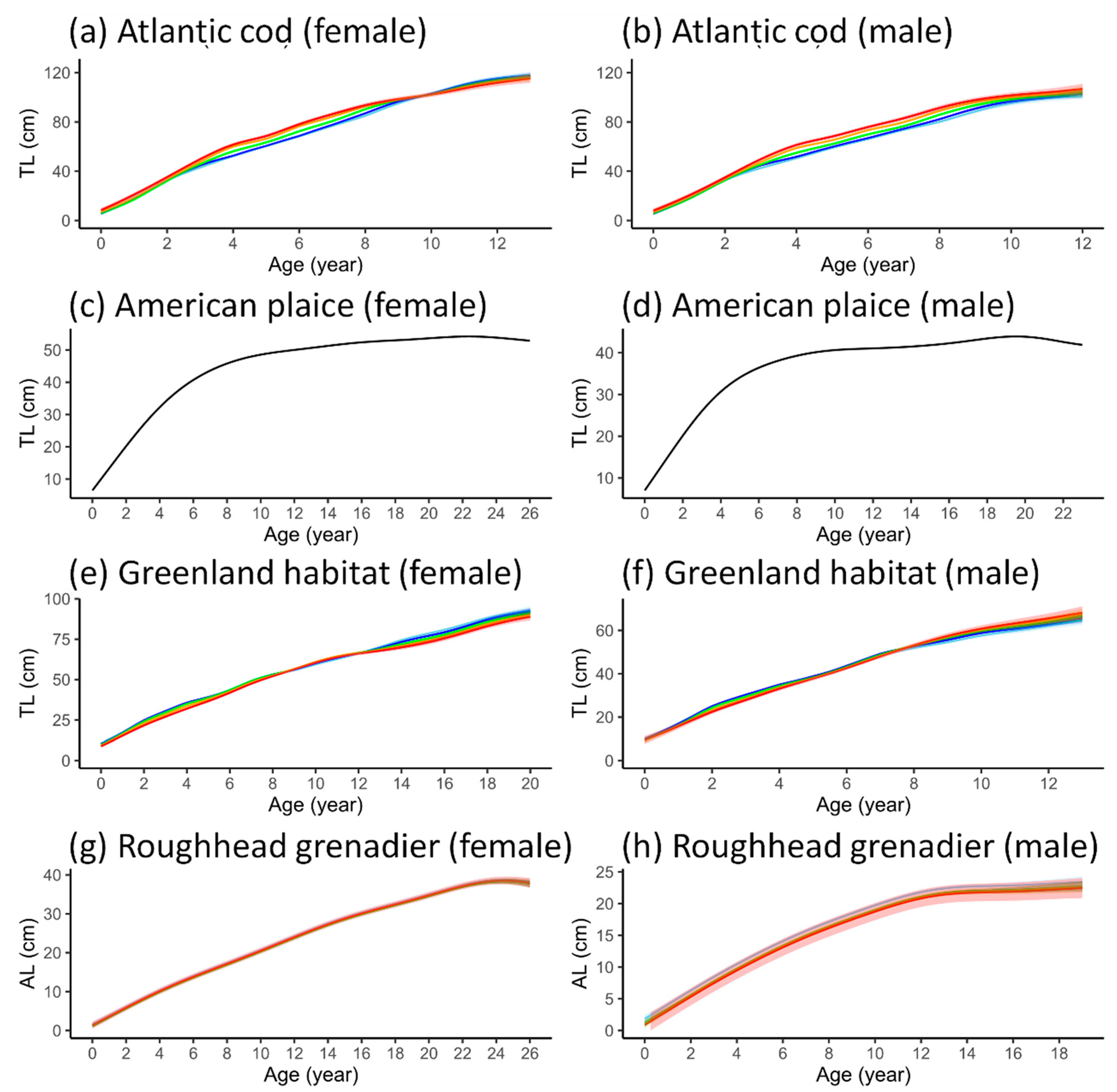
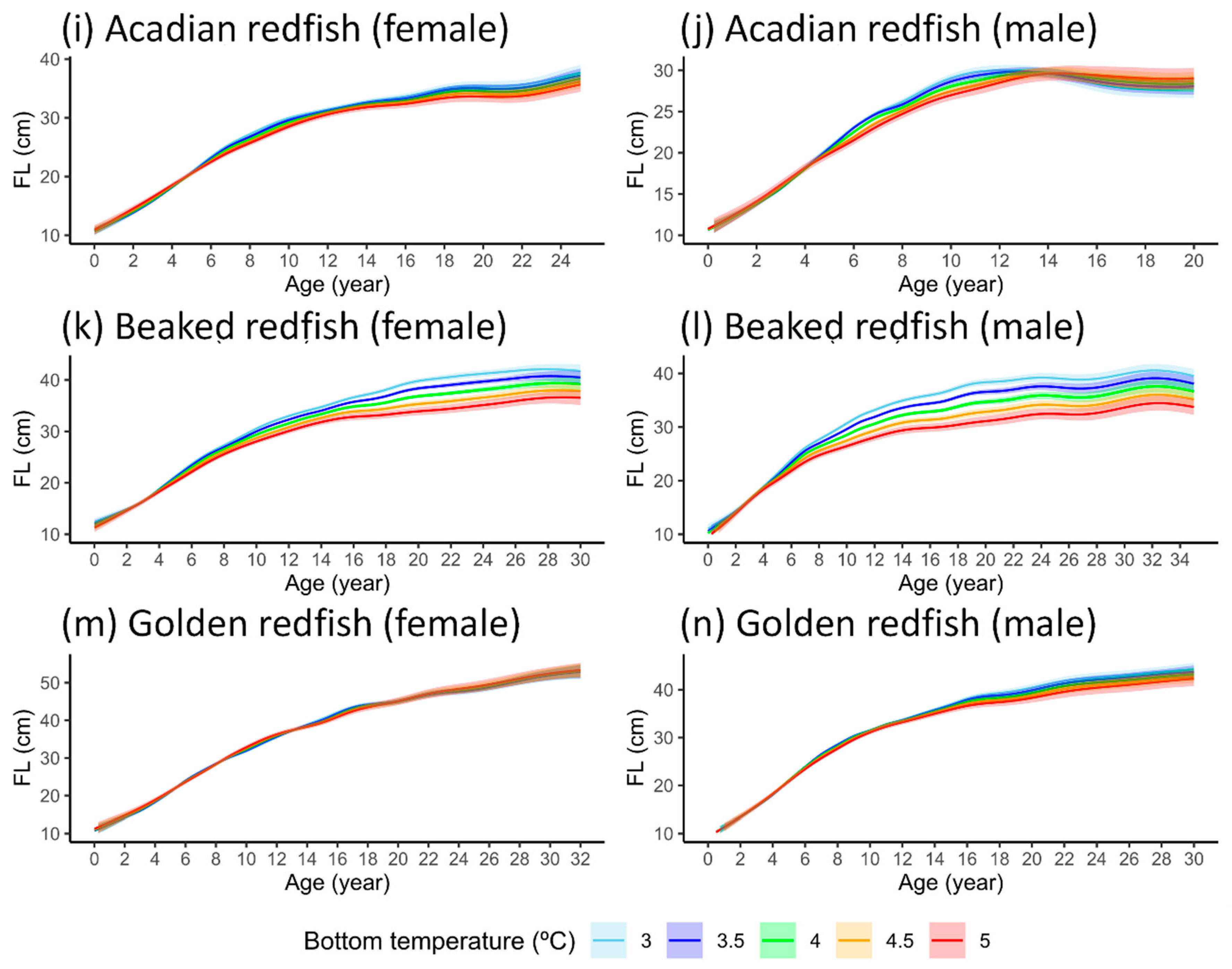
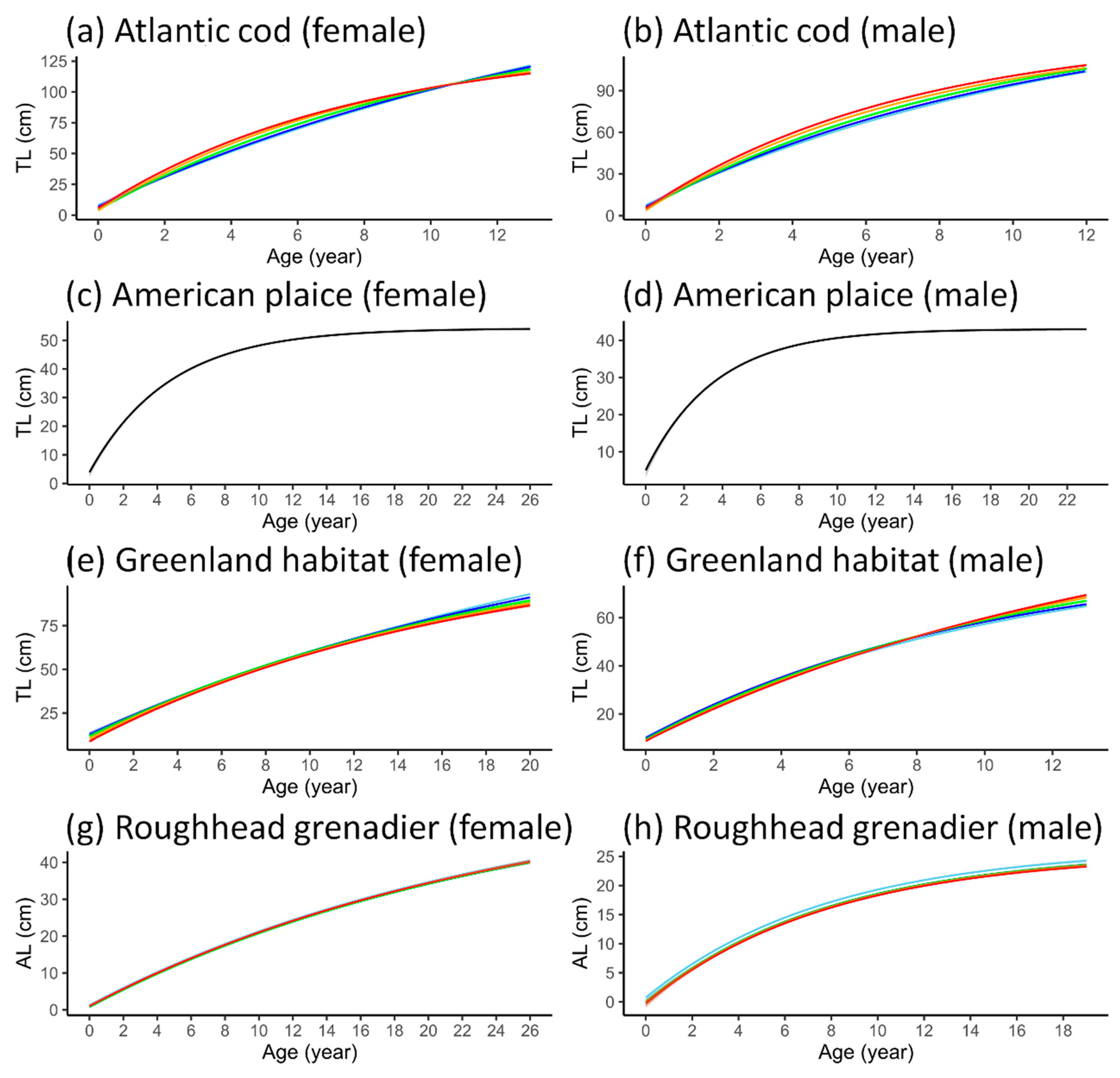

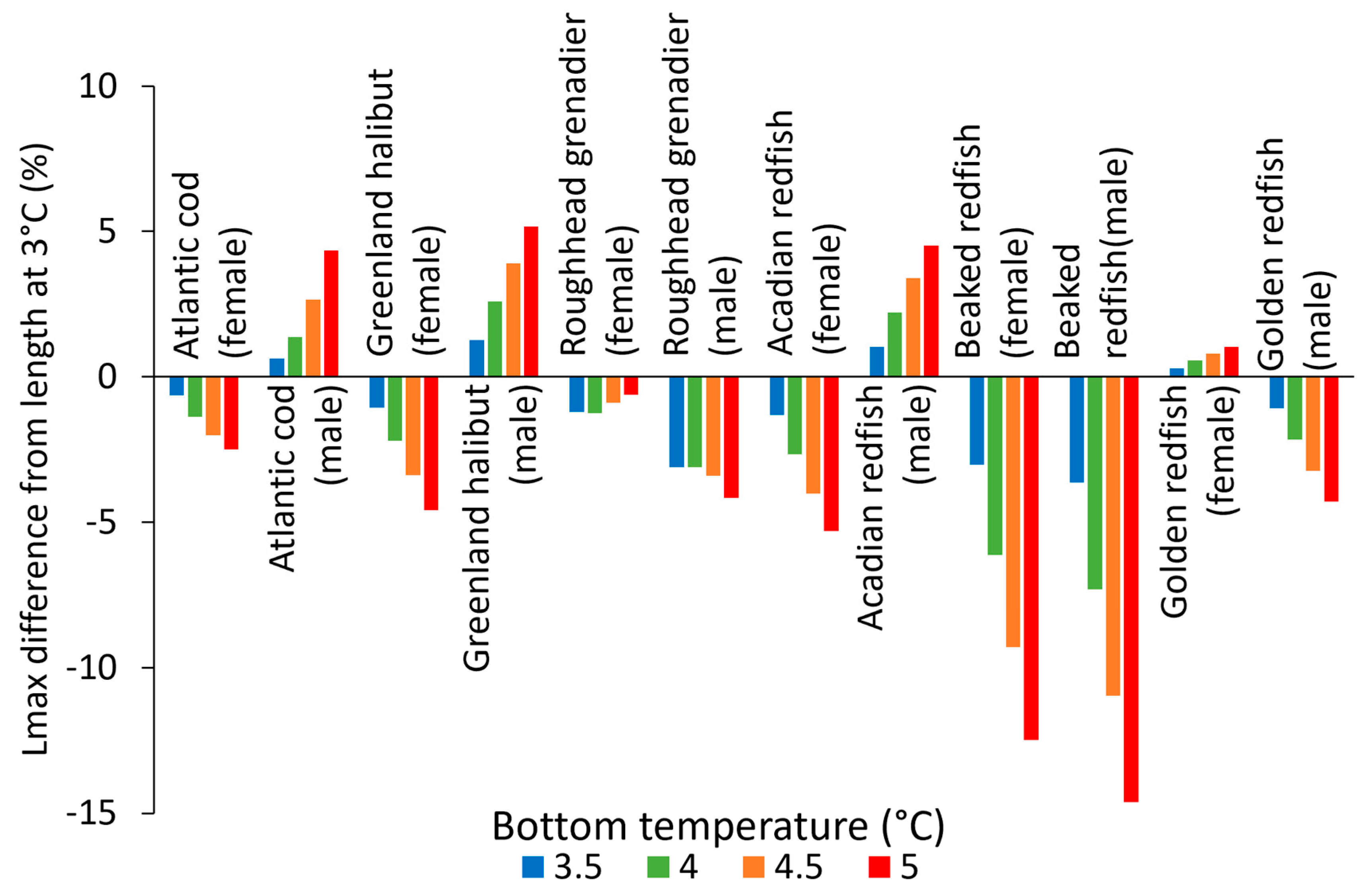
| Year | Atlantic Cod | American Plaice | Greenland Halibut | Roughhead Grenadier | Acadian Redfish | Beaked Redfish | Golden Redfish | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | Male | |
| 1993 | 917 | 896 | 533 | 459 | 465 | 391 | - | - | 239 | 242 | 185 | 225 | 176 | 201 |
| 1994 | 866 | 759 | 579 | 428 | 492 | 364 | 121 | 109 | 288 | 316 | 362 | 370 | 305 | 318 |
| 1995 | 703 | 768 | 396 | 345 | 356 | 244 | 260 | 224 | 398 | 346 | 344 | 341 | 381 | 454 |
| 1996 | 838 | 698 | 232 | 302 | 502 | 355 | 169 | 221 | 448 | 425 | 606 | 661 | 508 | 549 |
| 1997 | 762 | 569 | 211 | 210 | 694 | 568 | - | - | 49 | 78 | 243 | 279 | 150 | 121 |
| 1998 | 278 | 176 | 247 | 183 | 1040 | 875 | 304 | 289 | 182 | 196 | 257 | 234 | - | - |
| 1999 | 154 | 126 | 297 | 143 | 454 | 358 | 288 | 267 | 459 | 486 | 458 | 519 | 314 | 368 |
| 2000 | 131 | 111 | 184 | 190 | 634 | 524 | 304 | 296 | 37 | 38 | 232 | 233 | 105 | 75 |
| 2001 | 251 | 207 | 200 | 339 | 426 | 397 | 436 | 331 | - | - | 94 | 90 | - | - |
| 2002 | 162 | 139 | 155 | 67 | 447 | 400 | 179 | 146 | 117 | 102 | 181 | 171 | 58 | 71 |
| 2003 | 152 | 119 | 162 | 358 | 425 | 379 | 270 | 239 | - | - | 131 | 119 | - | - |
| 2004 | 399 | 277 | - | - | 541 | 449 | - | - | - | - | - | - | - | - |
| 2005 | 329 | 305 | 169 | 162 | 620 | 435 | 318 | 200 | - | - | 128 | 132 | - | - |
| 2006 | 534 | 505 | 116 | 183 | 635 | 487 | 191 | 121 | - | - | 69 | 80 | - | - |
| 2007 | 686 | 657 | 82 | 102 | 593 | 446 | 192 | 105 | - | - | 83 | 78 | - | - |
| 2008 | 802 | 839 | 204 | 201 | 569 | 397 | 183 | 118 | - | - | - | - | - | - |
| 2009 | 595 | 590 | 196 | 216 | 619 | 377 | 200 | 113 | 170 | 107 | 170 | 118 | - | - |
| 2010 | 532 | 430 | 187 | 204 | 479 | 350 | 525 | 343 | - | - | - | - | - | - |
| 2011 | 788 | 671 | 310 | 214 | 694 | 484 | 594 | 478 | 187 | 162 | 182 | 167 | - | - |
| 2012 | 698 | 592 | 358 | 377 | 455 | 253 | 387 | 278 | 276 | 153 | 282 | 164 | 339 | 174 |
| 2013 | 861 | 741 | 343 | 316 | 421 | 291 | 366 | 296 | 404 | 150 | 344 | 157 | 439 | 1 |
| 2014 | 416 | 320 | - | - | 77 | 51 | 144 | 107 | 112 | 37 | 85 | 66 | 161 | 1 |
| 2015 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 2016 | 793 | 616 | 461 | 469 | 408 | 257 | 379 | 300 | 152 | 111 | 166 | 99 | 376 | 123 |
| 2017 | 392 | 311 | - | - | 40 | 26 | 129 | 81 | 68 | 49 | 32 | 40 | 42 | 46 |
| 2018 | 211 | 196 | - | - | 87 | 43 | 127 | 88 | 43 | 34 | 46 | 47 | 68 | 10 |
| Total | 13,250 | 11,618 | 5622 | 5468 | 12,173 | 9201 | 6066 | 4750 | 3629 | 3032 | 4680 | 4390 | 3422 | 2512 |
| Species | Sex | tmax ± SE (years) |
|---|---|---|
| Atlantic cod | All | 13.3 ± 0.2 |
| Female | 13.3 ± 0.2 | |
| Male | 11.5 ± 0.2 | |
| American plaice | All | 25.6 ± 0.3 |
| Female | 25.5 ± 0.3 | |
| Male | 22.6 ± 0.5 | |
| Greenland halibut | All | 19.5 ± 0.4 |
| Female | 19.5 ± 0.4 | |
| Male | 12.6 ± 0.2 | |
| Roughhead grenadier | All | 26.4 ± 0.7 |
| Female | 26.4 ± 0.7 | |
| Male | 18.5 ± 0.5 | |
| Acadian redfish | All | 24.6 ± 0.9 |
| Female | 24.6 ± 0.9 | |
| male | 19.7 ± 0.5 | |
| Beaked redfish | All | 35.6 ± 1.1 |
| Female | 29.7 ± 1.0 | |
| Male | 35.0 ± 1.2 | |
| Golden redfish | All | 33.9 ± 1.3 |
| Female | 32.0 ± 1.3 | |
| Male | 30.2 ± 1.2 |
| Species | Female | Male | ||||||
|---|---|---|---|---|---|---|---|---|
| Model | edf | DE (%) | ΔAIC | Model | edf | DE (%) | ΔAIC | |
| Atlantic cod | s(Age, BT) | 27.12 | 87.9 | s(Age, BT) | 26.09 | 86.4 | ||
| s(Age) + s(BT) | 12.70 | 87.8 | 454.00 | s(Age) + s(BT) | 13.34 | 86.3 | 419.27 | |
| s(Age) | 7.97 | 86.8 | 547.70 | s(Age) | 7.97 | 85.1 | 522.57 | |
| American plaice | s(Age) | 8.43 | 90.4 | s(Age) | 8.36 | 80.6 | ||
| s(Age) + s(BT) | 10.33 | 90.4 | 3.60 | s(Age) + s(BT) | 9.79 | 80.6 | 7.60 | |
| s(Age, BT) | 25.94 | 90.4 | 18.21 | s(Age, BT) | 25.93 | 80.7 | 21.44 | |
| Greenland halibut | s(Age, BT) | 26.22 | 94.1 | s(Age, BT) | 24.05 | 90.7 | ||
| s(Age) + s(BT) | 13.70 | 94.1 | 106.53 | s(Age) + s(BT) | 13.95 | 90.7 | 13.67 | |
| s(Age) | 8.3 | 94.0 | 161.02 | s(Age) | 8.54 | 90.6 | 49.38 | |
| Roughhead grenadier | s(Age) + s(BT) | 11.52 | 96.0 | s(Age) + s(BT) | 11.48 | 91.6 | ||
| s(Age) | 8.26 | 96.0 | 6.56 | s(Age) | 7.26 | 91.3 | 4.31 | |
| s(Age, BT) | 24.44 | 96.0 | 60.96 | s(Age, BT) | 23.67 | 91.6 | 26.85 | |
| Acadian redfish | s(Age, BT) | 21.93 | 91.0 | s(Age, BT) | 22.23 | 86.8 | ||
| s(Age) + s(BT) | 11.01 | 90.9 | 30.09 | s(Age) + s(BT) | 8.42 | 86.3 | 54.10 | |
| s(Age) | 8.12 | 90.7 | 33.64 | s(Age) | 7.41 | 86.0 | 57.47 | |
| Beaked redfish | s(Age, BT) | 24.97 | 93.3 | s(Age, BT) | 25.25 | 91.5 | ||
| s(Age) + s(BT) | 10.91 | 93.1 | 86.35 | s(Age) + s(BT) | 8.71 | 90.9 | 144.51 | |
| s(Age) | 7.67 | 92.8 | 111.62 | s(Age) | 7.72 | 90.3 | 179.89 | |
| Golden redfish | s(Age, BT) | 21.07 | 92.2 | s(Age, BT) | 21.61 | 94.8 | ||
| s(Age) | 7.10 | 92.1 | 13.39 | s(Age) | 7.89 | 94.7 | 3.81 | |
| s(Age) + s(BT) | 8.10 | 92.1 | 20.66 | s(Age) + s(BT) | 8.91 | 94.7 | 10.37 | |
| Species | BT (°C) | Female | Male | ||||
|---|---|---|---|---|---|---|---|
| L∞ (cm) | k (Year−1) | t0 (Years) | L∞ (cm) | k (Year−1) | t0 (Years) | ||
| Atlantic cod | 3 | 234.53 | 0.05 | −0.63 | 158.78 | 0.08 | −0.55 |
| 3.5 | 205.31 | 0.07 | −0.5 | 147.79 | 0.1 | −0.44 | |
| 4 | 167.02 | 0.09 | −0.29 | 141.84 | 0.11 | −0.31 | |
| 4.5 | 158.64 | 0.12 | −0.24 | 132.64 | 0.13 | −0.24 | |
| 5 | 139.74 | 0.13 | −0.33 | 132.12 | 0.14 | −0.3 | |
| American plaice | NA | 54.22 | 0.21 | −0.37 | 43.09 | 0.27 | −0.47 |
| Greenland halibut | 3 | 173.02 | 0.03 | −2.26 | 87.42 | 0.09 | −1.33 |
| 3.5 | 150.62 | 0.04 | −2.11 | 86.37 | 0.1 | −1.22 | |
| 4 | 135.05 | 0.05 | −1.85 | 92.59 | 0.09 | −1.19 | |
| 4.5 | 126.57 | 0.06 | −1.56 | 101.69 | 0.08 | −1.19 | |
| 5 | 121.8 | 0.06 | −1.31 | 110.69 | 0.07 | −1.21 | |
| Roughhead grenadier | 3 | 61.66 | 0.04 | −0.53 | 26.59 | 0.13 | −0.24 |
| 3.5 | 61.2 | 0.04 | −0.34 | 25.87 | 0.13 | −0.02 | |
| 4 | 61.18 | 0.04 | −0.33 | 25.87 | 0.13 | −0.02 | |
| 4.5 | 61.32 | 0.04 | −0.39 | 25.8 | 0.13 | 0.01 | |
| 5 | 61.42 | 0.04 | −0.43 | 25.62 | 0.13 | 0.06 | |
| Acadian redfish | 3 | 38.75 | 0.11 | −2.15 | 29.99 | 0.2 | −1.43 |
| 3.5 | 38.2 | 0.12 | −2.17 | 30.28 | 0.19 | −1.52 | |
| 4 | 37.66 | 0.12 | −2.26 | 30.65 | 0.18 | −1.71 | |
| 4.5 | 37.14 | 0.12 | −2.38 | 31.07 | 0.16 | −1.98 | |
| 5 | 36.65 | 0.12 | −2.51 | 31.52 | 0.15 | −2.27 | |
| Beaked redfish | 3 | 46.06 | 0.08 | −2.87 | 41.23 | 0.11 | −2 |
| 3.5 | 43.9 | 0.09 | −2.86 | 39.46 | 0.11 | −2.12 | |
| 4 | 41.77 | 0.09 | −2.88 | 37.65 | 0.11 | −2.32 | |
| 4.5 | 39.76 | 0.1 | −2.89 | 35.85 | 0.11 | −2.53 | |
| 5 | 37.91 | 0.1 | −2.89 | 34.09 | 0.12 | −2.7 | |
| Golden redfish | 3 | 59.25 | 0.06 | −2.31 | 47 | 0.09 | −1.83 |
| 3.5 | 59.92 | 0.06 | −2.43 | 46.08 | 0.09 | −1.82 | |
| 4 | 60.6 | 0.06 | −2.56 | 45.24 | 0.1 | −1.82 | |
| 4.5 | 61.17 | 0.06 | −2.67 | 44.51 | 0.1 | −1.83 | |
| 5 | 61.65 | 0.06 | −2.79 | 43.89 | 0.1 | −1.86 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Songin, K.; Saborido-Rey, F.; Pierce, G.J. Bottom Temperature Effect on Growth of Multiple Demersal Fish Species in Flemish Cap, Northwest Atlantic. Animals 2025, 15, 1120. https://doi.org/10.3390/ani15081120
Songin K, Saborido-Rey F, Pierce GJ. Bottom Temperature Effect on Growth of Multiple Demersal Fish Species in Flemish Cap, Northwest Atlantic. Animals. 2025; 15(8):1120. https://doi.org/10.3390/ani15081120
Chicago/Turabian StyleSongin, Krerkkrai, Fran Saborido-Rey, and Graham J. Pierce. 2025. "Bottom Temperature Effect on Growth of Multiple Demersal Fish Species in Flemish Cap, Northwest Atlantic" Animals 15, no. 8: 1120. https://doi.org/10.3390/ani15081120
APA StyleSongin, K., Saborido-Rey, F., & Pierce, G. J. (2025). Bottom Temperature Effect on Growth of Multiple Demersal Fish Species in Flemish Cap, Northwest Atlantic. Animals, 15(8), 1120. https://doi.org/10.3390/ani15081120





