Effect of Alternative Splicing Euchromatic Histone Lysine Methyltransferase 2 (EHMT2/G9A) on Spermatogenesis in Mongolian Horses
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Testis Collection and Cell Culture
2.2. Immunofluorescence
2.3. Analysis of the Predicted Protein Sequence
2.4. Construction of the Lentiviral Vector
2.5. Cell Transfection
2.6. Cell Proliferation Detected Using the CCK8 Assay
2.7. RNA Isolation, cDNA Synthesis, and qRT–PCR Analysis
3. Results
3.1. EHMT2 Expression in Sertoli Cells
3.2. Predicted Secondary Structure of the EHMT2 Protein
3.3. Lentiviral Vector Construction
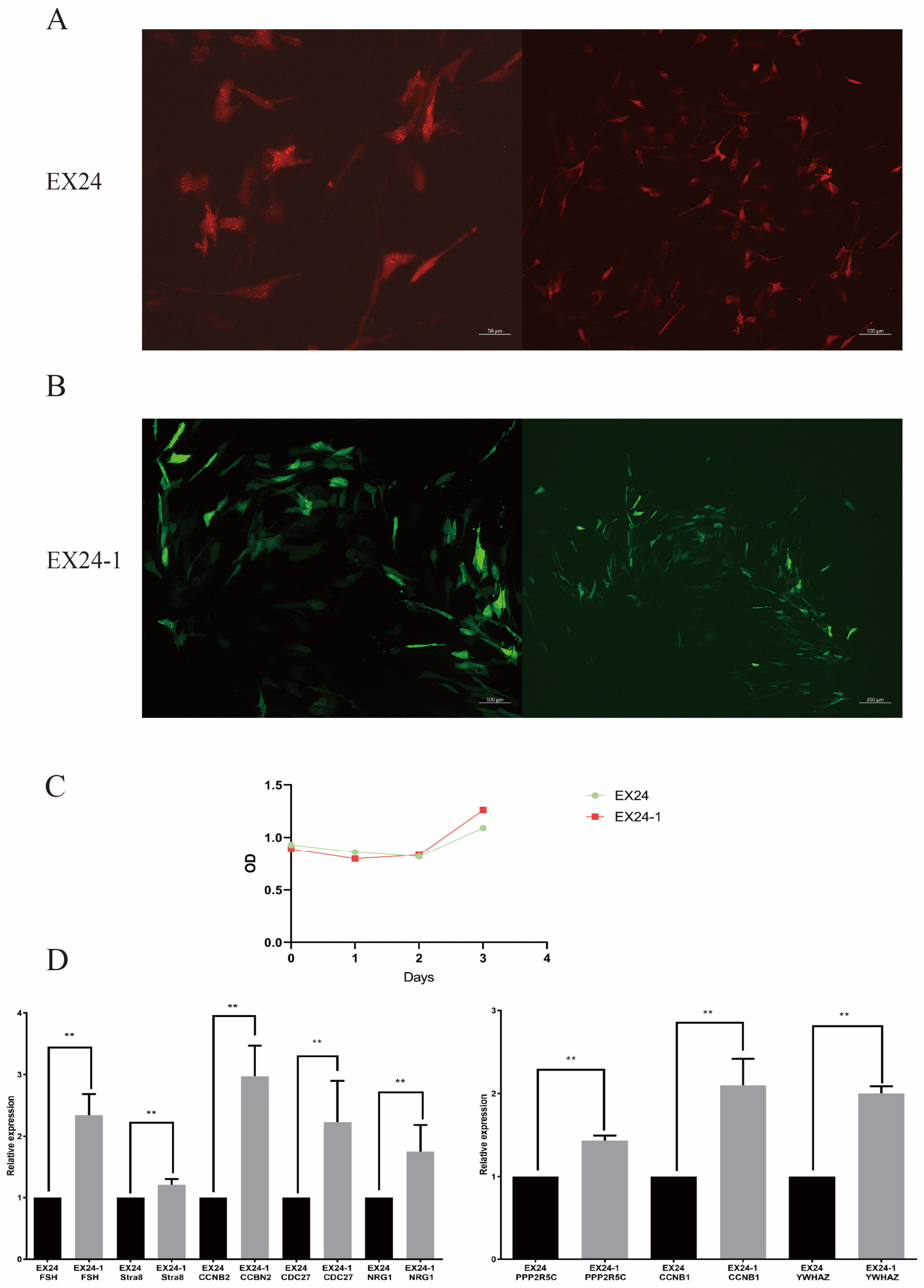
3.4. Effects of the Two Lentiviruses on SERTOLI Cells
3.5. Cell Proliferation Detected of Sertoli Cells by the CCK-8 Assay
3.6. qRT–PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Librado, P.; Khan, N.; Fages, A.; Kusliy, M.A.; Suchan, T.; Tonasso-Calvière, L.; Schiavinato, S.; Alioglu, D.; Fromentier, A.; Perdereau, A.; et al. The origins and spread of domestic horses from the Western Eurasian steppes. Nature 2021, 598, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Du, M.; Li, X.; Zhang, L.; Zhao, B.; Wang, N.; Dugarjaviin, M. Single-Cell Transcriptome Sequencing Reveals Molecular Expression Differences and Marker Genes in Testes during the Sexual Maturation of Mongolian Horses. Anim. Open Access J. 2024, 14, 1258. [Google Scholar] [CrossRef] [PubMed]
- Bou, T.; Ding, W.; Liu, H.; Gong, W.; Jia, Z.; Dugarjaviin, M.; Bai, D. A genome-wide landscape of mRNAs, miRNAs, lncRNAs, and circRNAs of skeletal muscles during dietary restriction in Mongolian horses. Comp. Biochem. Physiol. Part D Genom. Proteom. 2023, 46, 101084. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Randhawa, I.A.S.; MacHugh, D.E.; McGivney, B.A.; Katz, L.M.; Dugarjaviin, M.; Hill, E.W. Selection signatures for local and regional adaptation in Chinese Mongolian horse breeds reveal candidate genes for hoof health. BMC Genom. 2023, 24, 35. [Google Scholar] [CrossRef]
- Su, S.; Zhao, Y.; Liu, Z.; Liu, G.; Du, M.; Wu, J.; Bai, D.; Li, B.; Bou, G.; Zhang, X.; et al. Characterization and comparison of the bacterial microbiota in different gastrointestinal tract compartments of Mongolian horses. MicrobiologyOpen 2020, 9, 1085–1101. [Google Scholar] [CrossRef]
- Li, B.; He, X.; Zhao, Y.; Bai, D.; Li, D.; Zhou, Z.; Manglai, D. Analysis of the miRNA transcriptome during testicular development and spermatogenesis of the Mongolian horse. Reprod. Fertil. Dev. 2020, 32, 582–593. [Google Scholar] [CrossRef]
- Li, X.; Du, M.; Liu, Y.; Wang, M.; Shen, Y.; Xing, J.; Zhang, L.; Zhao, Y.; Bou, G.; Bai, D.; et al. Proteome and metabolomic profile of Mongolian horse follicular fluid during follicle development. Sci. Rep. 2024, 14, 19788. [Google Scholar] [CrossRef]
- Oatley, J.M.; Brinster, R.L. Regulation of spermatogonial stem cell self-renewal in mammals. Annu. Rev. Cell Dev. Biol. 2008, 24, 263–286. [Google Scholar] [CrossRef]
- Griswold, M.D. The central role of Sertoli cells in spermatogenesis. Semin. Cell Dev. Biol. 1998, 9, 411–416. [Google Scholar] [CrossRef]
- Johnson, J.M.; Castle, J.; Garrett-Engele, P.; Kan, Z.; Loerch, P.M.; Armour, C.D.; Santos, R.; Schadt, E.E.; Stoughton, R.; Shoemaker, D.D. Genome-wide survey of human alternative pre-mRNA splicing with exon junction microarrays. Science 2003, 302, 2141–2144. [Google Scholar] [CrossRef]
- Barbosa-Morais, N.L.; Irimia, M.; Pan, Q.; Xiong, H.Y.; Gueroussov, S.; Lee, L.J.; Slobodeniuc, V.; Kutter, C.; Watt, S.; Colak, R.; et al. The evolutionary landscape of alternative splicing in vertebrate species. Science 2012, 338, 1587–1593. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, T.W.; Graveley, B.R. Expansion of the eukaryotic proteome by alternative splicing. Nature 2010, 463, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Black, D.L. Mechanisms of alternative pre-messenger RNA splicing. Annu. Rev. Biochem. 2003, 72, 291–336. [Google Scholar] [CrossRef]
- Keren, H.; Lev-Maor, G.; Ast, G. Alternative splicing and evolution: Diversification, exon definition and function. Nat. Rev. Genet. 2010, 11, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Gan, H.; Cai, T.; Lin, X.; Wu, Y.; Wang, X.; Yang, F.; Han, C. Integrative proteomic and transcriptomic analyses reveal multiple post-transcriptional regulatory mechanisms of mouse spermatogenesis. Mol. Cell. Proteom. MCP 2013, 12, 1144–1157. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, H.; Guan, X.; Qin, D.; Zhou, J.; Wu, X. Loss of ESRP1 blocks mouse oocyte development and leads to female infertility. Development 2021, 148, dev196931. [Google Scholar] [CrossRef]
- Zagore, L.L.; Grabinski, S.E.; Sweet, T.J.; Hannigan, M.M.; Sramkoski, R.M.; Li, Q.; Licatalosi, D.D. RNA Binding Protein Ptbp2 Is Essential for Male Germ Cell Development. Mol. Cell. Biol. 2015, 35, 4030–4042. [Google Scholar] [CrossRef]
- Legrand, J.M.D.; Chan, A.L.; La, H.M.; Rossello, F.J.; Änkö, M.L.; Fuller-Pace, F.V.; Hobbs, R.M. DDX5 plays essential transcriptional and post-transcriptional roles in the maintenance and function of spermatogonia. Nat. Commun. 2019, 10, 2278. [Google Scholar] [CrossRef]
- Ushijima, Y.; Inoue, Y.H.; Konishi, T.; Kitazawa, D.; Yoshida, H.; Shimaji, K.; Kimura, H.; Yamaguchi, M. Roles of histone H3K9 methyltransferases during Drosophila spermatogenesis. Chromosome Res. 2012, 20, 319–331. [Google Scholar] [CrossRef]
- Tachibana, M.; Nozaki, M.; Takeda, N.; Shinkai, Y. Functional dynamics of H3K9 methylation during meiotic prophase progression. EMBO J. 2007, 26, 3346–3359. [Google Scholar] [CrossRef]
- Li, B.; He, X.; Zhao, Y.; Bai, D.; Du, M.; Song, L.; Liu, Z.; Yin, Z.; Manglai, D. Transcriptome profiling of developing testes and spermatogenesis in the Mongolian horse. BMC Genet. 2020, 21, 46. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Cui, Y.; Zhao, Y.; Bai, D.; Ren, X.; Te, R.; Mang, L.; Li, B. Isolation, Culture and Identification of Testis Sertoli Cells in Mongolian Horses in vitro. China Anim. Husb. Vet. Med. 2020, 47, 2751–2758. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Zhang, L.; Ge, R.; Mang, L.; Du, M. Comparison of testicular tissue morphology and spermatogenic epithelial cells between Mongolian horses before and after sexual maturity. Heilongjiang Anim. Sci. Vet. Med. 2024, 1–5+116. [Google Scholar] [CrossRef]
- Yi, M.; Tseweendolmaa, U.; Davshilt, T.; Wang, X.; Shen, Y.; Du, M.; Ren, H.; Mang, L.; Gerelchimeg, B. Research Progress on Function, Isolation, Purification and Identification of Sertoli Cells. China Anim. Husb. Vet. Med. 2021, 48, 2947–2956. [Google Scholar]
- Kramer, J.M.; Kochinke, K.; Oortveld, M.A.; Marks, H.; Kramer, D.; de Jong, E.K.; Asztalos, Z.; Westwood, J.T.; Stunnenberg, H.G.; Sokolowski, M.B.; et al. Epigenetic regulation of learning and memory by Drosophila EHMT/G9a. PLoS Biol. 2011, 9, e1000569. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, M.; Sugimoto, K.; Nozaki, M.; Ueda, J.; Ohta, T.; Ohki, M.; Fukuda, M.; Takeda, N.; Niida, H.; Kato, H.; et al. G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev. 2002, 16, 1779–1791. [Google Scholar] [CrossRef]
- Aitken, R.J.; Baker, M.A. Causes and consequences of apoptosis in spermatozoa; contributions to infertility and impacts on development. Int. J. Dev. Biol. 2013, 57, 265–272. [Google Scholar] [CrossRef]
- Kao, E.; Villalon, R.; Ribeiro, S.; Berger, T. Role for endogenous estrogen in prepubertal Sertoli cell maturation. Anim. Reprod. Sci. 2012, 135, 106–112. [Google Scholar] [CrossRef]
- Matzuk, M.M.; Lamb, D.J. The biology of infertility: Research advances and clinical challenges. Nat. Med. 2008, 14, 1197–1213. [Google Scholar] [CrossRef]
- Sharpe, R.M.; McKinnell, C.; Kivlin, C.; Fisher, J.S. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 2003, 125, 769–784. [Google Scholar] [CrossRef]
- Rebourcet, D.; Darbey, A.; Monteiro, A.; Soffientini, U.; Tsai, Y.T.; Handel, I.; Pitetti, J.L.; Nef, S.; Smith, L.B.; O’Shaughnessy, P.J. Sertoli Cell Number Defines and Predicts Germ and Leydig Cell Population Sizes in the Adult Mouse Testis. Endocrinology 2017, 158, 2955–2969. [Google Scholar] [CrossRef] [PubMed]
- Ewing, B.; Green, P. Analysis of expressed sequence tags indicates 35,000 human genes. Nat. Genet. 2000, 25, 232–234. [Google Scholar] [CrossRef]
- Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; Doyle, M.; FitzHugh, W.; et al. Initial sequencing and analysis of the human genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef]
- Venter, J.C.; Adams, M.D.; Myers, E.W.; Li, P.W.; Mural, R.J.; Sutton, G.G.; Smith, H.O.; Yandell, M.; Evans, C.A.; Holt, R.A.; et al. The sequence of the human genome. Science 2001, 291, 1304–1351. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Holt, I.; Pertea, G.; Karamycheva, S.; Salzberg, S.L.; Quackenbush, J. Gene index analysis of the human genome estimates approximately 120,000 genes. Nat. Genet. 2000, 25, 239–240. [Google Scholar] [CrossRef]
- Pennisi, E. Human Genome Project. And the gene number is…? Science 2000, 288, 1146–1147. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Tang, C.; Li, J.; Zhang, Y.; Bhetwal, B.P.; Zheng, H.; Yan, W. RAN-binding protein 9 is involved in alternative splicing and is critical for male germ cell development and male fertility. PLoS Genet. 2014, 10, e1004825. [Google Scholar] [CrossRef]
- Margolin, G.; Khil, P.P.; Kim, J.; Bellani, M.A.; Camerini-Otero, R.D. Integrated transcriptome analysis of mouse spermatogenesis. BMC Genom. 2014, 15, 39. [Google Scholar] [CrossRef]
- Sontag, E. Protein phosphatase 2A: The Trojan Horse of cellular signaling. Cell. Signal. 2001, 13, 7–16. [Google Scholar] [CrossRef]
- Brandeis, M.; Rosewell, I.; Carrington, M.; Crompton, T.; Jacobs, M.A.; Kirk, J.; Gannon, J.; Hunt, T. Cyclin B2-null mice develop normally and are fertile whereas cyclin B1-null mice die in utero. Proc. Natl. Acad. Sci. USA 1998, 95, 4344–4349. [Google Scholar] [CrossRef]
- Tang, J.X.; Li, J.; Cheng, J.M.; Hu, B.; Sun, T.C.; Li, X.Y.; Batool, A.; Wang, Z.P.; Wang, X.X.; Deng, S.L.; et al. Requirement for CCNB1 in mouse spermatogenesis. Cell Death Dis. 2017, 8, e3142. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Romer, K.A.; Anderson, E.L.; Baltus, A.E.; de Rooij, D.G.; Page, D.C. Periodic retinoic acid-STRA8 signaling intersects with periodic germ-cell competencies to regulate spermatogenesis. Proc. Natl. Acad. Sci. USA 2015, 112, E2347–E2356. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.T.; Niu, C.M.; Xia, J.; Shen, X.Y.; Xia, M.M.; Hu, Y.Q.; Zheng, Y. Stimulated by retinoic acid gene 8 (Stra8) plays important roles in many stages of spermatogenesis. Asian J. Androl. 2018, 20, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Puri, P.; Myers, K.; Kline, D.; Vijayaraghavan, S. Proteomic analysis of bovine sperm YWHA binding partners identify proteins involved in signaling and metabolism. Biol. Reprod. 2008, 79, 1183–1191. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baatar, T.; Song, D.; Weng, Y.; Wang, G.; Jin, L.; Guo, R.; Li, B.; Dugarjaviin, M. Effect of Alternative Splicing Euchromatic Histone Lysine Methyltransferase 2 (EHMT2/G9A) on Spermatogenesis in Mongolian Horses. Animals 2025, 15, 1106. https://doi.org/10.3390/ani15081106
Baatar T, Song D, Weng Y, Wang G, Jin L, Guo R, Li B, Dugarjaviin M. Effect of Alternative Splicing Euchromatic Histone Lysine Methyltransferase 2 (EHMT2/G9A) on Spermatogenesis in Mongolian Horses. Animals. 2025; 15(8):1106. https://doi.org/10.3390/ani15081106
Chicago/Turabian StyleBaatar, Tergel, Dailing Song, Yajuan Weng, Guoqing Wang, Liangyi Jin, Rui Guo, Bei Li, and Manglai Dugarjaviin. 2025. "Effect of Alternative Splicing Euchromatic Histone Lysine Methyltransferase 2 (EHMT2/G9A) on Spermatogenesis in Mongolian Horses" Animals 15, no. 8: 1106. https://doi.org/10.3390/ani15081106
APA StyleBaatar, T., Song, D., Weng, Y., Wang, G., Jin, L., Guo, R., Li, B., & Dugarjaviin, M. (2025). Effect of Alternative Splicing Euchromatic Histone Lysine Methyltransferase 2 (EHMT2/G9A) on Spermatogenesis in Mongolian Horses. Animals, 15(8), 1106. https://doi.org/10.3390/ani15081106




