Genetic Strategies for Enhancing Rooster Fertility in Tropical and Humid Climates: Challenges and Opportunities
Simple Summary
Abstract
1. Introduction
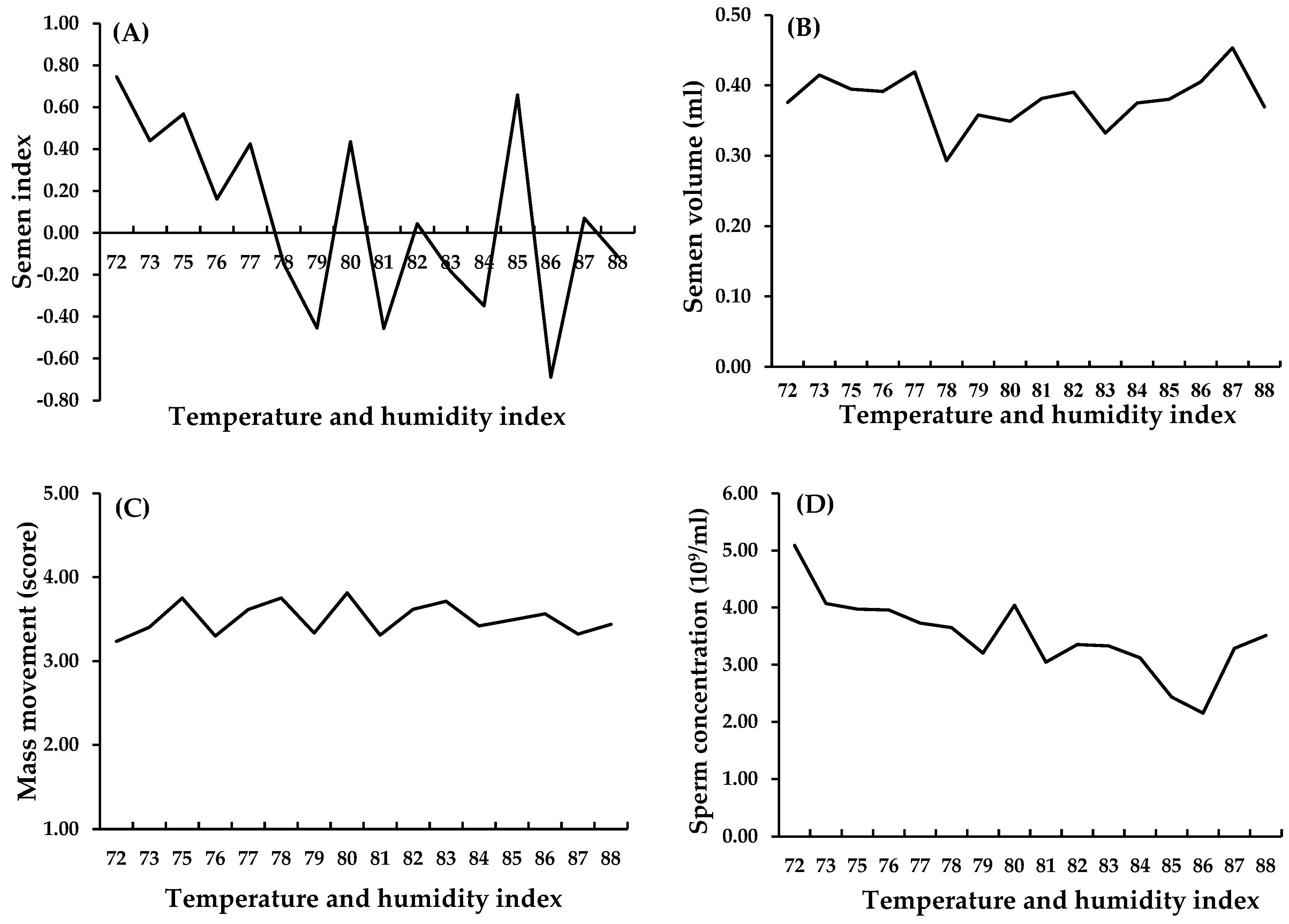
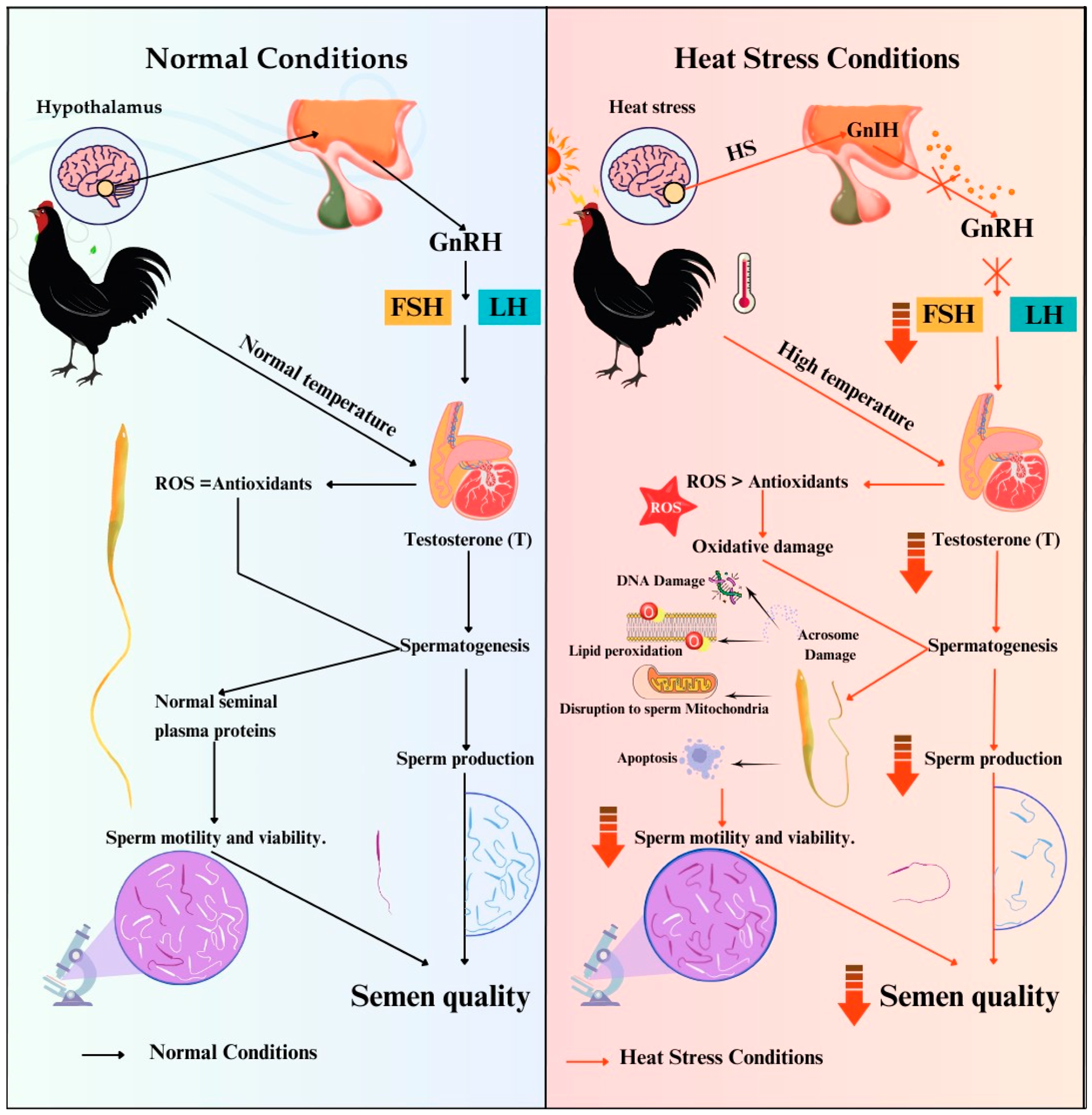
2. Effects of HS on Fertility Traits of Roosters
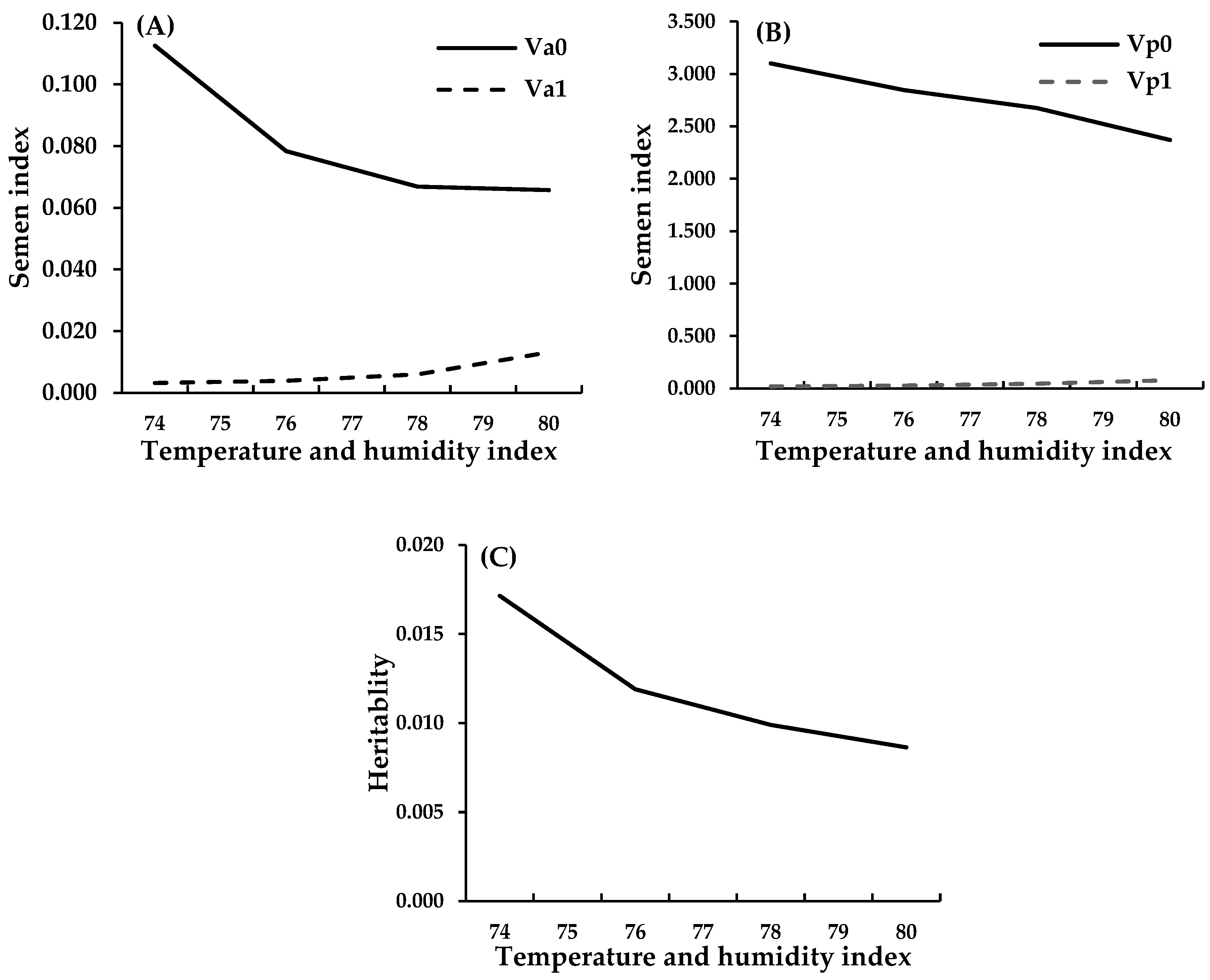
3. Genetic Improvement to Improve Production Efficiency in HS
4. Genomic Selection and Model Prediction
4.1. Genomic Best Linear Unbiased Prediction (GBLUP)
4.2. Single-Step Genomic Best Linear Unbiased Prediction (ssGBLUP)
4.3. Bayesian Approaches
4.4. Ridge Regression Genomic Best Linear Unbiased Prediction (RR-GBLUP)
4.5. Weighted Genomic Best Linear Unbiased Prediction (WGBLUP)
4.6. Multi-Trait Genomic Best Linear Unbiased Prediction (MTGBLUP)
5. Genome-Wide Association Study (GWAS)
6. Selection of Chicken Breeding Methods for Production Goals
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hafez, H.M.; Attia, Y.A. Challenges to the poultry industry: Current perspectives and strategic future after the COVID-19 Outbreak. Front. Vet. Sci. 2020, 7, 516. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-A.; Jung, Y.; Jo, C.; Park, J.-Y.; Nam, K.-C. Analysis of consumers’ preferences and price sensitivity to native chickens. Korean J. Food. Sci. Anim. Resour. 2017, 37, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Jaturasitha, S.; Srikanchai, T.; Kreuzer, M.; Wicke, M. Differences in carcass and meat characteristics between chicken indigenous to northern Thailand (black-boned and Thai native) and imported extensive breeds (Bresse and Rhode Island Red). Poult. Sci. 2008, 87, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Potue, P.; Chiangsaen, P.; Maneesai, P.; Khamseekaew, J.; Pakdeechote, P.; Chankitisakul, V.; Boonkum, W.; Duanghaklang, N.; Duangjinda, M. Effects of Thai native chicken breast meat consumption on serum uric acid level, biochemical parameters, and antioxidant activities in rats. Sci. Rep. 2022, 12, 14056. [Google Scholar] [CrossRef]
- Rikimaru, K.; Takahashi, H. Evaluation of the meat from Hinai-Jidori chickens and broilers: Analysis of general biochemical components, free amino acids, inosine 5′-monophosphate, and fatty acids. J. Appl. Poult. Res. 2010, 19, 327–333. [Google Scholar] [CrossRef]
- Lengkidworraphiphat, P.; Wongpoomchai, R.; Bunmee, T.; Chariyakornkul, A.; Chaiwang, N.; Jaturasitha, S. Taste-active and nutritional components of Thai native chicken meat: A perspective of consumer satisfaction. Food. Sci. Anim. Resour. 2021, 41, 237–246. [Google Scholar] [CrossRef]
- Jung, S.; Bae, Y.S.; Kim, H.J.; Jayasena, D.D.; Lee, J.H.; Park, H.B.; Heo, K.N.; Jo, C. Carnosine, anserine, creatine, and inosine 5′-monophosphate contents in breast and thigh meats from 5 lines of Korean native chicken. Poult. Sci. 2013, 92, 3275–3282. [Google Scholar] [CrossRef]
- Hidayat, C.; Asmarasari, S.A. Native chicken production in Indonesia: A review. JIIP 2015, 17, 1–11. [Google Scholar] [CrossRef]
- Park, S.; Kim, N.; Jang, Y.; Lee, D.; Moon, J. Poultry industry trends and consumer analysis in Korea: Native Korean chicken and processed chicken. Agribus. Inf. Manag. 2019, 11, 25–34. [Google Scholar] [CrossRef]
- Mogano, R.R.; Mpofu, T.J.; Mtileni, B.; Hadebe, K. South African indigenous chickens’ genetic diversity, and the adoption of ecological niche modelling and landscape genomics as strategic conservation techniques. Poult. Sci. 2025, 104, 104508. [Google Scholar] [CrossRef]
- Pym, R.; Bleich, E.G.; Hoffmann, I. The relative contribution of indigenous chicken breeds to poultry meat and egg production and consumption in the developing countries of Africa and Asia. In Proceedings of the 12th European Poultry Conference, Verona, Italy, 10–14 September 2006; pp. 10–14. [Google Scholar]
- Risk Status of Animal Genetic Resources. Domestic Animal Diversity Information System (DAD-IS); Food and Agriculture Organization (FAO) of the United Nations. Available online: https://www.fao.org/dad-is/risk-status-of-animal-genetic-resources/en/ (accessed on 21 February 2025).
- Besbes, B.; Tixier-Boichard, M.; Hoffmann, I.; Jain, G. Future trends for poultry genetic resources. In Poultry in the 21st Century: Avian Influenza and Beyond; FAO: Rome, Italy, 2007; pp. 299–323. [Google Scholar]
- McGary, S.; Estevez, I.; Bakst, M.R.; Pollock, D.L. Phenotypic traits as reliable indicators of fertility in male broiler breeders. Poult. Sci. 2002, 81, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Hafez, H.M.; Hauck, R. Genetic selection in turkeys and broilers and their impact on health conditions. In Proceedings of the 4th European Poultry Genetics Symposium, Dubrovnik, Croatia, 6–8 October 2005; World’s Poultry Science Association: Dubrovnik, Croatia, 2005; pp. 1–10. [Google Scholar]
- Barbarestani, S.Y.; Samadi, F.; Pirsaraei, Z.A.; Zaghari, M. Barley sprouts and D-aspartic acid supplementation improves fertility, hatchability, and semen quality in aging male broiler breeders by up-regulating StAR and P450SCC gene expressions. Poult. Sci. 2024, 103, 103664. [Google Scholar] [CrossRef] [PubMed]
- Tachiiri, K.; Silva Herran, D.; Su, X.; Kawamiya, M. Effect on the earth system of realizing a 1.5 °C warming climate target after overshooting to the 2 °C level. Environ. Res. Lett. 2019, 14, 124063. [Google Scholar] [CrossRef]
- Fernandes, E.; Raymundo, A.; Martins, L.L.; Lordelo, M.; de Almeida, A.M. The naked neck gene in the domestic chicken: A genetic strategy to mitigate the impact of heat stress in poultry production—A review. Animals 2023, 13, 1007. [Google Scholar] [CrossRef]
- Juiputta, J.; Chankitisakul, V.; Boonkum, W. Appropriate genetic approaches for heat tolerance and maintaining good productivity in tropical poultry production: A review. Vet. Sci. 2023, 10, 591. [Google Scholar] [CrossRef]
- El-Prollosy, A.; Iraqi, E.; Elsayed, N.; Khalil, H.; El-Saadany, A.; El-Sabrout, K. Impact of thermal manipulation during embryogenesis on thermotolerance and semen quality of mandarah roosters exposed to heat stress. Vet. World 2024, 17, 1311–1317. [Google Scholar] [CrossRef]
- Ratchamak, R.; Authaida, S.; Koedkanmark, T.; Boonkum, W.; Semaming, Y.; Chankitisakul, V. Dietary supplementation with ginseng extract enhances testicular function, semen preservation, and fertility rate of mature and aging Thai native roosters. Theriogenology 2024, 227, 31–40. [Google Scholar] [CrossRef]
- Long, C.; Shi, Y.-P.; Wang, Q.-Y.; Sheng, X.-H.; Wang, X.-G.; Xiao, L.-F.; Lin, Z.; Qi, X.-L. Dietary supplementation with lycopene improves semen quality and antioxidant status in breeder roosters. Poult. Sci. 2025, 104, 104658. [Google Scholar] [CrossRef]
- Juiputta, J.; Koedkanmark, T.; Chankitisakul, V.; Boonkum, W. Effect of heat stress on semen characteristics and genetics in Thai native grandparent roosters. Poult. Sci. 2024, 103, 104205. [Google Scholar] [CrossRef]
- Hu, J.; Chen, J.L.; Wen, J.; Zhao, G.P.; Zheng, M.Q.; Liu, R.R.; Liu, W.P.; Zhao, L.H.; Liu, G.F.; Wang, Z.W. Estimation of the genetic parameters of semen quality in Beijing-You chickens. Poult. Sci. 2013, 92, 2606–2612. [Google Scholar] [CrossRef]
- Wolc, A.; Arango, J.; Settar, P.; Fulton, J.E.; O’Sullivan, N.P.; Dekkers, J.C.M. Genetics of male reproductive performance in white leghorns. Poult. Sci. 2019, 98, 2729–2733. [Google Scholar] [CrossRef] [PubMed]
- Wolc, A.; Zhao, H.H.; Arango, J.; Settar, P.; Fulton, J.E.; O’Sullivan, N.P.; Preisinger, R.; Stricker, C.; Habier, D.; Fernando, R.L.; et al. Response and inbreeding from a genomic selection experiment in layer chickens. Genet. Sel. Evol. 2015, 47, 59. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yu, J.-Z.; Zhu, M.-Y.; Yang, F.-X.; Hao, J.-P.; He, Y.; Zhu, X.-L.; Hou, Z.-C.; Zhu, F. Optimizing breeding strategies for Peking ducks using genomic selection: Genetic parameter evaluation and selection progress analysis in reproductive traits. Appl. Sci. 2025, 15, 194. [Google Scholar] [CrossRef]
- Husien, H.M.; Saleh, A.A.; Hassanine, N.N.A.M.; Rashad, A.M.A.; Sharaby, M.A.; Mohamed, A.Z.; Abdelhalim, H.; Hafez, E.E.; Essa, M.O.A.; Adam, S.Y.; et al. The evolution and role of molecular tools in measuring diversity and genomic selection in livestock populations (traditional and up-to-date insights): A comprehensive exploration. Vet. Sci. 2024, 11, 627. [Google Scholar] [CrossRef]
- Durmuş, İ.; Kamanlı, S. Effects of cold and heat stress on egg quality traits of a newly developed native hybrid layer. TURJAF 2015, 3, 444–447. [Google Scholar] [CrossRef]
- Wang, S.-H.; Cheng, C.-Y.; Chen, C.-J.; Chen, H.-H.; Tang, P.-C.; Chen, C.-F.; Lee, Y.-P.; Huang, S.-Y. Changes in protein expression in testes of l2 strain Taiwan country chickens in response to acute heat stress. Theriogenology 2014, 82, 80–94. [Google Scholar] [CrossRef]
- Attia, Y.A.; El-Naggar, A.S.; Abou-Shehema, B.M.; Abdella, A.A. Effect of supplementation with trimethylglycine (Betaine) and/or vitamins on semen quality, fertility, antioxidant status, DNA repair and welfare of roosters exposed to chronic heat stress. Animals 2019, 9, 547. [Google Scholar] [CrossRef]
- Pimprasert, M.; Kheawkanha, T.; Boonkum, W.; Chankitisakul, V. Influence of semen collection frequency and seasonal variations on fresh and frozen semen quality in Thai native roosters. Animals 2023, 13, 573. [Google Scholar] [CrossRef]
- Jiang, D.L.; Pan, J.Q.; Li, J.Q.; Zhou, X.L.; Shen, X.; Xu, D.N.; Tian, Y.B.; Huang, Y.M. Effects of gonadotropin-inhibitory hormone on testicular development and reproduction-related gene expression in roosters. Anim. Biotechnol. 2023, 34, 4105–4115. [Google Scholar] [CrossRef]
- Robertson, A. The sampling variance of the genetic correlation coefficient. Biometrics 1959, 15, 469–485. [Google Scholar] [CrossRef]
- Ravagnolo, O.; Misztal, I. Genetic component of heat stress in dairy cattle, parameter estimation. J. Dairy Sci. 2000, 83, 2126–2130. [Google Scholar] [CrossRef] [PubMed]
- Bohlouli, M.; Alijani, S.; Naderi, S.; Yin, T.; König, S. Prediction accuracies and genetic parameters for test-day traits from genomic and pedigree-based random regression models with or without heat stress interactions. J. Dairy Sci. 2019, 102, 488–502. [Google Scholar] [CrossRef] [PubMed]
- Loengbudnark, W.; Chankitisakul, V.; Boonkum, W. The genetic impact of heat stress on the egg production of Thai native chickens (Pradu Hang Dum). PLoS ONE 2023, 18, e0281328. [Google Scholar] [CrossRef]
- Boonkum, W.; Chankitisakul, V.; Kananit, S.; Kenchaiwong, W. Heat stress effects on the genetics of growth traits in Thai native chickens (Pradu Hang dum). Anim. Biosci. 2024, 37, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.T.; Bowman, P.J.; Haile-Mariam, M.; Pryce, J.E.; Hayes, B.J. Genomic selection for tolerance to heat stress in Australian dairy cattle. J. Dairy Sci. 2016, 99, 2849–2862. [Google Scholar] [CrossRef]
- Meuwissen, T.H.E.; Hayes, B.J.; Goddard, M.E. Prediction of total genetic value using genome-wide dense marker maps. Genetics 2001, 157, 1819–1829. [Google Scholar] [CrossRef]
- Liu, R.; Zheng, M.; Wang, J.; Cui, H.; Li, Q.; Liu, J.; Zhao, G.; Wen, J. Effects of genomic selection for intramuscular fat content in breast muscle in Chinese local chickens. Anim. Genet. 2019, 50, 87–91. [Google Scholar] [CrossRef]
- Tan, X.; Liu, R.; Li, W.; Zheng, M.; Zhu, D.; Liu, D.; Feng, F.; Li, Q.; Liu, L.; Wen, J.; et al. Assessment the effect of genomic selection and detection of selective signature in broilers. Poult. Sci. 2022, 101, 101856. [Google Scholar] [CrossRef]
- Clark, S.A.; van der Werf, J. Genomic best linear unbiased prediction (gBLUP) for the estimation of genomic breeding values. In Genome-Wide Association Studies and Genomic Prediction. Methods in Molecular Biology, Vol 1019; Gondro, C., van der Werf, J., Hayes, B., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 321–330. [Google Scholar] [CrossRef]
- Tu, T.-C.; Lin, C.-J.; Liu, M.-C.; Hsu, Z.-T.; Chen, C.-F. Comparison of genomic prediction accuracy using different models for egg production traits in Taiwan country chicken. Poult. Sci. 2024, 103, 104063. [Google Scholar] [CrossRef]
- Daetwyler, H.D.; Pong-Wong, R.; Villanueva, B.; Woolliams, J.A. The impact of genetic architecture on genome-wide evaluation methods. Genetics 2010, 185, 1021–1031. [Google Scholar] [CrossRef]
- Erbe, M.; Hayes, B.J.; Matukumalli, L.K.; Goswami, S.; Bowman, P.J.; Reich, C.M.; Mason, B.A.; Goddard, M.E. Improving accuracy of genomic predictions within and between dairy cattle breeds with imputed high-density single nucleotide polymorphism panels. J. Dairy Sci. 2012, 95, 4114–4129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lourenco, D.; Aguilar, I.; Legarra, A.; Misztal, I. Weighting strategies for single-step genomic BLUP: An iterative approach for accurate calculation of GEBV and GWAS. Front. Genet. 2016, 7, 151. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Lee, J.H.; Gondro, C.; Koh, Y.J.; Lee, S.H. deepGBLUP: Joint deep learning networks and GBLUP framework for accurate genomic prediction of complex traits in Korean native cattle. Genet. Sel. Evol. 2023, 55, 56. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Misztal, I.; Aguilar, I.; Tsuruta, S.; Meuwissen, T.H.E.; Aggrey, S.E.; Wing, T.; Muir, W.M. Genome-wide marker-assisted selection combining all pedigree phenotypic information with genotypic data in one step: An example using broiler chickens. J. Anim. Sci. 2011, 89, 23–28. [Google Scholar] [CrossRef]
- Tu, T.-C.; Lin, C.-J.; Liu, M.-C.; Hsu, Z.-T.; Chen, C.-F. Genomic prediction and genome-wide association study for growth-related traits in Taiwan country chicken. Animals 2025, 15, 376. [Google Scholar] [CrossRef]
- Zhu, R.; Zhang, Y.; Jiang, Y.; Xu, Z.; Tai, Y.; Lian, Z.; Li, Z.; Wang, X.; Luo, N.; Zhao, G.; et al. Weighted single-step genome-wide association study identified genomic regions and candidate genes for growth and reproductive traits in Wenchang chicken. Poult. Sci. 2025, 104733. [Google Scholar] [CrossRef]
- Fang, X.; Ye, H.; Zhang, S.; Guo, L.; Xu, Y.; Zhang, D.; Nie, Q. Investigation of potential genetic factors for growth traits in yellow-feather broilers using weighted single-step genome-wide association study. Poult. Sci. 2023, 102, 103034. [Google Scholar] [CrossRef]
- Pan, R.; Qi, L.; Xu, Z.; Zhang, D.; Nie, Q.; Zhang, X.; Luo, W. Weighted single-step GWAS identified candidate genes associated with carcass traits in a Chinese yellow-feathered chicken population. Poult. Sci. 2024, 103, 103341. [Google Scholar] [CrossRef]
- Makanjuola, B.O.; Abdalla, E.A.; Wood, B.J.; Baes, C.F. Applicability of single-step genomic evaluation with a random regression model for reproductive traits in turkeys (Meleagris gallopavo). Front. Genet. 2022, 13, 923766. [Google Scholar] [CrossRef]
- Vandenplas, J.; ten Napel, J.; Darbaghshahi, S.N.; Evans, R.; Calus, M.P.L.; Veerkamp, R.; Cromie, A.; Mäntysaari, E.A.; Strandén, I. Efficient large-scale single-step evaluations and indirect genomic prediction of genotyped selection candidates. Genet. Sel. Evol. 2023, 55, 37. [Google Scholar] [CrossRef]
- Blasco, A. The Bayesian controversy in animal breeding. J. Anim. Sci. 2001, 79, 2023–2046. [Google Scholar] [CrossRef] [PubMed]
- Habier, D.; Fernando, R.L.; Kizilkaya, K.; Garrick, D.J. Extension of the Bayesian alphabet for genomic selection. BMC Bioinform. 2011, 12, 186. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Jang, E.-B.; Lee, H.-D.; Shin, D.-H.; Jang, J.-H.; Kim, J.-J. Performance of weighted genomic BLUP and Bayesian methods for Hanwoo carcass traits. Trop. Anim. Health Prod. 2025, 57, 38. [Google Scholar] [CrossRef] [PubMed]
- Gianola, D.; de los Campos, G.; Hill, W.G.; Manfredi, E.; Fernando, R. Additive genetic variability and the Bayesian alphabet. Genetics 2009, 183, 347–363. [Google Scholar] [CrossRef]
- Asgari, Z.; Ehsani, A.; Masoudi, A.A.; Torshizi, R.V. Bayes factors revealed selection signature for time to market body weight in chicken: A genome-wide association study using BayesCpi methodology. Ital. J. Anim. Sci. 2021, 20, 1468–1478. [Google Scholar] [CrossRef]
- Fernando, R.L.; Garrick, D. Bayesian methods applied to GWAS. Methods. Mol. Biol. 2013, 1019, 237–274. [Google Scholar] [CrossRef]
- Hayashi, T.; Iwata, H. A Bayesian method and its variational approximation for prediction of genomic breeding values in multiple traits. BMC Bioinform. 2013, 14, 34. [Google Scholar] [CrossRef]
- Wei, Z.; Conlon, E.M. Parallel Markov chain Monte Carlo for Bayesian hierarchical models with big data, in two stages. J. Appl. Stat. 2019, 46, 1917–1936. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, W.; Zhang, L.; Gong, D.; Shi, Q. Variational Bayesian dropout with a hierarchical prior. In Proceedings of the 2019 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), Long Beach, CA, USA, 15–20 June 2019; pp. 7124–7133. [Google Scholar]
- Coninck, A.D.; Fostier, J.; Maenhout, S.; De Baets, B. DAIRRy-BLUP: A high-performance computing approach to genomic prediction. Genetics 2014, 197, 813–822. [Google Scholar] [CrossRef]
- de los Campos, G.; Hickey, J.M.; Pong-Wong, R.; Daetwyler, H.D.; Calus, M.P.L. Whole-genome regression and prediction methods applied to plant and animal breeding. Genetics 2013, 193, 327–345. [Google Scholar] [CrossRef]
- VanRaden, P.M. Efficient methods to compute genomic predictions. J. Dairy Sci. 2008, 91, 4414–4423. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi-Arpanahi, R.; Morota, G.; Valente, B.D.; Kranis, A.; Rosa, G.J.M.; Gianola, D. Differential contribution of genomic regions to marked genetic variation and prediction of quantitative traits in broiler chickens. Genet. Sel. Evol. 2016, 48, 10. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Reif, J.C. Modeling epistasis in genomic selection. Genetics 2015, 201, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Wolc, A.; Dekkers, J.C.M. Application of Bayesian genomic prediction methods to genome-wide association analyses. Genet. Sel. Evol. 2022, 54, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, J.; Ding, X.; Bijma, P.; de Koning, D.-J.; Zhang, Q. Best linear unbiased prediction of genomic breeding values using a trait-specific marker-derived relationship matrix. PLoS ONE 2010, 5, e12648. [Google Scholar] [CrossRef]
- Romé, H.; Chu, T.T.; Marois, D.; Huang, C.-H.; Madsen, P.; Jensen, J. Accounting for genetic architecture for body weight improves accuracy of predicting breeding values in a commercial line of broilers. J. Anim. Breed. Genet. 2021, 138, 528–540. [Google Scholar] [CrossRef]
- Bradford, H.L.; Pocrnić, I.; Fragomeni, B.O.; Lourenco, D.A.L.; Misztal, I. Selection of core animals in the algorithm for proven and young using a simulation model. J. Anim. Breed. Genet. 2017, 134, 545–552. [Google Scholar] [CrossRef]
- Tang, X.; Xiao, S.; Ding, N.; Zhang, Z.; Huang, L. Comparative study of single-trait and multi-trait genomic prediction models. Animals 2024, 14, 2961. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, M.; Yue, B.; Wang, H.; Li, X.; Peng, W.; Jiang, M.; Zhong, J.; Kangzhu, Y.; Wang, J. Comparison of predictive ability of single-trait and multitrait genomic selection models for body growth traits in Meiwa yaks. Animal 2024, 18, 101350. [Google Scholar] [CrossRef]
- Zhang, X.; Tsuruta, S.; Andonov, S.; Lourenco, D.A.L.; Sapp, R.L.; Wang, C.; Misztal, I. Relationships among mortality, performance, and disorder traits in broiler chickens: A genetic and genomic approach. Poult. Sci. 2018, 97, 1511–1518. [Google Scholar] [CrossRef]
- Teng, J.; Zhai, T.; Zhang, X.; Zhao, C.; Wang, W.; Tang, H.; Wang, D.; Shang, Y.; Ning, C.; Zhang, Q. Improving multi-population genomic prediction accuracy using multi-trait GBLUP models which incorporate global or local genetic correlation information. Brief. Bioinform. 2024, 25, bbae276. [Google Scholar] [CrossRef] [PubMed]
- Karaman, E.; Lund, M.S.; Anche, M.T.; Janss, L.; Su, G. Genomic prediction using multi-trait weighted GBLUP accounting for heterogeneous variances and covariances across the genome. G3 2018, 8, 3549–3558. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, C.; Miller, S.; Schenkel, F. Multi-population genomic prediction using a multi-task Bayesian learning model. BMC Genet. 2014, 15, 53. [Google Scholar] [CrossRef] [PubMed]
- Dementieva, N.; Kudinov, A.; Pozovnikova, M.; Nikitkina, E.; Pleshanov, N.; Silyukova, Y.; Krutikova, A.; Plemyashov, K. Genome-wide association studies of cryostability of semen in roosters. Pol. J. Vet. Sci. 2020, 23, 461–463. [Google Scholar] [CrossRef]
- Dementieva, N.V.; Nikitkina, E.V.; Shcherbakov, Y.S.; Pleshanov, N.V.; Ryabova, A.E.; Azovtseva, A.I.; Silyukova, Y.L.; Musidray, A.A.; Griffin, D.K.; Romanov, M.N. Genome-wide analysis of genetic predispositions linked to damaged membranes and impaired fertility as indicators of compromised sperm–egg interaction mechanisms in frozen–thawed rooster semen. FBS 2025, 17, 26022. [Google Scholar] [CrossRef]
- Zhang, G.M.; Liu, P.H.; Chen, L.; Zheng, J.M.; Zhao, G.P.; Xing, W.H.; Wen, J.; Li, Q.H. Genome-wide association study identifies variants associated with semen volume in white-feathered broilers. Anim. Genet. 2023, 54, 803–807. [Google Scholar] [CrossRef]
- Menchaca, A.; dos Santos-Neto, P.C.; Mulet, A.P.; Crispo, M. CRISPR in livestock: From editing to printing. Theriogenology 2020, 150, 247–254. [Google Scholar] [CrossRef]
- Park, T.S. Gene-editing techniques and their applications in livestock and beyond. Anim. Biosci. 2023, 36, 333–338. [Google Scholar] [CrossRef]
- Zhuang, Z.-X.; Chen, S.-E.; Chen, C.-F.; Lin, E.-C.; Huang, S.-Y. Single-Nucleotide Polymorphisms in Genes Related to Oxidative Stress and Ion Channels in Chickens Are Associated with Semen Quality and Hormonal Responses to Thermal Stress. J. Therm. Biol. 2022, 105, 103220. [Google Scholar] [CrossRef]
- Dementieva, N.; Nikitkina, E.; Pleshanov, N.; Musidray, A.; Bogdanova, S.; Scherbakov, Y.; Krutikova, A.; Ryabova, A.; Reinbach, N. Search for Genome-Wide Associations with the Number of Damaged Membranes in Rooster Semen after Freezing. Anim. Reprod. Sci. 2022, 247, 107125. [Google Scholar] [CrossRef]
| Model | Trait | Process (Step) | Accuracy (%) | Time (Hour) | Program | Limit | Published |
|---|---|---|---|---|---|---|---|
| GBLUP | Production | 2 | ~70–85% | 3–5 | ASReml BLUPF90 | Assumes normal distribution | Yes |
| Fertility | |||||||
| ssGBLUP | Fertility | 1 | ~75–90% | 1–2 | ssGBLUP ASReml | Requires accurate pedigree data, higher RAM usage with large datasets | Yes |
| Diseases | |||||||
| Health | |||||||
| Environmental | |||||||
| Bayesian | Quality | 2–3 | ~80–95% | 12–48 | BayesR BGLR | Slowest computation time, prior SNP distribution setup needed | No |
| Fertility | |||||||
| Health | |||||||
| Health | |||||||
| RR-GBLUP | Production | 2 | ~65–80% | 3–5 | rrBLUP | Assumes no linkage disequilibrium | No |
| WGBLUP | Fertility | 2 | ~75–90% | 5–8 | ASReml BLUPF90 | Needs GWAS data for SNP weighting | No |
| Diseases | |||||||
| Health | |||||||
| Environmental | |||||||
| MTGBLUP | Production | 2 | ~80–95% | 5–8 | MTG2 BLUPF90 | Longer computation, requires ample multi-trait data | No |
| Quality | |||||||
| Fertility | |||||||
| Environmental |
| SNP Number | Gene | SNPs | Chromosome | Position | Trait | Reference |
|---|---|---|---|---|---|---|
| 60 K | LOXL1 | rs15557972 | 10 | 9810123 | Sperm motility | [80] |
| ENSGALG00000052127 | rs15751385 | 6 | 34380465 | Sperm motility | ||
| 600 K | TRPC1 | Affx-51823443 | 9 | 10568531 | Sperm concentration | [85] |
| Affx-51823444 | 9 | 10568594 | Sperm concentration | |||
| SLC9A9 | Affx-51824235 | 9 | 10856343 | Sperm concentration | ||
| Affx-51824375 | 9 | 10909735 | Sperm concentration | |||
| CUL3 | Affx-51873906 | 9 | 8433286 | Sperm concentration | ||
| MTF1 | Affx-51092352 | 23 | 3563615 | Sperm concentration | ||
| Affx-51092326 | 23 | 3557077 | Sperm concentration | |||
| 600 K | PHF14 | AX-76063628 | 2 | 26182792 | Sperm membrane | [86] |
| ARID1B | AX-76495998 | 3 | 51262693 | Sperm membrane | ||
| 55 K | FAPP1, OSBPL6, | Not specified | 7 | 13820000–16120000 | Semen volume | [82] |
| SESTD1, SSFA2 | ||||||
| 600 K | ENSGALG00000029931 | Not specified | Not specified | 168850183 | Sperm motility | [81] |
| KDELR3 | AX-75466971 | 1 | 50898160 | Sperm respiration | ||
| DDX17 | AX-75466971 | 1 | 50898160 | Sperm respiration | ||
| DMD | AX-75221789 | 1 | 116157001 | Sperm respiration | ||
| CDKL5 | AX-75231769 | 1 | 122024645 | Sperm respiration | ||
| DGAT2 | AX-75397985 | 1 | 196966714 | Sperm respiration | ||
| ST18 | AX-80992139 | 2 | 109830505 | Sperm respiration | ||
| FAM150A | AX-80992139 | 2 | 109830505 | Sperm respiration | ||
| DIAPH2 | AX-80778510 | 4 | 5664389 | Sperm respiration | ||
| MTMR7 | AX-76705102 | 4 | 63101468 | Sperm respiration | ||
| NAV2 | AX-76788932 | 5 | 1970758 | Sperm respiration | ||
| RAG2 | AX-76791651 | 5 | 20089359 | Sperm respiration | ||
| PDE11A | AX-76986124 | 7 | 15619919 | Sperm respiration | ||
| IFT70A | AX-76986304 | 7 | 15687930 | Sperm respiration | ||
| AGPS | AX-76986304 | 7 | 15687930 | Sperm respiration | ||
| WDFY1 | AX-77181439 | 9 | 8463525 | Sperm respiration | ||
| DEPDC5 | AX-75848147 | 15 | 9143016 | Sperm respiration | ||
| TSC1 | AX-75873724 | 17 | 7048201 | Sperm respiration | ||
| CASZ1 | AX-76244713 | 21 | 3983187 | Sperm respiration | ||
| PLEKHM2 | AX-76245698 | 21 | 4201372 | Sperm respiration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juiputta, J.; Chankitisakul, V.; Boonkum, W. Genetic Strategies for Enhancing Rooster Fertility in Tropical and Humid Climates: Challenges and Opportunities. Animals 2025, 15, 1096. https://doi.org/10.3390/ani15081096
Juiputta J, Chankitisakul V, Boonkum W. Genetic Strategies for Enhancing Rooster Fertility in Tropical and Humid Climates: Challenges and Opportunities. Animals. 2025; 15(8):1096. https://doi.org/10.3390/ani15081096
Chicago/Turabian StyleJuiputta, Jiraporn, Vibuntita Chankitisakul, and Wuttigrai Boonkum. 2025. "Genetic Strategies for Enhancing Rooster Fertility in Tropical and Humid Climates: Challenges and Opportunities" Animals 15, no. 8: 1096. https://doi.org/10.3390/ani15081096
APA StyleJuiputta, J., Chankitisakul, V., & Boonkum, W. (2025). Genetic Strategies for Enhancing Rooster Fertility in Tropical and Humid Climates: Challenges and Opportunities. Animals, 15(8), 1096. https://doi.org/10.3390/ani15081096






