Milk Yield, Major Milk Components and Macro Minerals in Blood Serum of Lactating Donkeys, as Affected by Dietary Trace Element Supplementation and Stage of Lactation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets, and Sampling
2.2. Blood Serum and Milk Analyses
2.3. Statistical Analysis
3. Results
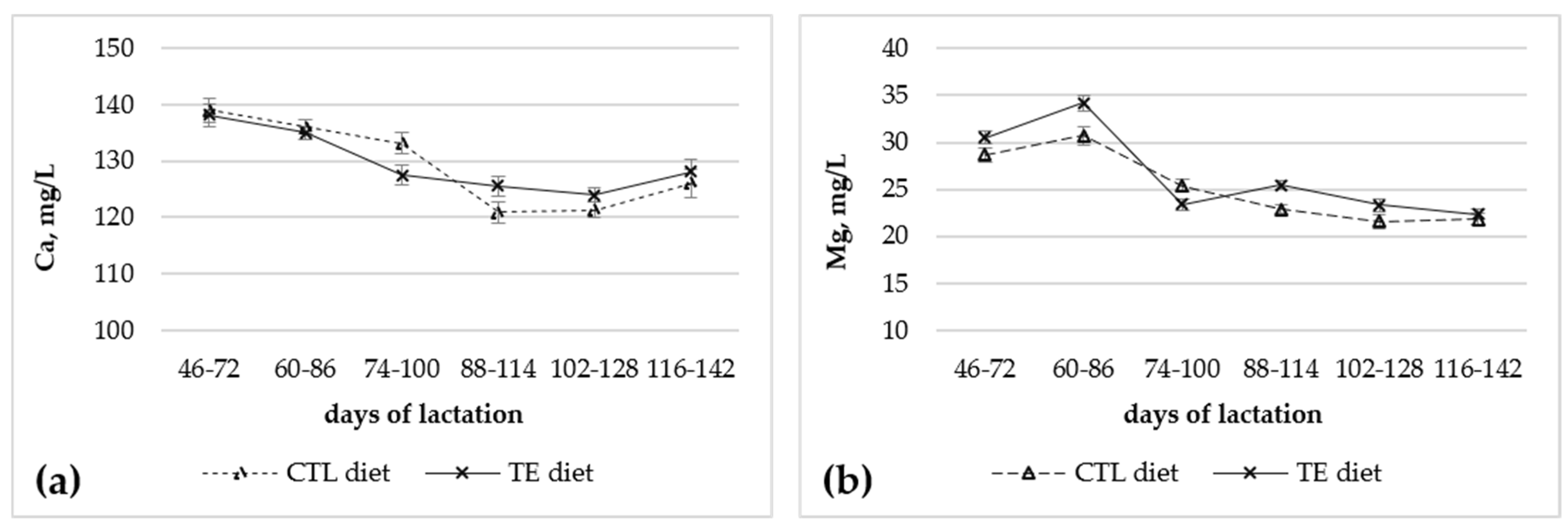
3.1. Effect of Dietary Trace Elements Supplementation on Milk Yield per Milking, Milk Gross Composition, and Serum Macro Minerals
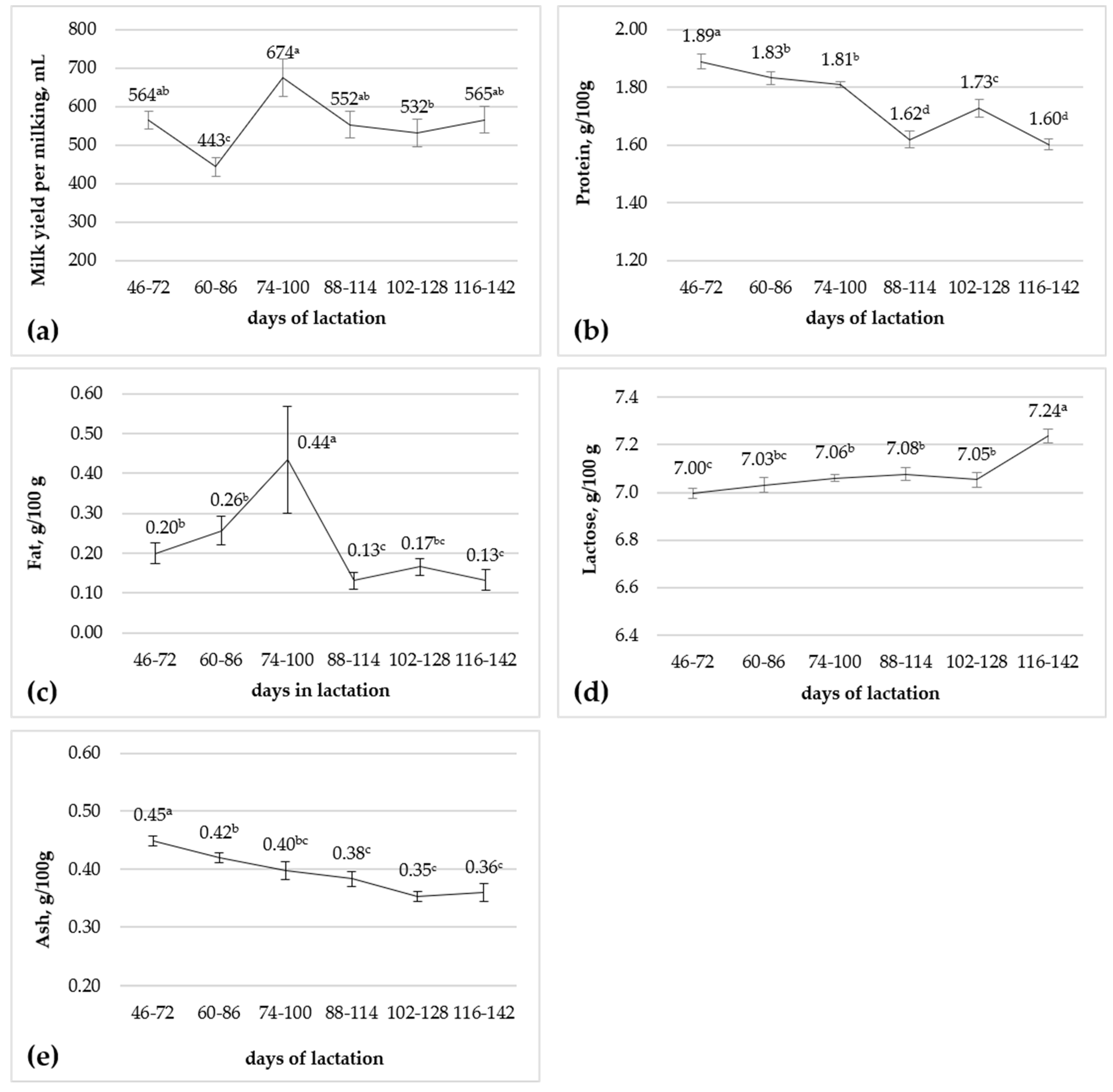
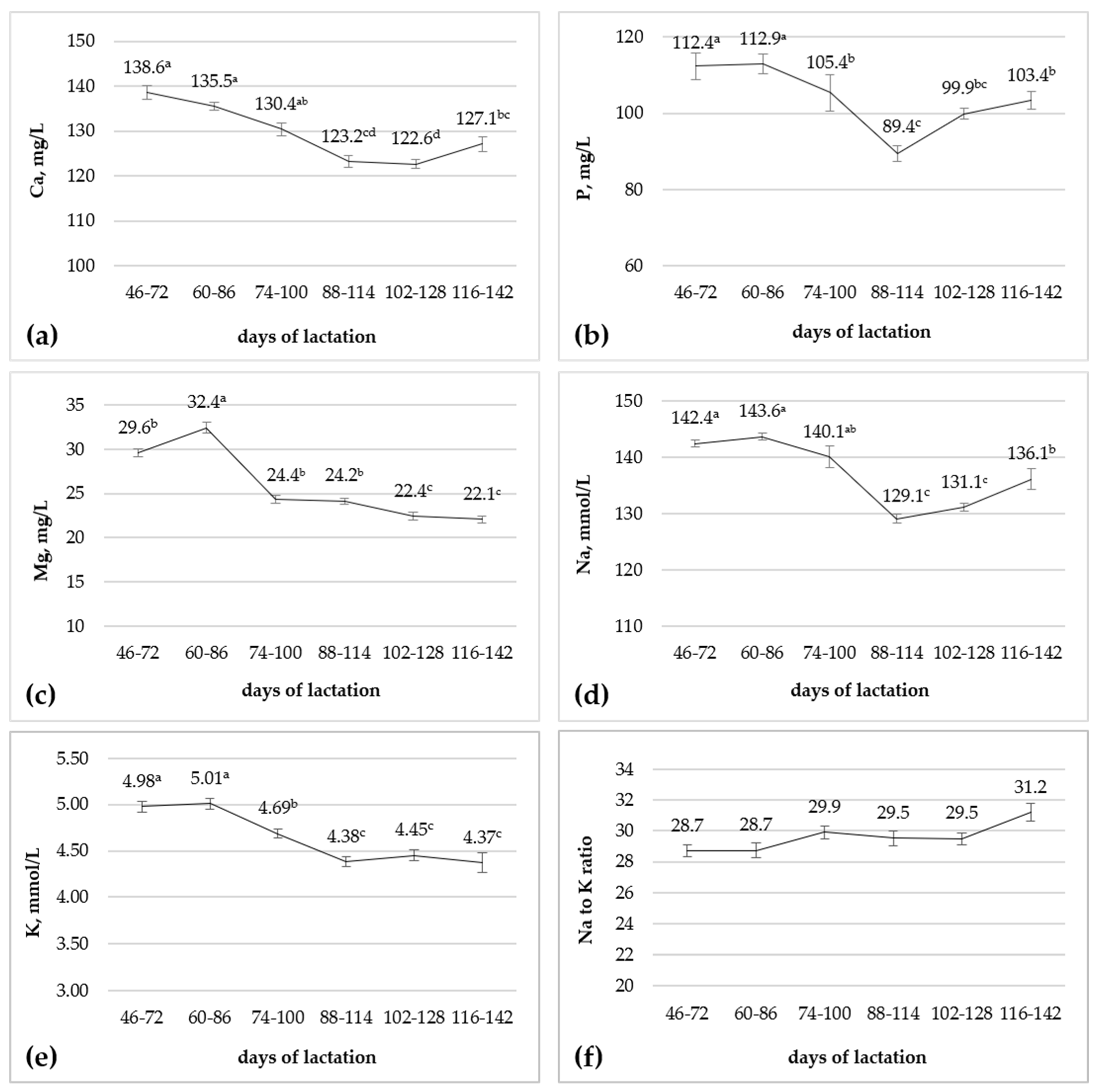
3.2. Effect of Stage of Lactation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salimei, E.; Fantuz, F. Donkey. Encyclopedia of Dairy Sciences, 3rd ed.; McSweeney, P.L.H., McNamara, J.P., Eds.; Elsevier/Academic Press: Amsterdam, The Netherlands; San Diego, CA, USA, 2022; Volume 1, pp. 65–76. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 25 March 2025).
- Salimei, E.; Fantuz, F. Equid milk for human consumption. Int. Dairy J. 2012, 24, 130–142. [Google Scholar] [CrossRef]
- Mansueto, P.; Iacono, G.; Taormina, G.; Seidita, A.; D’Alcamo, A.; Adragna, F.; Randazzo, G.; Carta, M.; Rini, G.; Carroccio, A. Ass’s milk in allergy to cow’s milk protein: A review. Acta Med. Mediter. 2013, 29, 153–160. [Google Scholar]
- Papademas, P.; Aspri, M.; Malissiova, E.; Fantuz, F.; Salimei, E. Donkey milk. In Encyclopedia of Dairy Sciences, 3rd ed.; McSweeney, P.L.H., McNamara, J.P., Eds.; Elsevier/Academic Press: Amsterdam, The Netherlands; San Diego, CA, USA, 2022; Volume 1, pp. 522–529. [Google Scholar] [CrossRef]
- Meena, S.; Meena, G.S.; Gautam, P.B.; Rai, D.C.; Kumari, S. A comprehensive review on donkey milk and its products: Composition, functionality and processing aspects. Food Chem. Adv. 2024, 4, 100647. [Google Scholar] [CrossRef]
- Salimei, E.; Fantuz, F. Horse and donkey milk. In Milk and Dairy Product in Human Nutrition: Production, Composition and Health; Park, Y.W., Haenlein, G.F.W., Eds.; John Wiley & Sons: Washington, DC, USA, 2013; pp. 594–613. [Google Scholar] [CrossRef]
- Miraglia, N.; Salimei, E.; Fantuz, F. Equine milk production and valorization of marginal areas—A review. Animals 2020, 10, 353. [Google Scholar] [CrossRef]
- Martin Rosset, W. Donkey nutrition and feeding: Nutrient requirements and recommended allowances—A review. J. Eq. Vet. Sci. 2018, 65, 75–85. [Google Scholar] [CrossRef]
- Raspa, F.; Cavallarin, L.; McLean, A.K.; Bergero, D.; Valle, E. A Review of the appropriate nutrition welfare criteria of dairy donkeys: Nutritional requirements, farm management requirements and animal-based indicators. Animals 2019, 9, 315. [Google Scholar] [CrossRef]
- Salari, F.; Licitra, R.; Altomonte, I.; Martini, M. Donkey feeding during maintenance, pregnancy, and lactation: Effects on body weight, milk production, and foal growth. J. Equine Vet. Sci. 2020, 91, 103131. [Google Scholar] [CrossRef]
- McDowel, L.R. General introduction. In Minerals in Animal and Human Nutrition, 2nd ed.; McDowel, L.R., Ed.; Elsevier Science: Amsterdam, The Netherlands; San Diego, CA, USA, 2003; pp. 1–32. [Google Scholar] [CrossRef]
- Coenen, M. Macro and trace elements in equine nutrition. In Equine Applied and Clinical Nutrition; Goer, R.J., Harris, P.A., Coenen, M., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2013; pp. 190–228. [Google Scholar] [CrossRef]
- Schweinzer, V.; Iwersen, M.; Drillich, M.; Wittek, T.; Tichy, A.; Mueller, A.; Krametter-Froetscher, R. Macromineral and Trace Element Supply in Sheep and Goats in Austria. Vet. Med. 2017, 62, 62–73. [Google Scholar] [CrossRef]
- Bonelli, F.; Rota, A.; Corazza, M.; Serio, D.; Sgorbini, M. Hematological and biochemical findings in pregnant, postfoaling, and lactating jennies. Theriogenology 2016, 85, 1233–1238. [Google Scholar] [CrossRef]
- Liao, Q.C.; Li, Z.; Han, Y.W.; Deng, L. Comparative analysis of serum mineral and biochemical parameter profiles between late pregnant and early lactating jennies. J. Equine Vet. Sci. 2021, 99, 103401. [Google Scholar] [CrossRef]
- Hui, F.; Tong, M.; Li, S.; Zhao, Y.; Guo, X.; Guo, Y.; Shi, B.; Yan, S. Effect of dietary energy level during late gestation on mineral contents in colostrum, milk, and plasma of lactating jennies. Animals 2024, 14, 2383. [Google Scholar] [CrossRef]
- Ghorbani, A.; Mohit, A.; Kuhi, H.D. Effects of dietary mineral intake on hair and serum mineral contents of horses. J Eq. Vet. Sci. 2015, 35, 295–300. [Google Scholar] [CrossRef]
- Fantuz, F.; Ferraro, S.; Todini, L.; Mariani, P.; Piloni, R.; Salimei, E. Essential trace elements in milk and blood serum of lactating donkeys as affected by lactation stage and dietary supplementation with trace elements. Animal 2013, 7, 1893–1899. [Google Scholar] [CrossRef] [PubMed][Green Version]
- National Research Council. Nutrient Requirements of Horses, 6th ed.; The National Academies: Washington, DC, USA, 2007. [Google Scholar]
- Salimei, E.; Fantuz, F.; Coppola, R.; Chiofalo, B.; Polidori, P.; Varisco, G. Composition and characteristics of ass’s milk. Anim. Res. 2004, 53, 67–78. [Google Scholar] [CrossRef]
- Fantuz, F.; Ferraro, S.; Todini, L.; Piloni, R.; Mariani, P.; Salimei, E. Donkey milk concentration of calcium, phosphorus, potassium, sodium, and magnesium. Int. Dairy J. 2012, 24, 143–145. [Google Scholar] [CrossRef]
- Fantuz, F.; Ferraro, S.; Todini, L.; Piloni, R.; Mariani, P.; Malissiova, E.; Salimei, E. Minor and potentially toxic trace elements in milk and blood serum of dairy donkeys. J. Dairy Sci. 2015, 98, 5125–5132. [Google Scholar] [CrossRef]
- Todini, L.; Salimei, E.; Malfatti, A.; Ferraro, S.; Fantuz, F. Thyroid hormones in milk and blood of lactating donkeys as affected by stage of lactation and dietary supplementation with trace elements. J. Dairy Res. 2012, 79, 232–237. [Google Scholar] [CrossRef]
- Todini, L.; Salimei, E.; Malfatti, A.; Brunetti, V.; Fantuz, F. Thyroid hormones in donkey blood and milk: Correlations with milk yield and environmental temperatures. Ital. J. Anim. Sci. 2015, 14, 4089. [Google Scholar] [CrossRef]
- Salimei, E.; Fantuz, F.; Varisco, G.; Maglieri, C.; Polidori, M. Different fibre sources in dairy ass’s diet: Effects on milk yield and composition. Ital. J. Anim. Sci. 2005, 4, 430–432. [Google Scholar] [CrossRef]
- Salimei, E.; Rosi, F.; Maglieri, C.; Magistrelli, D.; Fantuz, F. Leptin in milk and plasma of dairy asses. Ital. J. Anim. Sci. 2007, 6, 654–656. [Google Scholar] [CrossRef]
- Fantuz, F.; Maglieri, C.; Casamassima, D.; Palazzo, M.; Chiofalo, B.; Salimei, E. Nutritional status of dairy asses managed with different machine milking strategies. Ital. J. Anim. Sci. 2007, 6, 647–649. [Google Scholar] [CrossRef][Green Version]
- Fantuz, F.; Ferraro, S.; Todini, L.; Cimarelli, L.; Fatica, A.; Marcantoni, F.; Salimei, E. Distribution of calcium, phosphorus, sulfur, magnesium, potassium, and sodium in major fractions of donkey milk. J. Dairy Sci. 2020, 103, 8741–8749. [Google Scholar] [CrossRef] [PubMed]
- Alabiso, M.; Giosuè, C.; Alicata, M.L.; Mazza, F.; Iannolino, G. The effects of different milking intervals and milking times per day in jennet milk production. Animal 2009, 3, 543–547. [Google Scholar] [CrossRef]
- D’Alessandro, A.G.; Martemucci, G. Lactation curve and effects of milking regimen on milk yield and quality, and udder health in Martina Franca jennies (Equus asinus). J. Anim. Sci. 2012, 90, 669–681. [Google Scholar] [CrossRef]
- Fantuz, F.; Salimei, E.; Papademas, P. Macro- and micro-nutrients in non-cow milk and products and their impact on human health. In Non-Bovine Milk and Milk Products; Tsakalidou, E., Papadimitriou, K., Eds.; Academic Press: London, UK, 2016; pp. 209–261. [Google Scholar] [CrossRef]
- Lopez, I.; Estepa, J.C.; Mendoza, F.J.; Rodriguez, M.; Aquilera-Teiero, E. Serum concentrations of calcium, phosphorus, magnesium and calciotropic hormones in donkeys. Am. J. Vet. Res. 2006, 67, 1333–1336. [Google Scholar] [CrossRef]
- Bazydlo, L.A.L.; Needham, M.; Harris, N.S. Calcium, magnesium, and phosphate. Lab Med. 2014, 45, e44–e50. [Google Scholar] [CrossRef]
- Burden, F.A.; Hazell-Smith, E.; Mulugeta, G.; Patrick, V.; Trawford, R.; Brooks Brownlie, H.W. Reference intervals for biochemical and haematological parameters in mature domestic donkeys (Equus asinus) in the UK. Equine Vet. Educ. 2016, 28, 134–139. [Google Scholar] [CrossRef]
- Goodrich, E.L.; Webb, J.L. Complete blood count and biochemistry reference intervals for healthy adult donkeys in the United States. Animals 2024, 14, 2018. [Google Scholar] [CrossRef]
- Trimboli, F.; De Amicis, I.; Di Loria, A.; Ceniti, C.; Carluccio, A. Reference ranges for hematological and biochemical profile of Martina Franca donkeys. Front. Vet. Sci. 2020, 7, 1054. [Google Scholar] [CrossRef]
- Gloria, A.; Veronesi, M.C.; Carluccio, R.; Parrillo, S.; De Amicis, I.; Contri, A. Biochemical blood analysis along pregnancy in Martina Franca jennies. Theriogenology 2018, 115, 84–89. [Google Scholar] [CrossRef]
- Caldin, M.; Furlanello, T.; Solano-Gallego, L.; De Lorenzi, D.; Carli, E.; Tasca, S.; Lubas, G. Reference ranges for haematology, biochemical profile and electrophoresis in a single herd of Ragusana donkeys from Sicily (Italy). Comp. Clin. Pathol. 2005, 14, 5–12. [Google Scholar] [CrossRef]
- Sow, A.; Kalandi, K.M.; Ndiaye, N.P.; Bathily, A.; Sawadogo, G.J. Clinical biochemical parameters of Burkinabese local donkeys’ breeds. Int. Res. J. Biochem. Bioinform. 2012, 2, 84–89. [Google Scholar]
- da Silva, G.B.; da Silva, C.J.F.L.; de Souza, L.A.; Hunka, M.M.; Ferreira, L.M.C.; Manso, H.E.C.C.; Manso Filho, H.C. Hematological and blood chemistry values of donkeys (Equus africanus asinus) in different management systems. Pferdeheilkunde 2018, 34, 253–259. [Google Scholar] [CrossRef]
- Silva, G.; Silvestre-Ferreira, A.C.; Leiva, B.; Queiroga, F.L. Serum Biochemistry Parameters of the Endangered Miranda’s Donkey Breed: Reference Intervals and the Influence of Gender and Age. Animals 2024, 14, 805. [Google Scholar] [CrossRef]
- Grace, N.D.; Pearce, S.G.; Firth, E.C.; Fennessy, P.F. Concentrations of macro- and micro-elements in the milk of pasture-fed Thoroughbred mares. Aust. Vet. J. 1999, 3, 177–180. [Google Scholar] [CrossRef]
- Yue, Y.; Li, L.; Tong, M.; Li, S.; Zhao, Y.; Guo, X.; Guo, Y.; Shi, B.; Yan, S. Effect of varying dietary crude protein level on milk production, nutrient digestibility, and serum metabolites by lactating donkeys. Animals 2022, 12, 2066. [Google Scholar] [CrossRef]
- Huang, F.; Du, X.; Ma, Z.; Liu, G.; Wang, C.; Zhou, M. Effects of methionine on milk performance and milk constituents of lactating donkeys. Animals 2024, 14, 3027. [Google Scholar] [CrossRef]
- Liang, X.S.; Yue, Y.X.; Zhao, Y.L.; Guo, Y.M.; Guo, X.Y.; Shi, B.L.; Yan, S.M. Effects of dietary concentrate to forage ratio on milk performance, milk amino acid composition and milk protein synthesis of lactating donkeys. Anim. Feed Sci. Technol. 2022, 292, 115444. [Google Scholar] [CrossRef]
- Overton, T.R.; Yasui, T. Practical applications of trace minerals for dairy cattle. J. Anim. Sci. 2014, 92, 416–426. [Google Scholar] [CrossRef]
- Marchand, C.; Royer, I.; Gervais, R.; Girard, C.L.; Benchaar, C.; Hassanat, F.; Zastepa, A.; Crevecoeur, S.; Duplessis, M. Effects of feeding sulfate trace minerals above recommendations on nutrient digestibility, rumen fermentation, lactational performance, and trace mineral excretion in dairy cows. J. Dairy Sci. 2024, 107, 7983–7995. [Google Scholar] [CrossRef]
- Baucus, K.L.; Ralston, S.L.; Rich, G.A.; and Squires, E.L. The effect of copper and zinc supplementation on mineral content of mare’ milk. Equine Vet. Sci. 1989, 9, 206–209. [Google Scholar] [CrossRef]
- Yur, F.; Dede, S.; Deger, Y.; Kilicalp, D. Effects of vitamin E and selenium on serum trace and major elements in horses. Biol. Trace Elem. Res. 2008, 125, 223–228. [Google Scholar] [CrossRef]
- Stewart, A.J. Magnesium disorders in horses. Vet. Clin. Eq. 2011, 27, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Blaine, J.; Chonchol, M.; Levi, M. Renal control of calcium, phosphate, and magnesium Homeostasis. Clin. J. Am. Soc. Nephrol. 2014, 17, 1886. [Google Scholar] [CrossRef] [PubMed]
- Curry, N.; Yu, A.S.L. Magnesium handling in the kidney. Adv. Chronic Kidney Dis. 2018, 25, 236–243. [Google Scholar] [CrossRef]
- de Baaij, J.H.F.; Hoenderop, J.G.J.; Bindels, J.M. Regulation of magnesium balance: Lessons learned from human genetic disease. Clin. Kidney J. 2012, 5, i15–i24. [Google Scholar] [CrossRef]
- van der Wijst, J.; Bindels, R.J.M.; Hoenderop, J.G.J. Mg2+ homeostasis: The balancing act of TRPM6. Curr. Opin. Nephrol. Hypertens. 2014, 23, 361–369. [Google Scholar] [CrossRef]
- Guo, H.; Pang, K.; Zhang, X.; Zhao, L.; Chen, S.; Dong, M.; Ren, F. Composition, physiochemical properties, nitrogen fraction distribution, and amino acid profile of donkey milk. J. Dairy Sci. 2007, 90, 1635–1643. [Google Scholar] [CrossRef]
- Giosuè, C.; Alabiso, M.; Russo, G.; Alicata, M.L.; Torrisi, C. Jennet milk production during the lactation in a Sicilian farming system. Animal 2008, 2, 1491–1495. [Google Scholar] [CrossRef]
- Bordonaro, S.; Dimauro, C.; Criscione, A.; Marletta, D.; Macciotta, N.P. The mathematical modeling of the lactation curve for dairy traits of the donkey (Equus asinus). J. Dairy Sci. 2013, 96, 4005–4014. [Google Scholar] [CrossRef]
- Muhatai, G.; Cheng, L.; Rugoho, I.; Xiao, G.; Chen, G.; Hodge, S.; Zhou, X. Effect of parity, milking time and stage of lactation on milk yield of Jiangyue donkey (Equus asinus) in North West China. J. Dairy Res. 2017, 84, 23–26. [Google Scholar] [CrossRef]
- Malacarne, M.; Criscione, A.; Franceschi, P.; Bordonaro, S.; Formaggioni, P.; Marletta, D.; Summer, A. New insights into chemical and mineral composition of donkey milk throughout nine months of lactation. Animals 2019, 9, 1161. [Google Scholar] [CrossRef] [PubMed]
- Salari, F.; Mariti, C.; Altomonte, I.; Gazzano, A.; Martini, M. Impact of variability factors on hair cortisol, blood count and milk production of donkeys. Animals 2022, 12, 3009. [Google Scholar] [CrossRef] [PubMed]
- Aroua, M.; Fatica, A.; Ben Said, S.; Mahouachi, M.; Salimei, E. Preserving Mediterranean Donkeys: A study on milk production and nutritional benefits. Animals 2024, 14, 3713. [Google Scholar] [CrossRef]
- Martini, M.; Altomonte, I.; Salari, F.; Caroli, A.M. Short communication: Monitoring nutritional quality of Amiata donkey milk: Effects of lactation and productive season. J. Dairy Sci. 2014, 97, 6819–6822. [Google Scholar] [CrossRef]
- Barbosa dos Santos, I.C.; Rangel, A.H.N.; Ribeiro, C.V.D.M.; Oliveira, C.A.A. Donkey milk composition is altered by lactation stage and jennies age. J. Food Compos. Anal. 2023, 115, 104971. [Google Scholar] [CrossRef]
- Capuco, A.V.; Akers, R.M. Galactopoiesis, Effects of Hormones and Growth Factors. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Fox, P.F., McSweeney, P.L.H., Eds.; Academic Press: San Diego, CA, USA, 2011; Volume 3, pp. 26–31. [Google Scholar] [CrossRef]
- Harvey, J.W.; Pate, M.G.; Kivipelto, J.; Asquith, R.L. Clinical biochemistry of pregnant and nursing mares. Vet. Clin. Pathol. 2005, 34, 248–254. [Google Scholar] [CrossRef]
| Treatments 1 | ||||
|---|---|---|---|---|
| Item | CTL | TE | SEM 2 | p Value |
| Milk yield per milking, mL | 528 | 583 | 29.1 | 0.21 |
| Protein, g/100 g | 1.73 | 1.76 | 0.02 | 0.49 |
| Fat, g/100 g | 0.19 | 0.25 | 0.05 | 0.42 |
| Lactose, g/100 g | 7.09 | 7.06 | 0.03 | 0.59 |
| Ash, g/100 g | 0.40 | 0.39 | 0.01 | 0.36 |
| Treatments 1 | ||||
|---|---|---|---|---|
| Element | CTL | TE | SEM 2 | p Value |
| Ca, mg/L | 129.4 | 129.7 | 0.91 | 0.83 |
| P, mg/L | 105.0 | 102.8 | 3.33 | 0.67 |
| Mg, mg/L | 25.0 | 26.6 | 0.41 | 0.031 |
| Na, mmol/L | 137.2 | 137.0 | 0.48 | 0.80 |
| K, mmol/L | 4.62 | 4.67 | 0.05 | 0.49 |
| Na to K ratio | 29.6 | 29.5 | 0.43 | 0.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fantuz, F.; Todini, L.; Salimei, E.; Fatica, A.; Mariani, P.; Marcantoni, F.; Ferraro, S. Milk Yield, Major Milk Components and Macro Minerals in Blood Serum of Lactating Donkeys, as Affected by Dietary Trace Element Supplementation and Stage of Lactation. Animals 2025, 15, 1073. https://doi.org/10.3390/ani15081073
Fantuz F, Todini L, Salimei E, Fatica A, Mariani P, Marcantoni F, Ferraro S. Milk Yield, Major Milk Components and Macro Minerals in Blood Serum of Lactating Donkeys, as Affected by Dietary Trace Element Supplementation and Stage of Lactation. Animals. 2025; 15(8):1073. https://doi.org/10.3390/ani15081073
Chicago/Turabian StyleFantuz, Francesco, Luca Todini, Elisabetta Salimei, Antonella Fatica, Pierluigi Mariani, Fausto Marcantoni, and Stefano Ferraro. 2025. "Milk Yield, Major Milk Components and Macro Minerals in Blood Serum of Lactating Donkeys, as Affected by Dietary Trace Element Supplementation and Stage of Lactation" Animals 15, no. 8: 1073. https://doi.org/10.3390/ani15081073
APA StyleFantuz, F., Todini, L., Salimei, E., Fatica, A., Mariani, P., Marcantoni, F., & Ferraro, S. (2025). Milk Yield, Major Milk Components and Macro Minerals in Blood Serum of Lactating Donkeys, as Affected by Dietary Trace Element Supplementation and Stage of Lactation. Animals, 15(8), 1073. https://doi.org/10.3390/ani15081073





