Towards Practical Conservation Cloning: Understanding the Dichotomy Between the Histories of Commercial and Conservation Cloning
Simple Summary
Abstract
1. Introduction
2. The Complete History of Successfully Cloned Animals
2.1. Measuring Success, and Optimizing Efficiency of Cloning Efforts
2.2. Health, Fertility, and Longevity of Clones
3. Discussion
3.1. Trends in Conservation-Related Cloning Efforts
3.2. Factors Hindering Progress for Conservation Cloning
3.3. Considerations for Applied Conservation Cloning in Practice
- Cloning research should be advanced using taxa for which cloning can provide benefits to conservation and management. Individuals to be cloned should be selected for their strategic value for long-term management of taxa-specific genetic variation for many generations into the future. Conservation cloning efforts would ideally meet the overlapping criteria shown in Figure 2. If existing knowledge is not extensive, but the value of cloning for conservation is significant and urgent, research would preferably be designed to fill such knowledge gaps as cloning trials are performed.
- Biopsies to obtain cells from living donors should be minimally invasive, and in the case of ex situ animals should be obtained opportunistically during routine medical exams or other procedures to minimize handling that could induce stress. Donor cells should be cultured, expanded, and cryopreserved to save cells for future conservation purposes. If possible, cells should be reprogrammed to yield induced pluripotent stem cells, establishing a nearly inexhaustible resource [181].
- Oocytes and recipient surrogate mothers of common, non-threatened taxa with well-understood reproductive biology and husbandry should receive highest consideration. Not only does this practice alleviate welfare concerns for the focal taxa, but provides the ability to expand populations independent of natural breeding seasons, as domestic taxa can be bred continuously [154].
- The number of recipients and embryos transferred to establish pregnancies for mammalian taxa should be performed at scales in which live births can be expected. Commercial cloning companies and private ranchers can provide valuable knowledge and capacity to achieve success. For example, private ranches can be of significant value for cloning wild ungulates. Private ranches possess significantly larger herds of numerous exotic ungulates than zoos [182].
- Collection of data to build comprehensive baselines of normal and pathological embryonic, prenatal, perinatal, and postnatal development should be undertaken, incorporating control studies to enable research to identify developmental abnormalities in the cloning process.
- The results of cloning efforts need to be reported through peer-reviewed, open source outlets. If authors can show genetic evidence to support cloning claims, then journals need to allow the publishing of observations of cloned individuals despite the proprietary methods that may be employed to produce cloned embryos.
- Observations and analysis of health, behavior, and longevity should be made and reported throughout the lifetime of clones and at least one generation of offspring.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Benirschke, K. The frozen zoo concept. Zoo Biol. 1984, 3, 325–328. [Google Scholar] [CrossRef]
- Ryder, O.A.; Benirschke, K. The potential use of “cloning” in the conservation effort. Zoo Biol. 1997, 16, 295–300. [Google Scholar] [CrossRef]
- USFWS. Advancements for Black-footed Ferret Conservation Continue with New Offspring from Cloned Ferret. Press Release 2024. Available online: https://www.fws.gov/press-release/2024-11/advancements-black-footed-ferret-conservation-continue-new-offspring-cloned (accessed on 1 November 2024).
- Novak, B.J.; Gober, P.; Bortner, R.; Garelle, D.; Wright, M.; Novak, J.; Houck, M.L.; Ryder, O.A.; Milutinovich, D.; Benavidez, J.; et al. First endangered black-footed ferrets, Mustela nigripes, cloned for genetic rescue. bioRxiv 2024. [Google Scholar] [CrossRef]
- Wisely, S.M.; Ryder, O.A.; Santymire, R.M.; Engelhardt, J.F.; Novak, B.J. A Road Map for 21st Century Genetic Restoration: Gene Pool Enrichment of the Black-Footed Ferret. J. Hered. 2015, 106, 581–592. [Google Scholar] [PubMed]
- Howard, J.G.; Lynch, C.; Santymire, R.M.; Marinari, P.E.; Wildt, D.E. Recovery of gene diversity using long-term cryopreserved spermatozoa and artificial insemination in the endangered black-footed ferret. Anim. Conserv. 2016, 19, 102–111. [Google Scholar]
- Cohen, J. Six Cloned Horses Help Rider Win Prestigious Polo Match. Science. 2016. Available online: https://www.sciencemag.org/news/2016/12/six-cloned-horses-help-rider-win-prestigious-polo-match (accessed on 25 November 2024).
- Gambini, A.; Maserati, M. A journey through horse cloning. Reprod. Fertil. Dev. 2017, 30, 8–17. [Google Scholar]
- Campbell, J. Two of a Kind: China’s First Pet Cloning Service Duplicates Star Pooch. Reuters. 2018. Available online: https://www.reuters.com/article/lifestyle/two-of-a-kind-chinas-first-pet-cloning-service-duplicates-star-pooch-idUSKBN1OG11I/ (accessed on 25 November 2024).
- Kim, M.J.; Oh, H.J.; Hwang, S.Y.; Hur, T.Y.; Lee, B.C. Health and temperaments of cloned working dogs. J. Vet. Sci. 2018, 19, 585–591. [Google Scholar]
- Chaudhary, S.B. World’s First Cloned Female Camel Celebrates 10th Birthday in Dubai. Gulf News UAE/Science. 2019. Available online: https://gulfnews.com/uae/science/worlds-first-cloned-female-camel-celebrates-10th-birthday-in-dubai-1.63194680 (accessed on 25 November 2024).
- van der Berg, J.P.; Kleter, G.A.; Kok, E.J. Regulation and safety considerations of somatic cell nuclear transfer-cloned farm animals and their offspring used for food production. Theriogenology 2019, 135, 85–93. [Google Scholar] [CrossRef]
- Wells, D.N.; Misica, P.M.; Tervit, H.R.; Vivanco, W.H. Adult somatic cell nuclear transfer is used to preserve the last surviving cow of the Enderby Island cattle breed. Reprod. Fertil. Dev. 1998, 10, 369–378. [Google Scholar]
- Backus, L. Enderby Island Cattle: A Breeding Strategy for Genetic Conservation. 2006. Available online: https://rarebreeds.co.nz/enderbycattle.pdf (accessed on 21 November 2024).
- Qingqing, C. Update: World’s First Cloned Endangered Xizang Cattle Species Born Chen Qingqing. Global Times China/Society. 2024. Available online: https://www.globaltimes.cn/page/202401/1306259.shtml (accessed on 21 November 2024).
- Alberio, R.; Wolf, E. 25th Anniversary of cloning by somatic-cell nuclear transfer: Nuclear transfer and the development of genetically modified/gene edited livestock. Reproduction 2021, 162, F59–F68. [Google Scholar]
- Galli, C.; Lazzari, G. 25th anniversary of cloning by somatic-cell nuclear transfer: Current applications of SCNT in advanced breeding and genome editing in livestock. Reproduction 2021, 162, F23–F32. [Google Scholar] [PubMed]
- Greenfield, A. 25th anniversary of cloning by somatic-cell nuclear transfer: Cloning, mitochondrial replacement and genome editing: 25 years of ethical debate since Dolly. Reproduction 2021, 162, F69–F78. [Google Scholar]
- Klinger, B.; Schnieke, A. 25th anniversary of cloning by somatic-cell nuclear transfer Twenty-five years after Dolly: How far have we come? Reproduction 2021, 162, F1–F10. [Google Scholar] [PubMed]
- Loi, P.; Palazzese, L.; Scapolo, P.A.; Fulka, J.; Fulka, H.; Czernik, M. 25th anniversary of cloning by somatic-cell nuclear transfer: Anniversary of cloning by somatic-cell nuclear transfer: Scientific and technological approaches to improve SCNT efficiency in farm animals and pets. Reproduction 2021, 162, F33–F43. [Google Scholar]
- Ogura, A.; Matoba, S.; Inoue, K. 25th anniversary of cloning by somatic-cell nuclear transfer: Epigenetic abnormalities associated with somatic cell nuclear transfer. Reproduction 2021, 162, F45–F58. [Google Scholar]
- Polejaeva, I.A. 25th anniversary of cloning by somatic cell nuclear transfer: Generation of genetically engineered livestock using somatic cell nuclear transfer. Reproduction 2021, 162, F11–F22. [Google Scholar]
- Dinnyés, A.; Sousa, P.; King, T.; Wilmut, I. Somatic Cell Nuclear Transfer: Recent Progress and Challenges. Cloning Stem Cells 2002, 4, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Di Berardino, M.A. Origin and Progress of Nuclear Transfer In Nonmammalian Animals. In Nuclear Transfer Protocols: Cell Reprogramming and Transgenesis; Verma, P.J., Trounson, A.O., Eds.; Humana Press: Totowa, NJ, USA, 2006; pp. 3–34. [Google Scholar]
- Ogura, A.; Inoue, K.; Wakayama, T. Recent advancements in cloning by somatic cell nuclear transfer. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 13–16. [Google Scholar] [CrossRef]
- Keefer, C.L. Artificial cloning of domestic animals. Proc. Natl. Acad. Sci. USA 2015, 112, 8874–8878. [Google Scholar] [CrossRef]
- Matoba, S.; Zhang, Y. Somatic Cell Nuclear Transfer Reprogramming: Mechanisms and Applications. Cell Stem Cell 2018, 23, 471–485. [Google Scholar] [CrossRef]
- Inoue, K. Mouse somatic cell nuclear transfer: What has changed and remained unchanged in 25 years. J. Reprod. Dev. 2023, 69, 129–138. [Google Scholar] [PubMed]
- Ryder, O.A. Cloning advances and challenges for conservation. Trends Biotechnol. 2002, 20, 231–232. [Google Scholar] [PubMed]
- Holt, W.V.; Pickard, A.R.; Prather, R.S. Wildlife conservation and reproductive cloning. Reproduction 2004, 127, 317–324. [Google Scholar]
- Loi, P.; Modlinski, J.A.; Ptak, G. Interspecies somatic cell nuclear transfer: A salvage tool seeking first aid. Theriogenology 2011, 76, 217–228. [Google Scholar]
- Loi, P.; Iuso, D.; Czernik, M.; Ogura, A. A New, Dynamic Era for Somatic Cell Nuclear Transfer? Trends Biotechnol. 2016, 34, 791–797. [Google Scholar]
- Borges, A.A.; Pereira, A.F. Potential role of intraspecific and interspecific cloning in the conservation of wild mammals. Zygote 2019, 27, 111–117. [Google Scholar] [PubMed]
- Swegen, A.; Appeltant, R.; Williams, S.A. Cloning in action: Can embryo splitting, induced pluripotency and somatic cell nuclear transfer contribute to endangered species conservation? Biol. Rev. 2023, 98, 1225–1249. [Google Scholar]
- Mastromonaco, G.F. A quarter century of CANDES: The state of embryo technologies in companion animals, non-domestic and endangered species. Theriogenology Wild. 2023, 4, 100069. [Google Scholar] [CrossRef]
- Cowl, V.B.; Comizzoli, P.; Appeltant, R.; Bolton, R.L.; Browne, R.K.; Holt, W.V.; Penfold, L.M.; Swegen, A.; Walker, S.L.; Williams, S.A. Cloning for the Twenty-First Century and Its Place in Endangered Species Conservation. Annu. Rev. Anim. Biosci. 2024, 12, 91–112. [Google Scholar]
- Briggs, R.; King, T.J. Transplantation of living nuclei from blastula cells into enucleated frogs’ eggs. Proc. Natl. Acad. Sci. USA 1952, 38, 455–463. [Google Scholar]
- Briggs, R.; King, T.J. 1Changes in the nuclei of differentiating endoderm cells as revealed by nuclear tansplantation. J. Morhpology 1957, 100, 269–311. [Google Scholar] [CrossRef]
- Sambuichi, H. The roles of the nucleus and the cytoplasm in development I. An interspecific hybrid frog, developed from a combination of Rana nigro-maculata nigromaculata cytoplasm with a diploid nucleus of Rana nigromaculata brevipoda. J. Sci. Hiroshima Univ. Ser. Biol. Div 1957, 17, 33–41. [Google Scholar]
- Tecirlioglu, R.T.; Guo, J.; Trounson, A.O. Interspecies somatic cell nuclear transfer and preliminary data for horse-cow/mouse iSCNT. Stem Cell Rev. 2006, 2, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Beyhan, Z.; Iager, A.E.; Cibelli, J.B. Interspecies Nuclear Transfer: Implications for Embryonic Stem Cell Biology. Cell Stem Cell 2007, 1, 502–512. [Google Scholar] [CrossRef] [PubMed]
- McKinnell, R.G. Intraspecific Nuclear Transplantation in Frogs. J. Hered. 1962, 53, 199–207. [Google Scholar] [CrossRef]
- Gurdon, J.B. The developmental capacity of nuclei taken from differentiating endoderm cells of Xenopus laevis. J. Embryol. Exp. Morphol. 1960, 8, 505–526. [Google Scholar] [PubMed]
- Gurdon, J.B. The transplantation of nuclei between two subspecies of Xenopus laevis. Heredity 1961, 16, 305–315. [Google Scholar] [CrossRef]
- Kawamura, T.; Nishioka, M.; Myorei, Y. Reproductive capacity of autotetraploid males in brown frogs. J. Sci. Hiroshima Univ. Ser. Biol. Div. 1963, 1, 15–24. [Google Scholar]
- Nishioka, M. Reciprocal nucleo-cytoplasmic hybrids between Rana brevipoda and Rana plancyi chosenica. Sci. Rep. Lab. Amphib. Biol. Hiroshima Univ. 1972, 1, 259–275. [Google Scholar]
- Nishioka, M. Reciprocol Nucleo-cytoplasmic Hybrids between Rana esculenta and Rana brevipoda. Sci. Rep. Lab. Amphib. Biol. Hiroshima Univ. 1972, 1, 245–257. [Google Scholar]
- Nishioka, M. Nucleo-cytoplasmic Hybrids between Rana japonica and Rana temporaria temporaria. J. Sci. Hiroshima Univ. Ser. Biol. Div. 1972, 1, 211–243. [Google Scholar]
- Tong, D.; Shangqin, W.; Ye, Y.; Yan, S.; Du, M.; Lu, D. Nuclear transplantation of fish. Chin. Sci. Bull. 1963, 7, 60–61. [Google Scholar]
- Signoret, J. Transplantations nucléaires et différenciation embryonnaire. Arch. Biol. 1965, 71, 591–606. [Google Scholar]
- Aimar, C. Analyse par la greffe nucléaire des propriétés morphogéné- tiques des noyaux embryonnaires chez Pleurodeles waltlii (Amphibien Urodèle). Application à l’étude de la gémellarité expérimentale. Ann. Embryol. Morphog. 1972, 5, 5–42. [Google Scholar]
- Tong, D.; Ye, Y.; Miao, D. Nuclear transplantation in teleosts: Hybrid fish produced by injecting carp nucleus into crucian cytoplasm. Chin. Sci. 1980, 4, 376–380. [Google Scholar]
- Illmensee, K.; Hoppe, P.C. Nuclear transplantation in mus musculus: Developmental potential of nuclei from preimplantation embryos. Cell 1981, 23, 9–18. [Google Scholar] [PubMed]
- Yan. S.Y.; Lu, D.Y.; Du, M.; U, G.S.; Han, Z.; Yang, H.Y.; Wu, Z.A. Nuclear transplantation in teleosts. Nuclear transplantation between different subfamilies–hybrid fish from the nucleus of grass carp (Ctenopharyngodon idellus) and the cytoplasm of blunt-snout bream (Mega/obrama amblycaephala). Chin. J. Biotech. 1985, 1, 15–26. [Google Scholar]
- Willadsen, S.M. Nuclear transplantation in sheep embryos. Nature 1986, 320, 63–65. [Google Scholar]
- Prather, R.S.; Barnes, F.L.; Sims, M.M.; Robl, J.M.; Eyestone, W.H.; and First, N.L. Nuclear transplantation in the bovine embryo: Assessment of donor nuclei and recipient oocyte. Biol. Reprod. 1987, 37, 859–866. [Google Scholar]
- Stice, S.L.; Robl, J.M. Nuclear reprogramming in nuclear transplant rabbit embryos. Biol. Reprod. 1988, 39, 657–664. [Google Scholar]
- Prather, R.S.; Sims, M.M.; First, N.L. Nuclear transplantation in early pig embryos. Biol. Reprod. 1989, 41, 414–418. [Google Scholar] [PubMed]
- Yan, S.Y.; Tu, M.; Yang, H.Y.; Mao, Z.G.; Zhao, Z.Y.; Fu, L.J.; Li, G.S.; Huang, G.P.; Li, S.H.; Jin, G.Q. Developmental incompatibility between cell nucleus and cytoplasm as revealed by nuclear transplantation experiments in teleost of different families and orders. Int. J. Dev. Biol. 1990, 34, 255–266. [Google Scholar]
- Yong, Z.; Jianchen, W.; Jufen, Q.; Zhiming, H. Nuclear transplantation in goats. Theriogenology 1991, 35, 289. [Google Scholar]
- Meng, L.; Ely, J.J.; Stouffer, R.L.; Wolf, P.; Rhesus, D. Monkeys produced by nuclear transfer. Biol. Reprod. 1997, 57, 454–459. [Google Scholar]
- Kang, Y.; Dai, S.; Zeng, Y.; Wang, F.; Yang, P.; Yang, Z.; Pu, Y.; Li, Z.; Chen, X.; Tian, B.; et al. Cloning and base editing of GFP transgenic rhesus monkey and off-target analysis. Sci. Adv. 2022, 8, eabo3123. [Google Scholar] [PubMed]
- Liao, Z.; Zhang, J.; Sun, S.; Li, Y.; Xu, Y.; Li, C.; Cao, J.; Nie, Y.; Niu, Z.; Liu, J.; et al. Reprogramming mechanism dissection and trophoblast replacement application in monkey somatic cell nuclear transfer. Nat. Commun. 2024, 15, 5. [Google Scholar] [PubMed]
- Wakamatsu, Y.; Ju, B.; Pristyaznhyuk, I.; Niwa, K.; Ladygina, T.; Kinoshita, M.; Araki, K.; and Ozato, K. Fertile and diploid nuclear transplants derived from embryonic cells of a small laboratory fish, medaka (Oryzias latipes). Proc. Natl. Acad. Sci. USA 2001, 98, 1071–1076. [Google Scholar]
- Bubenshchikova, E.; Kaftanovskaya, E.; Motosugi, N.; Fujimoto, T.; Arai, K.; Kinoshita, M.; Hashimoto, H.; Ozato, K.; Wakamatsu, Y. Diploidized eggs reprogram adult somatic cell nuclei to pluripotency in nuclear transfer in medaka fish (Oryzias latipes). Dev. Growth Differ. 2007, 49, 699–709. [Google Scholar]
- Hill, J.R.; Winger, Q.A.; Long, C.R.; Looney, C.R.; Thompson, J.A.; Westhusin, M.E. Development rates of male bovine nuclear transfer embryos derived from adult and fetal cells. Biol. Reprod. 2000, 62, 1135–1140. [Google Scholar] [PubMed]
- Meirelles, F.V.; Bordignon, V.; Watanabe, Y.; Watanabe, M.; Dayan, A.; Lôbo, R.B.; Garcia, J.M.; Smith, L.C. Complete replacement of the mitochondrial genotype in a Bos indicus calf reconstructed by nuclear transfer to a bos taurus oocyte. Genetics 2001, 158, 351–356. [Google Scholar]
- Yu, M.; Muteti, C.; Ogugo, M.; Ritchie, W.A.; Raper, J.; Kemp, S. Cloning of the African indigenous cattle breed Kenyan Boran. Anim. Genet. 2016, 47, 510–511. [Google Scholar]
- Lanza, R.P.; Cibelli, J.B.; Diaz, F.; Moraes, C.T.; Farin, P.W.; Farin, C.E.; Hammer, C.J.; West, M.D.; Damiani, P. Cloning of an endangered species (Bos gaurus) using interspecies nuclear transfer. Cloning 2000, 2, 79–90. [Google Scholar]
- Srirattana, K.; Imsoonthornruksa, S.; Laowtammathron, C.; Sangmalee, A.; Tunwattana, W.; Thongprapai, T.; Chaimongkol, C.; Ketudat-Cairns, M.; Parnpai, R. Full-term development of gaur-bovine interspecies somatic cell nuclear transfer embryos: Effect of Trichostatin A treatment. Cell Reprogramming 2012, 14, 248–257. [Google Scholar]
- Loi, P.; Ptak, G.; Barboni, B.; Fulka, J., Jr.; Cappai, P.; Clinton, M. Genetic rescue of an endangered mammal by cross-species nuclear transfer using post-mortem somatic cells. Nat. Biotechnol. 2001, 19, 962–964. [Google Scholar]
- Shin, T.; Kraemer, D.; Pryor, J.; Liu, L.; Rugila, J.; Howe, L.; Buck, S.; Murphy, K.; Lyons, L.; Westhusin, M. A cat cloned by nuclear transplantation. Nature 2002, 415, 859. [Google Scholar]
- Hu, W.; Wang, Y.; Chen, S.; Zhu, Z. Nuclear transplantation in different strains of zebrafish. Chin. Sci. Bull. 2002, 47, 1277–1280. [Google Scholar]
- Lee, K.Y.; Huang, H.; Ju, B.; Yang, Z.; Lin, S. Cloned zebrafish by nuclear transfer from long-term-cultured cells. Nat. Biotechnol. 2002, 20, 795–799. [Google Scholar]
- Zhou, Q.; Renard, J.P.; Le Friec, G.; Brochard, V.; Beaujean, N.; Cherifi, Y.; Fraichard, A.; Cozzi, J. Generation of fertile cloned rats by regulating oocyte activation. Science 2003, 302, 1179. [Google Scholar]
- Folch, J.; Cocero, M.J.; Chesné, P.; Alabart, J.L.; Domínguez, V.; Cognié, Y.; Roche, A.; Fernández-Arias, A.; Martí, J.I.; Sánchez, P.; et al. First birth of an animal from an extinct subspecies (Capra pyrenaica pyrnaica) by cloning. Theriogenology 2009, 71, 1026–1034. [Google Scholar]
- Janssen, D.L.; Edwards, M.L.; Koster, J.A.; Lanza, R.P.; Ryder, O.A. 206 postnatal management of chryptorchid banteng calves cloned by nuclear transfer utilizing frozen fibroblast cultures and enucleated cow ova. Reprod. Fertil. Dev. 2003, 16, 224. [Google Scholar]
- Galli, C.; Lagutina, I.; Crotti, G.; Colleoni, S.; Turini, P.; Ponderato, N.; Duchi, R.; Lazzari, G. A cloned horse born to its dam twin. Nature 2003, 424, 635. [Google Scholar] [PubMed]
- CVM Researchers First to Clone White-Tailed Deer. Texas A&M University Veterinary Medicine & Biomedical Science. 2003. Available online: https://vetmed.tamu.edu/news/press-releases/cvm-researchers-first-to-clone-white-tailed-deer/ (accessed on 25 November 2014).
- Hlavaty, C. Now a Grandfather, First Clone of His Kind Turns 10 at A&M. Houston Crhonicale. 2013. Available online: https://www.chron.com/news/houston-texas/article/now-a-grandfather-first-clone-of-his-kind-turns-4542735.php (accessed on 25 November 2024).
- Berg, D.K.; Li, C.; Asher, G.; Wells, D.N.; Oback, B. Red deer cloned from antler stem cells and their differentiated progeny. Biol. Reprod. 2007, 77, 384–394. [Google Scholar]
- Haigh, A.J.; MacDonald, W.A.; Lloyd, V.K. The generation of cloned Drosophila melanogaster. Genetics 2005, 169, 1165–1167. [Google Scholar]
- Gómez, M.C.; Pope, C.E.; Giraldo, A.M.; Lyons, L.; Harris, R.F.; King, A.; Cole, A.; Godke, R.A.; Dresser, B.L. 38 birth of african wild cat cloned kittens. Reprod. Fertil. Dev. 2003, 16, 141–142. [Google Scholar]
- Gómez, M.C.; Pope, C.E.; Kutner, R.H.; Ricks, D.M.; Lyons, L.A.; Ruhe, M.; Dumas, C.; Lyons, J.; López, M.; Dresser, B.L.; et al. Nuclear transfer of sand cat cells into enucleated domestic cat oocytes is affected by cryopreservation of donor cells. Cloning Stem Cells 2008, 10, 469–484. [Google Scholar]
- Jang, G.; Lee, B.C. Update on the first cloned dog and outlook for canine cloning. Cell Reprogramming 2015, 17, 325–326. [Google Scholar]
- Kim, M.K.; Jang, G.; Oh, H.J.; Yuda, F.; Kim, H.J.; Hwang, W.S.; Hossein, M.S.; Kim, J.J.; Shin, N.S.; Kang, S.K.; et al. Endangered wolves cloned from adult somatic cells. Cloning Stem Cells 2007, 9, 130–137. [Google Scholar]
- Oh, H.J.; Kim, M.K.; Jang, G.; Kim, H.J.; Hong, S.G.; Park, J.E.; Park, K.; Park, C.; Sohn, S.H.; Kim, D.Y.; et al. Cloning endangered gray wolves (Canis lupus) from somatic cells collected postmortem. Theriogenology 2008, 70, 638–647. [Google Scholar] [CrossRef]
- Li, Z.; Sun, X.; Chen, J.; Liu, X.; Wisely, S.M.; Zhou, Q.; Renard, J.P.; Leno, G.H.; Engelhardt, J.F. Cloned ferrets produced by somatic cell nuclear transfer. Dev. Biol. 2006, 293, 439–448. [Google Scholar]
- Shi, D.; Lu, F.; Wei, Y.; Cui, K.; Yang, S.; Wei, J.; Liu, Q. Buffalos (Bubalus bubalis) cloned by nuclear transfer of somatic cells. Biol. Reprod. 2007, 77, 285–291. [Google Scholar]
- Selokar, N.L.; Sharma, P.; Saini, M.; Sheoran, S.; Rajendran, R.; Kumar, D.; Sharma, R.K.; Motiani, R.K.; Kumar, P.; Jerome, A.; et al. Successful cloning of a superior buffalo bull. Sci. Rep. 2019, 9, 11366. [Google Scholar]
- Wani, N.A.; Wernery, U.; Hassan, F.A.H.; Wernery, R.; Skidmore, J.A. Production of the first cloned camel by somatic cell nuclear transfer. Biol. Reprod. 2010, 82, 373–379. [Google Scholar] [CrossRef]
- First Cloned Camel Gives Birth to First Cloned Offspring in Dubai. The National UAE. 2015. Available online: https://www.thenational.ae/uae/first-cloned-camel-gives-birth-to-first-cloned-offspring-in-dubai-1.65260 (accessed on 25 November 2024).
- Hwang, I.; Jeong, Y.W.; Kim, J.J.; Lee, H.J.; Kang, M.; Park, K.B.; Park, J.H.; Kim, Y.W.; Kim, W.T.; Shin, T.; et al. Successful cloning of coyotes through interspecies somatic cell nuclear transfer using domestic dog oocytes. Reprod. Fertil. Dev. 2013, 25, 1142–1148. [Google Scholar] [PubMed]
- Entrepreneurs Clone Animals: Local Rancher, Veterinarian Supplement Deer Herd. Amarillo Globe-News. 2013. Available online: https://www.amarillo.com/news/local-news/2013-10-25/entreprenuers-clone-animals-local-rancher-veternarian-supplement-deer (accessed on 25 November 2024).
- Hajian, M.; Hosseini, S.M.; Forouzanfar, M.; Abedi, P.; Ostadhosseini, S.; Hosseini, L.; Moulavi, F.; Gourabi, H.; Shahverdi, A.H.; Vosough Taghi Dizaj, A.; et al. “Conservation cloning” of vulnerable Esfahan mouflon (Ovis orientalis isphahanica): In vitro and in vivo studies. Eur. J. Wildl. Res. 2011, 57, 959–969. [Google Scholar] [CrossRef]
- Dehghan, S.K. Scientists in Iran Clone Endangered Mouflon–Born to Domestic Sheep. The Guardian. 2015. Available online: https://www.theguardian.com/science/2015/aug/05/iran-scientists-clone-endangered-mouflon-domestic-sheep (accessed on 25 November 2024).
- Wani, N.A.; Vettical, B.S.; Hong, S.B. First cloned Bactrian camel (Camelus bactrianus) calf produced by interspecies somatic cell nuclear transfer: A step towards preserving the critically endangered wild Bactrian camels. PLoS ONE 2017, 12, e0177800. [Google Scholar] [CrossRef]
- DOJ, Office of Public Affairs. Montana Man Stentence for Federal Wildlife Trafficking Charges as Part of Yearslong Effort to Create Giant Hybrid Sheep for Captive Hunting. Press Release Number: 24-1233. 2024. Available online: https://www.justice.gov/opa/pr/montana-man-sentenced-federal-wildlife-trafficking-charges-part-yearslong-effort-create (accessed on 24 November 2024).
- Gomez, J.; Limehouse, J. Montana Rancher Gets 6 Months in Prison for Creating Hybrid Sheep for Captive Hunting. USA TODAY. 2024. Available online: https://www.usatoday.com/story/news/nation/2024/10/01/montana-rancher-sentenced-giant-sheep-hybrid-hunting/75463964007/ (accessed on 25 November 2024).
- Liu, Z.; Cai, Y.; Wang, Y.; Nie, Y.; Zhang, C.; Xu, Y.; Zhang, X.; Lu, Y.; Wang, Z.; Poo, M.; et al. Cloning of macaque monkeys by somatic cell nuclear transfer. Cell 2018, 172, 881–887. [Google Scholar] [CrossRef]
- Liu, Z.; Cai, Y.; Liao, Z.; Xu, Y.; Wang, Y.; Wang, Z.; Jiang, X.; Li, Y.; Lu, Y.; Nie, Y.; et al. Cloning of a gene-edited macaque monkey by somatic cell nuclear transfer. Natl. Sci. Rev. 2019, 6, 101–108. [Google Scholar] [CrossRef]
- Novak, B.J.; Ryder, O.A.; Houck, M.L.; Walker, K.; Russell, L.; Russell, B.; Walker, S.; Arenivas, S.S.; Aston, L.; Veneklasen, G.; et al. Endangered Przewalski’s Horse, Equus przewalskii, Cloned from Historically Cryopreserved Cells. Animals 2025, 15, 613. [Google Scholar] [CrossRef] [PubMed]
- World′s First Cloned Arctic Wolf Was Born! Sinogene Empowers Species Diversity Conservation. Sinogene. 2022. Available online: https://www.sinogenepets.com/worlds-first-cloned-arctic-wolf-was-born-sinogene-empowers-species-diversity-conservation.html (accessed on 25 November 2024).
- Sliwa, A.; Ghadirian, T.; Appel, A.; Banfield, L.; Sher Shah, M.; Watcher, T. Felis margarita. IUCN Red List. Threat. Species 2016, e.T8541A50651884. [Google Scholar]
- White, K.L.; Bunch, T.D.; Mitalipov, S.; Reed, W.A. Establishment of pregnancy after the transfer of nuclear transfer embryos produced from the fusion of argali (Ovis ammon) nuclei into domestic sheep (Ovis aries) enucleated oocytes. Cloning 1999, 1, 47–54. [Google Scholar] [CrossRef]
- Williams, B.; Shin, T.; Liu, L.; Flores-Foxworth, G.; Romano, J.; Rugila, J.; Dean, D.; Burghardt, R.; Westhusin, M.; Kraemer, D. Abstracts for Poster Presentation-Cloning/Nuclear Transfer-Interspecies Nuclear Transfer of Desert Bighorn Sheep (Ovis canadensis mexicana). Theriogenology 2002, 57, 457. [Google Scholar]
- Williams, J.B.; Shin, T.; Liu, L.; Flores-Foxworth, G.; Romano, J.; Blue-McClendon, A.; Kraemer, D.; Westhusin, M.E. Cloning of Exotic/Endangered Species: Desert Bighorn Sheep. Methods Mol. Biol. 2006, 348, 169–182. [Google Scholar] [PubMed]
- Friese, C. Cloning Wildlife: Zoos, Captivity, and the Future of Endangered Animals; New York University Press: New York, NY, USA, 2013. [Google Scholar]
- Hwang, W. Interspecies somatic cell nuclear transfer for the production of endangered Korean tiger (Pantera Tigris Altaica). Theriogenology 2001, 55, 271. [Google Scholar]
- Damiani, P. Development of giant eland (Taurotragus oryx) and bovine (Bos taurus) oocytes. Theriogenology 2003, 59, 390. [Google Scholar]
- Gómez, M.C.; Pope, C.E.; Ricks, D.M.; Lyons, J.; Dumas, C.; Dresser, B.L. Cloning endangered felids using heterospecific donor oocytes and interspecies embryo transfer. Reprod. Fertil. Dev. 2009, 21, 76–82. [Google Scholar]
- Li, Y.; Dai, Y.; Du, W.; Zhao, C.; Wang, L.; Wang, H.; Liu, Y.; Li, R.; Li, N. In vitro development of yak (Bos grunniens) embryos generated by interspecies nuclear transfer. Anim. Reprod. Sci. 2007, 101, 45–59. [Google Scholar]
- Lafsky, M. What Happens When One Cloned Wolf Mates With Another Cloned Wolf? Discover Magazine. 2009. Available online: https://www.discovermagazine.com/technology/what-happens-when-one-cloned-wolf-mates-with-another-cloned-wolf (accessed on 25 November 2024).
- Yin, X.; Lee, Y.; Lee, H.; Kim, N.; Kim, L.; Shin, H.; Kong, I. In vitro production and initiation of pregnancies in inter-genus nuclear transfer embryos derived from leopard cat (Prionailurus bengalensis) nuclei fused with domestic cat (Felis silverstris catus) enucleated oocytes. Theriogenology 2006, 66, 275–282. [Google Scholar]
- Imsoonthornruksa, S.; Sangmalee, A.; Srirattana, K.; Parnpai, R.; Ketudat-Cairns, M. Development of intergeneric and intrageneric somatic cell nuclear transfer (SCNT) cat embryos and the determination of telomere length in cloned offspring. Cell Reprogramming 2012, 14, 79–87. [Google Scholar]
- Yong, E. Resurrecting the Extinct Frog With a Stomach for a Womb. National Geographic. 2013. Available online: https://www.nationalgeographic.com/science/article/resurrecting-the-extinct-frog-with-a-stomach-for-a-womb (accessed on 25 November 2024).
- Fatira, E.; Havelka, M.; Labbé, C.; Depincé, A.; Iegorova, V.; Pšenička, M.; Saito, T. Application of interspecific Somatic Cell Nuclear Transfer (iSCNT) in sturgeons and an unexpectedly produced gynogenetic sterlet with homozygous quadruple haploid. Sci. Rep. 2018, 8, 5997. [Google Scholar]
- Fatira, E.; Havelka, M.; Labbé, C.; Depincé, A.; Pšenička, M.; Saito, T. A newly developed cloning technique in sturgeons; an important step towards recovering endangered species. Sci. Rep. 2019, 9, 10453. [Google Scholar]
- Kumar, S.; Suleski, M.; Craig, J.E.; Kasprowicz, A.E.; Sanderford, M.; Li, M.; Stecher, G.; Hedges, S.B. TimeTree 5: An Expanded Resource for Species Divergence Times. Mol. Biol. Evol. 2022, 39, msac174. [Google Scholar] [CrossRef] [PubMed]
- Botigué, L.R.; Song, S.; Scheu, A.; Gopalan, S.; Pendleton, A.L.; Oetjens, M.; Taravella, A.M.; Seregély, T.; Zeeb-Lanz, A.; Arbogast, R.M.; et al. Ancient European dog genomes reveal continuity since the Early Neolithic. Nat. Commun. 2017, 8, 16082. [Google Scholar] [CrossRef] [PubMed]
- Orlando, L. Ancient Genomes Reveal Unexpected Horse Domestication and Management Dynamics. BioEssays 2020, 42, 1900164. [Google Scholar] [CrossRef] [PubMed]
- Wilmut, I.; Schnieke, A.E.; McWhir, J.; Kind, A.J.; Campbell, K.H.S. Viable offspring derived from fetal and adult mammalian cells. Nature 1997, 385, 810–813. [Google Scholar] [CrossRef]
- Lagutina, I.; Fulka, H.; Lazzari, G.; Galli, C. Interspecies somatic cell nuclear transfer: Advancements and problems. Cell Reprogramming 2013, 15, 374–384. [Google Scholar] [CrossRef]
- Mrowiec, P.; Bugno-Poniewierska, M.; Młodawska, W. The perspective of the incompatible of nucleus and mitochondria in interspecies somatic cell nuclear transfer for endangered species. Reprod. Domest. Anim. 2021, 56, 199–207. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Gao, Y.; Su, J.; Zhang, J.; Xing, X.; Zhou, C.; Yao, K.; An, Q.; Zhang, Y. H3K9 demethylase KDM4E is an epigenetic regulator for bovine embryonic development and a defective factor for nuclear reprogramming. Development 2018, 145, dev158261. [Google Scholar] [CrossRef]
- Matoba, S.; Wang, H.; Jiang, L.; Lu, F.; Iwabuchi, K.A.; Wu, X.; Inoue, K.; Yang, L.; Press, W.; Lee, J.T.; et al. Loss of H3K27me3 imprinting in somatic cell nuclear transfer embryos disrupts post-implantation development. Cell Stem Cell 2018, 23, 343–354. [Google Scholar] [CrossRef]
- Ruan, D.; Peng, J.; Wang, X.; Ouyang, Z.; Zou, Q.; Yang, Y.; Chen, F.; Ge, W.; Wu, H.; Liu, Z.; et al. XIST derepression in active X chromosome hinders pig somatic cell nuclear transfer. Stem Cell Rep. 2018, 10, 494–508. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Su, J.; Luo, Y.; Quan, F.; Zhang, Y. Nuclear Donor Cell Lines Considerably Influence Cloning Efficiency and the Incidence of Large Offspring Syndrome in Bovine Somatic Cell Nuclear Transfer. Reprod. Domest. Anim. 2013, 48, 660–664. [Google Scholar] [CrossRef]
- Sinclair, K.D.; Corr, S.A.; Gutierrez, C.G.; Fisher, P.A.; Lee, J.H.; Rathbone, A.J.; Choi, I.; Campbell, K.H.S.; Gardner, D.S. Healthy ageing of cloned shee. Nat. Commun. 2016, 7, 12359. [Google Scholar] [PubMed]
- Wakayama, S.; Kohda, T.; Obokata, H.; Tokoro, M.; Li, C.; Terashita, Y.; Mizutani, E.; Nguyen, V.T.; Kishigami, S.; Ishino, F.; et al. Successful serial recloning in the mouse over multiple generations. Cell Stem Cell 2013, 12, 293–297. [Google Scholar]
- Kim, M.J.; Oh, H.J.; Kim, G.A.; Setyawan, E.M.N.; Bin Choi, Y.; Lee, S.H.; Petersen-Jones, S.M.; Ko, C.M.J.; Lee, B.C. Birth of clones of the world’s first cloned dog. Sci. Rep. 2017, 7, 3–6. [Google Scholar] [CrossRef]
- Li, J.; Chen, D.; Han, Z.; Zhu, Z.; Wen, D.; Sun, Q.; Liu, Z.; Wang, M.; Lian, L.; Du, J.; et al. Serial nuclear transfer improves the development of interspecies reconstructed giant panda (Aluropoda melanoleuca) embryos. Chin. Sci. Bull. 2002, 47, 467–469. [Google Scholar]
- Sun, X.; Yan, Z.; Yi, Y.; Li, Z.; Lei, D.; Rogers, C.S.; Chen, J.; Zhang, Y.; Welsh, M.J.; Leno, G.H.; et al. Adeno-associated virus–targeted disruption of the CFTR gene in cloned ferrets. J. Clin. Investig. 2008, 118, 1578–1583. [Google Scholar] [PubMed]
- Gurdon, J.B. Nuclear transplantation in eggs and oocytes. J. Cell Sci. 1986, 4, 287–318. [Google Scholar]
- Gurdon, J.B. The Control of Gene Expression in Animal Development; Oxford and Harvard University Presses: Oxford, UK, 1974. [Google Scholar]
- Burgstaller, J.P.; Brem, G. Aging of Cloned Animals: A Mini-Review. Gerontology 2017, 63, 417–425. [Google Scholar]
- FDA. Animal Cloning: A Risk Assessment. 2008. Available online: https://downloads.regulations.gov/FDA-2008-N-0033-0004/content.pdf (accessed on 25 November 2024).
- Watanabe, S.; Nagai, T. Death losses due to stillbirth, neonatal death and diseases in cloned cattle derived from somatic cell nuclear transfer and their progeny: A result of nationwide survey in Japan. Anim. Sci. J. 2009, 80, 233–238. [Google Scholar] [CrossRef]
- First Cloned Mouse Dies Of Old Age. CBS News. 2000. Available online: https://www.cbsnews.com/news/first-cloned-mouse-dies-of-old-age/ (accessed on 25 November 2024).
- Myers, M.; World’s First Cloned Cat Dies. Texas A&M Today. 2020. Available online: https://today.tamu.edu/2020/03/04/worlds-first-cloned-cat-dies/ (accessed on 25 November 2024).
- World’s 1st Cloned Cow Dies in Central Japan at 21. Kyodo News. 2019. Available online: https://english.kyodonews.net/news/2019/10/6a9f77a90f6b-worlds-1st-cloned-cow-dies-in-central-japan-at-21.html (accessed on 25 November 2024).
- Ji-Sook, B. 1st Clond Wolf Found Dead at Zoo. The Korea Times. 2009. Available online: https://www.koreatimes.co.kr/www/nation/2024/11/113_51074.html (accessed on 25 November 2024).
- Ptak, G.; Clinton, M.; Barboni, B.; Muzzeddu, M.; Cappai, P.; Tischner, M.; Loi, P. Preservation of the wild European mouflon: The first example of genetic management using a complete program of reproductive biotechnologies. Biol. Reprod. 2002, 66, 796–801. [Google Scholar]
- Michel, S.; Ghoddousi, A. Ovis gmelini. IUCN Red List. Threat. Species 2020, 2020, e.T54940218A22147055. [Google Scholar] [CrossRef]
- Casinello, J. CABI International, Ovis Aries Musimon (European mouflon). 2022. Available online: https://www.cabidigitallibrary.org/doi/full/10.1079/cabicompendium.71353 (accessed on 25 November 2024).
- Chen, D.Y.; Wen, D.C.; Zhang, Y.P.; Sun, Q.Y.; Han, Z.M.; Liu, Z.H.; Shi, P.; Li, J.S.; Xiangyu, J.G.; Lian, L.; et al. Interspecies implantation and mitochondria fate of panda-rabbit cloned embryos. Biol. Reprod. 2002, 67, 637–642. [Google Scholar] [PubMed]
- Guanfu, H.; Guiwei, Z.; Xiangjiang, L.; Hongwei, L.; Zhong, L.; Shaona, H. The carp-goldfish nucleocytoplasmic hybrid has mitochondria from the carpal as the nuclear donor species. Gene 2014, 536, 265–271. [Google Scholar]
- Farris, S.M. The rise to dominance of genetic model organisms and the decline of curiosity-driven organismal research. PLoS ONE 2020, 15, e0243088. [Google Scholar]
- Comizzoli, P.; Holt, W.V. Breakthroughs and new horizons in reproductive biology of rare and endangered animal species. Biol. Reprod. 2019, 101, 514–525. [Google Scholar]
- Bolton, R.L.; Mooney, A.; Pettit, M.T.; E Bolton, A.; Morgan, L.; Drake, G.J.; Appeltant, R.; Walker, S.L.; Gillis, J.D.; Hvilsom, C. Resurrecting biodiversity: Advanced assisted reproductive technologies and biobanking. Reprod. Fertil. 2022, 3, R121–R146. [Google Scholar] [CrossRef]
- Whitham, J.C.; Wielebnowski, N. New directions for zoo animal welfare science. Appl. Anim. Behav. Sci. 2013, 147, 247–260. [Google Scholar]
- Myths About Cloning. U.S. Food & Drug Administration. 2020. Available online: https://public4.pagefreezer.com/browse/FDA/06-04-2023T20:24/https://www.fda.gov/animal-veterinary/animal-cloning/myths-about-cloning (accessed on 10 October 2020).
- Yin, Y.; Dong, Y.; Wang, K.; Wang, D.; Jones, B.F. Public use and public funding of science. Nat. Hum. Behav. 2022, 6, 1344–1350. [Google Scholar] [CrossRef]
- Pizzutto, C.S.; Colbachini, H.; Jorge-Neto, P.N. One Conservation: The integrated view of biodiversity conservation. Anim. Reprod. 2021, 18, e20210024. [Google Scholar]
- Wildt, D.E.; Rall, W.F.; Critser, J.K.; Monfort, S.L.; Seal, U.S. Living collections for biodiversity conservation. Bioscience 1997, 47, 1–20. [Google Scholar]
- Lee, K. Can cloning save endangered species? Curr. Biol. 2001, 11, 245–246. [Google Scholar]
- Ryder, O.A. Opportunities and Challenges for Conserving Small Populations: How Zoos Can Hel. In The Ark and Beyond: The Evolution of Zoo and Aquarium Conservation, 1st ed.; Minteer, B.A., Maienshein, J., Collins, J.P., Rabb, G., Eds.; University of Chicago Press: Chicago, IL, USA, 2018. [Google Scholar]
- Hayashi, K.; Ohta, H.; Kurimoto, K.; Aramaki, S.; Saitou, M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell 2011, 146, 519–532. [Google Scholar] [PubMed]
- Hayashi, K.; Ogushi, S.; Kurimoto, K.; Shimamoto, S.; Ohta, H.; Saitou, M. Offspring from oocytes derived from in vitro primordial germ cell-like cells in mice. Science 2012, 338, 971–975. [Google Scholar]
- Conservation Planning Specialist Grou (n.d.). The One Plan Approach to Conservation International Union for the Conservation of Nature. Available online: https://www.cpsg.org/our-approach/one-plan-approach-conservation (accessed on 2 October 2024).
- Pickrell, J. Australian Fires Have Incinerated the Habitats of up to 100 Threatened Species. Science News. 2020. Available online: https://www.sciencenews.org/article/australian-fires-incinerated-habitats-threatened-species-ecological-disaster (accessed on 24 November 2024).
- Readfearn, G. “Triple Whammy”: Drought, Fires and Floods Push Australian Rivers into Crisis. The Guardian. 2020. Available online: https://www.theguardian.com/environment/2020/feb/12/triple-whammy-hits-push-australian-rivers-crisis (accessed on 24 November 2024).
- Readfearn and Graham. “A Moment of Complete Despair”: Last Population of Macquarie Perch All but Wiped Out in NSW River Carnage. The Guardian. 2020. Available online: https://www.theguardian.com/environment/2020/feb/15/last-population-macquarie-perch-nsw-river-carnage-bushfire-ash-fish-species (accessed on 24 November 2024).
- Ceballos, G.; Ehrlich, P.R.; Dirzo, R. Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc. Natl. Acad. Sci. USA 2017, 114, E6089–E6096. [Google Scholar]
- Ceballos, G.; Ehrlich, P.R.; Raven, P.H. Vertebrates on the brink as indicators of biological annihilation and the sixth mass extinction. Proc. Natl. Acad. Sci. USA 2020, 117, 13596–13602. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.; Abeli, T.; Bruns, E.B.; Dalrymple, S.E.; Foster, J.; Gilbert, T.C.; Hogg, C.J.; Lloyd, N.A.; Meyer, A.; Moehrenschlager, A.; et al. Extinct in the wild: The precarious state of Earth’s most threatened group of species. Science 2023, 379, eadd2889. [Google Scholar]
- Houck, M.L.; Lear, T.L.; Charter, S.J. Animal Cytogenetics. In The AGT Cytogenetics Laboratory Manual, 4th ed.; Arsham, M.S., Barch, M.J., Lawce, H.J., Eds.; John Wiley & Sons, Inc. eBooks: Hoboken, NJ, USA, 2017; pp. 1055–1102. [Google Scholar]
- González-del-Pliego, P.; Freckleton, R.P.; Edwards, D.P.; Koo, M.S.; Scheffers, B.R.; Pyron, R.A.; Jetz, W. Phylogenetic and Trait-Based Prediction of Extinction Risk for Data-Deficient Amphibians. Curr. Biol. 2019, 29, 1557–1563.e3. [Google Scholar] [PubMed]
- Luedtke, J.A.; Chanson, J.; Neam, K.; Hobin, L.; Maciel, A.O.; Catenazzi, A.; Borzée, A.; Hamidy, A.; Aowphol, A.; Jean, A.; et al. Ongoing declines for the world’s amphibians in the face of emerging threats. Nature 2023, 622, 308–314. [Google Scholar]
- Howell, L.G.; Frankham, R.; Rodger, J.C.; Witt, R.R.; Clulow, S.; Upton, R.M.O.; Clulow, J. Integrating Biobanking Minimises Inbreeding and Produces Significant Cost Benefits for a Threatened Frog Captive Breeding Programs. Conserv. Lett. 2021, 14, e12776. [Google Scholar]
- Browne, R.K.; Luo, Q.; Wang, P.; Mansour, N.; Kaurova, S.A.; Gakhova, E.N.; Shishova, N.V.; Uteshev, V.K.; Kramarova, L.I.; Venu, G.; et al. The Sixth Mass Extinction and Amphibian Species Sustainability Through Reproduction and Advanced Biotechnologies, Biobanking of Germplasm and Somatic Cells, and Conservation Breeding Programs (RBCs). Animals 2024, 14, 3395. [Google Scholar] [CrossRef]
- Frankham, R.; Ballou, J.D.; Ralls, K.; Eldridge, M.D.B.; Dudash, M.R.; Fenster, C.B.; Lacy, R.C.; Sunnocks, P. Genetic Management of Fragmented Animal and Plant Populations, 1st ed.; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Bell, D.A.; Robinson, Z.L.; Funk, W.C.; Fitzpatrick, S.W.; Allendorf, F.W.; Tallmon, D.A.; Whiteley, A.R. The Exciting Potential and Remaining Uncertainties of Genetic Rescue. Trends Ecol. Evol. 2019, 34, 1070–1079. [Google Scholar]
- Frankham, R. Genetic rescue of small inbred populations: Meta-analysis reveals large and consistent benefits of gene flow. Mol. Ecol. 2015, 24, 2610–2618. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, A.; Tonkin, Z.; Pearce, L.; Robledo-Ruiz, D.; Lintermans, M.; Ingram, B.; Lyon, J.; Beitzel, M.; Broadhurst, B.; Rourke, M.; et al. A shift to metapopulation genetic management for persistence of a species threatened by fragmentation: The case of an endangered Australian freshwater fish. Authorea Prepr. 2024. [Google Scholar] [CrossRef]
- Pavlova, A.; Beheregaray, L.B.; Coleman, R.; Gilligan, D.; Harrisson, K.A.; Ingram, B.A.; Kearns, J.; Lamb, A.M.; Lintermans, M.; Lyon, J.; et al. Severe consequences of habitat fragmentation on genetic diversity of an endangered Australian freshwater fish: A call for assisted gene flow. Evol. Appl. 2017, 10, 531–550. [Google Scholar] [CrossRef] [PubMed]
- Kriesner, P.; Weeks, A.R.E.; Sunnucks, P. Assessing genetic risks to Victorian flora and fauna. In For Department of Environment, Land, Water and Planning; Monash University: Clayton, VIC, Australia, 2020; ISBN 978-1-76105-313-9. [Google Scholar]
- Hardy, M.A.; Hull, S.D.; Zuckerberg, B. Swift action increases the success of population reinforcement for a declining prairie grouse. Ecol. Evol. 2018, 8, 1906–1917. [Google Scholar] [CrossRef]
- van de Kerk, M.; Onorato, D.P.; Hostetler, J.A.; Bolker, B.M.; Oli, M.K. Dynamics, persistence, and genetic management of the endangered Florida Panther Population. Wildl. Monogr. 2019, 203, 3–35. [Google Scholar] [CrossRef]
- Czernik, M.; Anzalone, D.A.; Palazzese, L.; Oikawa, M.; Loi, P. Somatic cell nuclear transfer: Failures, successes and the challenges ahead. Int. J. Dev. Biol. 2019, 63, 123–130. [Google Scholar]
- Hutchinson, A.M.; Appeltant, R.; Burdon, T.; Bao, Q.; Bargaje, R.; Bodnar, A.; Chambers, S.; Comizzoli, P.; Cook, L.; Endo, Y.; et al. Advancing stem cell technologies for conservation of wildlife biodiversity. Development 2024, 151, dev203116. [Google Scholar] [CrossRef]
- Wildt, D.; Miller, P.; Koepfli, K.P.; Pukazhenthi, B.; Palfrey, K.; Livingston, G.; Beetem, D.; Shurter, S.; Gregory, J.; Takács, M.; et al. Breeding centers, private ranches, and genomics for creating sustainable wildlife populations. BioScience 2019, 69, 928–943. [Google Scholar]
- Wisely, S.M.; Statham, M.J.; Fleischer, R.C. Pleistocene Refugia and Holocene Expansion of a Grassland-Dependent Species, the Black-Footed Ferret (Mustela nigripes). J. Mammal. 2008, 89, 87–96. [Google Scholar] [CrossRef]
- Der Sarkissian, C.; Ermini, L.; Schubert, M.; Yang, M.A.; Librado, P.; Fumagalli, M.; Jónsson, H.; Bar-Gal, G.K.; Albrechtsen, A.; Vieira, F.G.; et al. Evolutionary genomics and conservation of the endangered Przewalski’s horse. Curr. Biol. 2015, 25, 2577–2583. [Google Scholar] [CrossRef]
- Taylor, H.R.; Colbourne, R.M.; Robertson, H.A.; Nelson, N.J.; Allendorf, F.W.; Ramstad, K.M. Cryptic inbreeding depression in a growing population of a long-lived species. Mol. Ecol. 2017, 26, 799–813. [Google Scholar] [PubMed]
- Wiedenfeld, D.A.; Alberts, A.C.; Angulo, A.; Bennett, E.L.; Byers, O.; Contreras-MacBeath, T.; Drummond, G.; da Fonseca, G.A.; Gascon, C.; Harrison, I.; et al. Conservation resource allocation, small population resiliency, and the fallacy of conservation triage. Conserv. Biol. 2021, 35, 1388–1395. [Google Scholar] [PubMed]
- Ryder, O.A.; Friese, C.; Greely, H.T.; Sandler, R.; Saragusty, J.; Durrant, B.S.; Redford, K.H. Exploring the limits of saving a subspecies: The ethics and social dynamics of restoring northern white rhinos (Ceratotherium simum cottoni). Conserv. Sci. Pract. 2020, 2, e241. [Google Scholar]
- Sandler, R.L.; Moses, L.; Wisely, S.M. An ethical analysis of cloning for genetic rescue: Case study of the black-footed ferret. Biol. Conserv. 2021, 257, 109118. [Google Scholar]
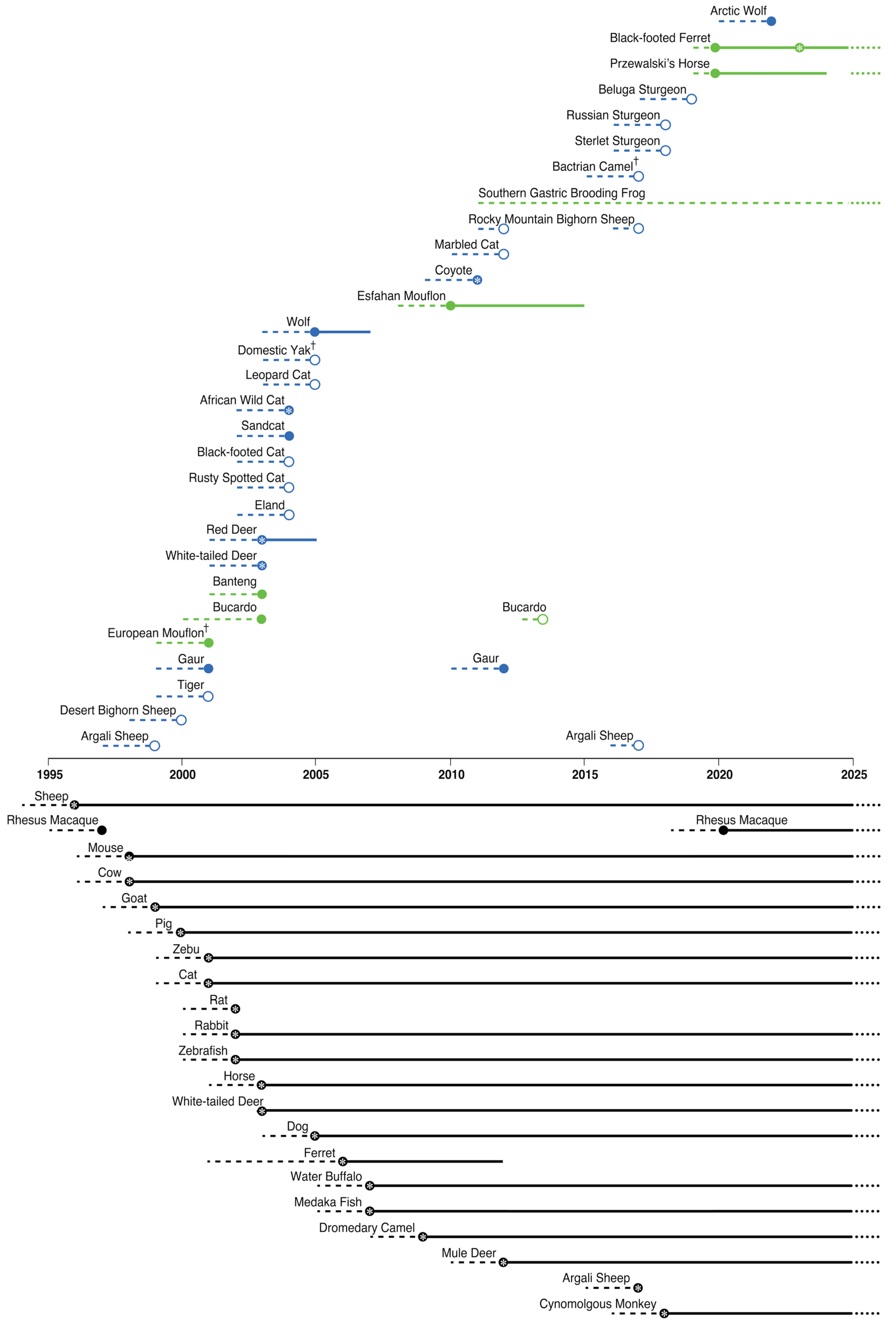

| Common Name | Species Name | Year of First Live Birth/Hatching | Higher Classification | Wild or Domestic | Current IUCN Status | Same-Species Cloning | Cross-Species Cloning | Healthy Adult Clone(s) Produced | Evaluation of Reproductive Capability | Reference(s) | Nature of Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dark-spotted frog | Pelophylax nigromaculatus (formerly P. nigromaculata) | 1957 | Amphibian | Wild | Near Threatened (assessed 2004); species was cloned before IUCN red list was formed | × | Yes | Fertile | [39] | Peer-reviewed publication. | |
| Northern leopard frog | Lithobates pipiens (formerly Rana pipiens) | 1957 | Amphibian | Wild | Least Concern | × | Yes | Fertile | [38,42] | Peer-reviewed publication. | |
| African clawed frog | Xenopus laevis laevis | 1960 | Amphibian | Domestic | Least Concern | × | × | Yes | Fertile | [43] | Peer-reviewed publication. |
| Mwanza frog | Xenopus victorianus | 1961 | Amphibian | Wild | Least Concern | × | Yes | Fertile | [44] | Peer-reviewed publication. | |
| Durama pond frog | Pelophylax porosus (formerly P. p. brevipoda) | 1963 | Amphibian | Wild | Least Concern | × | × | Yes | Fertile | [45,46,47] | Peer-reviewed publication. |
| Japanese brown frog | Rana japonica | 1963 | Amphibian | Wild | Least Concern | × | Yes | Fertile | [45] | Peer-reviewed publication. | |
| Montane brown frog | Rana ornativentris (formerly R. ornaventris) | 1963 | Amphibian | Wild | Least Concern | × | Yes | Unknown (Original reference could not be obtained, information gleaned from other papers) | [46,48] | Peer-reviewed publication. | |
| Goldfish | Carassisus auratus auratus | 1963 | Fish | Domestic | Least Concern | × | Yes | Fertile | [49] | Peer-reviewed publication. | |
| Bitterling | Rhodeus sinensis | 1963 | Fish | Wild | Least Concern | × | × (cross-genus) | Yes | Fertile | [49] | Peer-reviewed publication. |
| Common Asian carp | Cyprinus carpio | 1963 | Fish | Domestic | Wild populations Vulnerable (assessed 1996) | × | × (cross-species and cross-genus) | Yes | Fertile | [49] | Peer-reviewed publication. |
| Axolotl | Ambystoma mexicanum | 1965 | Amphibian | Domestic | Wild populations Critically Endangered (assessed 1986) | × | Yes | Fertile | [50] | Peer-reviewed publication. | |
| Peter’s platanna | Xenopus petersii | 1966 | Amphibian | Wild | Least Concern | × | Yes | Unknown (Original reference could not be obtained, information gleaned from other papers) | [46] | Peer-reviewed publication. | |
| Iberian ribbed newt | Pleurodeles waltl | 1970 | Amphibian | Wild | Near Threatened (assessed 2006) | × | × | Yes | Fertile | [51] | Peer-reviewed publication. |
| Edough ribbed newt | Pleurodeles poireti | 1970 | Amphibian | Wild | Endangered (assessed 2004) | × | × | Yes | Unknown (Original reference could not be obtained, information gleaned from other papers) | [51] | Peer-reviewed publication. |
| Gold-spotted pond frog | Pelophylax plancyi (formerly P. chosenicus) | 1972 | Amphibian | Wild | Vulnerable (assessed 2004) | × | Yes | Fertile | [46] | Peer-reviewed publication. | |
| Common frog | Rana temporaria | 1972 | Amphibian | Wild | Least Concern | × | Yes | Infertile | [48] | Peer-reviewed publication. | |
| Edible frog | Pelophylax esculenta (possibly. P. ridibundus or P. lessonae) | 1972 | Amphibian | Wild | Not classified, considered common | × | No | NA | [47] | Peer-reviewed publication. | |
| Wild goldfish | Carassius auratus | 1980 | Fish | Wild | Least Concern | × | × (cross-subspecies) | Yes | Fertile | [52] | Peer-reviewed publication. |
| Mouse | Mus musculus | 1981 | Mammal | Domestic | Least Concern | × | Yes | Fertile | [53] | Peer-reviewed publication. | |
| Grass Carp | Ctenopharyngoden idellus | 1984 | Fish | Wild | Not classified, considered common | × (cross-genus) | Yes | Fertile | [54] | Peer-reviewed publication. | |
| Sheep | Ovis aries | 1986 | Mammal | Domestic | Not Applicable | × | Yes | Fertile | [55] | Peer-reviewed publication. | |
| Cattle | Bos taurus | 1987 | Mammal | Domestic | Not classified, not applicable | × | Yes | Fertile | [56] | Peer-reviewed publication. | |
| Rabbit | Oryctolagus cuniculus domestica | 1988 | Mammal | Domestic | Not classified, not applicable | × | Yes | Fertile | [57] | Peer-reviewed publication. | |
| Pig | Sus scrofa domesticus | 1989 | Mammal | Domestic | Not classified, not applicable | × | Yes | Fertile | [58] | Peer-reviewed publication. | |
| Loach | Paramisgurnus dabryanus | 1990 | Fish | Wild | Not classified, considered common | × | Yes | Fertile | [59] | Peer-reviewed publication. | |
| Goat | Capra hircus | 1991 | Mammal | Domestic | Not Applicable | × | Yes | Fertile | [60] | Peer-reviewed publication. | |
| Rhesus macaque | Macaca mulatta | 1997 | Mammal | Wild | Least Concern | × | Clones born in 2020 are still alive and nearing age of sexual maturity. | NA | [61,62,63] | Peer reviewed publications, current status of clones provided by Dr. Qiang Sun. | |
| Medaka | Oryzias latipes | 1999 | Fish | Domestic | Least Concern | × | Yes | Fertile | [64,65] | Peer-reviewed publication. | |
| Zebu | Bos indicus | 1999 | Mammal | Domestic | Least Concern | × | Yes | Fertile | [66,67,68] | Peer-reviewed publications. | |
| Gaur | Bos gaurus | 2001 | Mammal | Wild | Vulnerable | × | No | NA | [69,70] | Peer-reviewed publication. | |
| European mouflon | Ovis aries musimon | 2001 | Mammal | Feral Domestic | Not classified, not applicable (was considered a wild species at time of cloning, O. gmelini musimon, which was classified as Near Threatened) | × | Yes | Reached weaning age, but not sexual maturity | [71] | Peer-reviewed publication. Fertility information provided by Dr. Pasqualino Loi. | |
| Domestic Cat | Felis catus | 2001 | Mammal | Domestic | Not classified, not applicable | × | Yes | Fertile | [72] | Peer-reviewed publication. | |
| Zebrafish | Danio rerio | 2002 | Fish | Domestic | Least Concern | × | Yes | Fertile | [73,74] | Peer-reviewed publication. | |
| Rat | Rattus norvegicus domestica | 2002 | Mammal | Domestic | Not classified, not applicable | × | Yes | Fertile | [75] | Peer-reviewed publication. | |
| Bucardo (died) | Capra pyrenaica pyrenaica | 2003 | Mammal | Wild | Extinct (nominate species Least Concern) | × | No | NA | [76] | Peer-reviewed publication. | |
| Banteng | Bos javanicus | 2003 | Mammal | Wild | Endangered | × | Yes | Infertile | [77] | Conference abstract. Fertility reported here for the first time. | |
| Horse | Equus caballus | 2003 | Mammal | Domestic | Not classified, not applicable | × | Yes | Fertile | [78] | Peer-reviewed publication. | |
| White-tailed deer | Ococoileus virginianus | 2003 | Mammal | Wild | Least Concern | × | Yes | Fertile | [79,80] | All information reported in popular press. | |
| Red deer | Cervus elaphus | 2003 | Mammal | Wild | Least Concern | × | Yes | Fertile | [81] | Peer-reviewed publication. Fertility information provided by Dr. Debbie Berg. | |
| Fruitfly | Drosophila melanogaster | 2004 | Insect | Domestic | Not classified, not applicable | × | Yes | Fertile | [82] | Peer-reviewed publication. | |
| African wild cat | Felis sylvestris lybica | 2004 | Mammal | Wild | Least Concern | × | Yes | Fertile | [83] | Peer-reviewed publication. | |
| Sand cat * | Felis margarita | 2004 | Mammal | Wild | Least Concern (Near Threatened at time of cloning) | × | No | NA | [84] | Peer-reviewed publication. | |
| Domestic Dog | Canis lupus familiaris | 2005 | Mammal | Domestic | Not classified, not applicable | × | Yes | Fertile | [85] | Peer-reviewed publication. | |
| Gray Wolf | Canis lupus lupus | 2005 | Mammal | Wild | Least Concern | × (cross-subspecies) | Yes | Unknown | [86,87] | Peer-reviewed publication. | |
| Domestic Ferret | Mustela putorius furo | 2006 | Mammal | Domestic | Least Concern | × | Yes | Fertile | [88] | Peer-reviewed publication. Fertility information provided by Dr. John Engelhardt. | |
| Water Buffalo | Bubalus bubalis | 2007 | Mammal | Domestic | Not classified, not applicable | × | Yes | Fertile | [89,90] | Peer-reviewed publication | |
| Dromedary Camel | Camelus dromadarius | 2009 | Mammal | Domestic | Not classified, not applicable | × | Yes | Fertile | [91,92] | Peer-reviewed publication. | |
| Coyote | Canis latrans | 2011 | Mammal | Wild | Least Concern | × | Yes | Fertile | [93] | Peer-reviewed publication. Fertility information provided by Dr. Hwang Suk. | |
| Mule deer | Ococoileus hemionus | 2012 | Mammal | Wild | Least Concern | × | Yes | Fertile | [94] | Reported in popular press. Fertility information provided by Shawn Walker. | |
| Esfahan mouflon | Ovis gmelini isphahanica | 2015 | Mammal | Wild | Near Threatened | × | Unknown | Unknown | [95,96] | Live births reported in peer-reviewed publication in 2010, single live birth reported by researchers to press in 2015. | |
| Bactrian camel | Camelus bactrianus | 2017 | Mammal | Domestic | Not classified, not applicable | × | No | NA | [97] | Peer-reviewed publication. | |
| Argali | Ovis ammon | 2017 | Mammal | Wild | Near Threatened | × | Yes | Fertile | [98,99] | Reported in popular press and government press release from court case documents. | |
| Cynomolgus monkeys | Macaca fascicularis | 2018 | Mammal | Wild | Endangered (assessment published 2020); species was listed as Least Concern at the time of cloning | × | Yes | Fertile | [100,101] | Peer reviewed publication. Current status of clones provided by Dr. Qiang Sun | |
| Przewalksi’s Horse | Equus przewalskii | 2020 | Mammal | Wild | Endangered | × | Clone born in 2020 is alive and expected to reach sexual maturity in 2025; second clone will reach sexual maturity in 2028 | Cannot be evaluated yet | [102] | Peer-reviewed publication. | |
| Black-footed ferret | Mustela nigripes | 2020 | Mammal | Wild | Endangered | × | Yes | Fertile | [3,4] | Preprint and government press release. | |
| Arctic wolf | Canis lupus arctos | 2022 | Mammal | Wild | Least Concern | × | Unknown | Not yet reported | [103] | Press release. |
| Common Name | Species Name | Status | Method | Year(s) | Oocyte Donor | Somatic/Oocyte Donor Evolutionary Divergence Estimated (Range) MYA * | Recipient Surrogate Mothers | Number of Embryos Transferred/Fostered for Development | Number of Live Births/Hatching | Percentage Implanted/Fostered Embryos Resulting in Live Births/Hatching | Number of Clones Reaching Weaning Age | Reproductive Capability | References | Nature of References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Argali | Ovis ammon | Near Threatened | Cross-species | 1999, 2017 | Ovis aries (domestic), O. c. canadensis × O. aries hybrid | 0.96 (0.4730–1.4406) | Ovis aries (domestic), O. c. canadensis x O. aries hybrid, O. c. canadensis x O. aries/O. gmelini (Rocky Mountain Bighorn x Texas Dall Sheep hybrid) | 28, 165 | 0, 1 | 0, 0.6% | 0, 1 | Cloned argali was used to produce >50 offspring via hybridization with Rocky Mountain Bighorn Sheep, Ovis canadensis canadensis | [98,99,105] | Peer reviewed publication, court case documents |
| Desert Bighorn Sheep | Ovis canadensis nelsonii (formerly O. c. mexicana) | Least Concern | Cross-species | 2000, 2017 | Ovis aries (domestic) | 3.14 (2.30–4.97) | Ovis aries (domestic) | 223 | 0 | 0 | 0 | NA | [106,107] | Conference abstract, book series, and information provided by Dr. Mike Kjelland. |
| Gaur | Bos gaurus | Vulnerable | Cross-species | 2001, 2012 | Bost taurus | 3.76 (2.86–5.42) | Bos taurus | 44 ** | 1, 1 | 2.4% (averaged); 0–11% per cell line/treatment | 0 | NA | [69,70,108] | Peer reviewed publication reports of viable cloned pregnancies in 2000, live birth reported to press by San Diego Zoo Global, Advanced Cell Technologies, and TransOva Genetics. Live births from another research group were Fertile in peer-reviewed literature in 2012. |
| Tiger | Panthera tigris altaica | Endangered | Cross-species | 2001 | Could not obtain original reference. | - | - | - | 0 | 0 | 0 | NA | [109] | Conference abstract is referenced in other papers, but original reference could not be obtained to extract data regarding each step of the cloning process. |
| Banteng | Bos javanicus | Endangered | Cross-species | 2003 | Bos taurus | 3.76 (2.86–5.42) | Bos taurus | NA | 2 | NA | 1 | Infertile | [108] | Abstract from conference. Reproductive capability and Reason for program termination presented in this publication by co-authors of previous peer-reviewed report. |
| Bucardo | Capra pyrenaica pyrenaica | Subspecies Extinct (Evolutionarily Torpid), Nominate species Least Concern | Cross-species | 2003, 2013 | Capra hircus | 2.85 (1.25–5.61) | Capra pyrainaica hispanica and Capra pyrenaica hispanica x Capra hircus hybrids | 154, NA | 1, 0 | 0.6% (total) | 0 | NA | [76] | Peer-reviewed publication. The research team renewed efforts in 2013, but produced no pregnancies. Personal communication Dr. Alberto Fernándaz-Arias. |
| Red deer | Cervus elaphus | Least Concern | Same-species | 2003–2005 | Cervus elaphus | - | Cervus elaphus | 84 | 10 | 12% (averaged) | 8 | Clones produced normal sperm, were never bred. | [81] | Peer-reviewed publication, personal communication Debbie Berg. |
| White-tailed Deer | Odocoileus virginianis | Least Concern | Same-species | 2003 onward | Odocoileus virginianis | - | Odocoileus virginianis | NA | NA | NA | 1 | Cloned deer was reported to produce offspring, with up to three generations of descendants. | [79,80] | First clone Fertile by Texas A&M University and ViaGen Pets & Equine, but never published in peer-review. Total number of adult clones produced provided by ViaGen Pet’s and Equine. Personal communication Shawn Walker. |
| Eland | Tragelaphus oryx | Least Concern | Cross-species | 2003 | Bos taurus | 17.3 (14.3–19.3) | - | - | 0 | 0 | 0 | NA | [110] | Conference abstract is referenced in other papers, but original reference could not be obtained to extract data regarding each step of the cloning process. |
| Sandcat | Felis margarita | Least Concern | Cross-species | 2004 | Felis catus | 3.6 (1.13–5.10) | Felis catus | 1600 | 14 | 0.3–1.8% per treatment/cell line | 0 | NA | [84,111] | Peer-reviewed publications. |
| Black-footed cat | Felis nigripes | Vulnerable | Cross-species | 2004 | Felis catus | 4.35 (3.36–5.92) | Felis catus | 698 | 0 | 0 | 0 | NA | [84,111] | Peer-reviewed publications. |
| Rusty spotted cat | Prionailurus rubiginosus | Near Threatened | Cross-species | 2004 | Felis catus | 9 (5.6–11.9) | Felis catus | 201 | 0 | 0 | 0 | NA | [84,111] | Peer-reviewed publications. |
| African wildcat | Felis sylvestris lybica | Least Concern | Cross-species | 2004 | Felis catus | 2.8 (1.40–5.10) | Felis catus | 1627 | 17 | 1% (averaged) | 8 | Clones were bred, one generation of descendants published. | [111] | Peer-reviewed publication. |
| Domestic Yak *** | Bos grunniens | Not Listed, Not applicable | Cross-species | 2005 | Bos taurus | 4.36 (2.72–6.46) | Bos taurus | 324 | 0 | 0 | 0 | NA | [112] | Peer-reviewed publication. |
| Gray Wolf | Canis lupus lupus | Least Concern | Cross-subspecies | 2005, 2007 | Canis lupus familiaris | (0.02–0.04) | Canis lupus familiaris | 251, 372 | 2, 6 | 0.8%; 0–9.5% per oocyte donor/recipient combination | 2,3 | NA | [86,87,113] | Two independent research groups published successful results in peer-reviewed literature. Reports of intent to breed in popular press. |
| Leopard cat | Prionailurus bengalensis | Least Concern | Cross-species | 2005 | Felis catus | 9 (5.6–11.9) | Felis catus | 435 | 0 | 0 | 0 | NA | [114] | Peer-reviewed publication. |
| Esfahan Mouflon | Ovis gmelini isphahanica | Near Threatened | Cross-species | 2010–2015 | Ovis aries (domestic) | 1.17 (0.47–8.10) | Ovis aries (domestic) | 12 | 2, NA | 16% (averaged); NA | 0, NA | Unknown | [95,96] | Two live births reported in peer-reviewed publication in 2010, single live birth reported by researchers to press in 2015. |
| Coyote | Canis latrans | Least Concern | Cross-species | 2011 | Canis lupus familiaris | 3.04 (1.39–4.51) | Canis lupus familiaris | 320 | 8 | 2.5% (averaged) | 8 | Clones were bred, two litters have been born to cloned parents to date. | [93] | Peer-reviewed publication. Reproduction information provided by Dr. Hwang Woo-Suk. |
| Rocky Mountain Bighorn Sheep | Ovis canadensis canadensis | Least Concern | Cross-species | 2012 | Ovis aries | 3.14 (2.30–4.97) | Ovis aries (domestic) | NA | 0 | 0 | 0 | NA | NA | Information provided by Dr. Mike Kjelland. |
| Marbled cat | Pardofelis marmorata | Near Threatened | Cross-species | 2012 | Felis catus | 15.2 (12.2–16.6) | Felis catus | 461 | 0 | 0 | 0 | NA | [115] | Peer-reviewed publication. |
| Southern Gastric Brooding Frog | Rheobatrachus silus | Extinct | Cross-species | 2013- | Mixophyes fasciolatus | 141 (123–149) | - | NA | 0 | 0 | 0 | NA | [116] | Popular press. Current status provided by Dr. Michael Archer |
| Bactrian Camel *** | Camelus bactrianus | Not Listed, Not applicable | Cross-species | 2017 | Camelus dromedarius | 6.4 (2.4–9.5) | Camelus dromedarius | 26 | 1 | 3.8% (total) | 0 | NA | [97] | Peer-reviewed publication. |
| Russian Sturgeon | Acipenser gueldenstaedtii | Critically Endangered | Cross-species | 2018, 2019 | Acipenser ruthenus | 71 (8–71) | - | 266 | 0 | 0 | 0 | NA | [117,118] | Peer reviewed publications. |
| Sterlet | Acipenser ruthenus | Vulnerable | Same-species | 2018 | Acipenser ruthenus | - | - | 129 | 0 | 0 | 0 | NA | [117] | Peer-reviewed publication. |
| Beluga | Huso huso | Critically Endangered | Cross-species | 2019 | Acipenser ruthenus | 158 (16–172) | - | 130 | 0 | 0 | 0 | NA | [117,118] | Peer reviewed publications. |
| Przewalksi’s Horse | Equus przewalskii | Endangered | Cross-species | 2020, 2023 | Equus caballus | (0.035–0.055) | Equus caballus | 11 | 2 | 18% (total) | 2 | NA | [102] | Peer-reviewed publication.. |
| Black-footed ferret | Mustela nigripes | Endangered | Cross-species | 2020 onward | Mustela putorius furo | 0.5 | Mustela putorius furo | 163 | 3 | 0–2.4% | 3 | One clone has produced 2 kits to date | [4,5] | Preprint and USFWS press release. |
| Arctic wolf | Canis lupus arctos | Least Concern | Cross-subspecies | 2022 | Canis lupus familiaris | (0.02–0.04) | Canis lupus familiaris | 85 | 1 | 0.1% | 1 | NA | [103] | Press Release Announcement by SinoGene, China. |
| Common Name | Species Name | Primary Purpose for Cloning | Reason for Program Termination | Longevity | Typical Lifespan | Causes of Death and Post-Natal Information. | References | Nature of Reference |
|---|---|---|---|---|---|---|---|---|
| Coyote | Canis latrans | Research: to advance conservation cloning science | Obtaining live birth satisfied end goal. | One lived 4 years, two lived 8 years, one lived 9 years. Four clones were still alive in 2020 at 9 years of age. | 13–15 years | The first death was caused by wounds incurred from fighting, causes of two deaths unknown, one death due to heartworm parasitic infection. Clones were bred and produced six F1 offspring, one of which was cannibalized at less than one year of age, another died less than one year of age, cause unknown, and the remaining four were alive at six and four years of age in 2020. | Personal communication. | Information provided by Dr. Hwang Woo-Suk. |
| African wildcat | Felis sylvestris lybica | Research: to advance conservation cloning science | No response to inquiry; assumption that live births satisfied research end-goals | Three clones lived < 36 h, six lived < 30 days, one lived 1 month, one lived 3 years, two were terminated at 14 years. Four clones were still alive in 2020 at 16 years of age. | 13–14 years | Two perinatal deaths due to bacterial infections. One perinatal death due to acute pneumonia from aspiration from bottle-feeding. Remaining perinatal deaths due to respiratory failure, potentially due to underdeveloped lungs. One clone died at 1 month of age due to poor fitness. One clone died at 3 years of age due to trauma. Two clones were euthanized due to age related issues at extreme old age. Four clones (50%) were alive and exceeding life expectancy in 2020. These clones produced eight F1 offspring, two of which died at one year of age (unknown reasons, suspected accident in enclosure), one euthanized at age 14 due to age related complications, and the remaining five were alive at 15 years of age in 2020. | [111] | Peer-reviewed publication, additional information provided by Lisa Murphy. |
| Gaur | Bos gaurus | Research: to advance conservation cloning science | Both studies achieved scientific end-goals; cloning the gaur by San Diego Zoo Global, Advanced Cell Technologies, and TransOva Genetics was an initial proof of concept for endangered bovids. Cloning additional gaur by San Diego Zoo Global was not pursued because it would not serve significant conservation value. | >48 h | Up to 26 years (typical range unknown) | The single clone died of dysentery. | [70,108] | Live birth and death in 2000 reported in press. Reason for termination reported from interviews in published book. |
| European mouflon | Ovis aries musimon | Research and Application: to advance conservation cloning science and recover deceased individuals within a small managed population. At the initiation of the work the species was classified as a distinct wild taxon, but now is considered a feral subspecies of domestic sheep. | Limited funding prevented further attempts. When initiated, Italy prohibited cloning of domestic animals unless for transgenic research, wild animals were exempted from the ban. Cloning mouflon presented an opportunity to advance cloning research as well as to recover the genotypes of deceased individuals within a small island population on Sicily. | 14 months | 8–12 years | The single clone was released to the same management area that the somatic donor lived. It died of pulmonary adenomatosis induced by infection. This was the same disease that caused the death of the somatic cell donor, and therefore susceptibility to the disease was not due to poorer fitness of the clone. Pulmonary infections are a major cause of multiple sheep taxa mortalities worldwide. Until onset of infection the clone was healthy and developed normally. | Personal communication. | Information provided by Dr. Pasqualino Loi. |
| Bucardo | Capra pyrenaica pyrenaica | Application: to recover a subspecies that had gone extinct from cryopreserved cell lines. | Limited funding and opposition from specific political and conservation stakeholders. A recent successful reintroduction of a related Ibex subspecies to the bucardo’s former range now precludes significant ecological value of recovering the extinct subspecies. | ~7 minutes | 16 years | The clone died due to complications arising from an extra, abnormal lung lobe. This was likely due to problematic early embryonic nuclear reprogramming or later gestational complications. | [76] | Peer-reviewed publication. Reason for program termination provided by Dr. Alberto Fernándaz-Arias. |
| Banteng | Bos javanicus | Application: genetic management of under-represented individual in ex situ zoo populations | Although cloning will be of value long-term, other lower cost genetic management options are currently adequate, negating justification to continue efforts to produce a fertile clone currently. | One clone lived 7 days, the second lived 7 years | Up to 27 years (typical range unknown) | The first clone was large and failed to gain strength, an abnormality in domestic cattle that occurs in higher frequencies in clones. The second individual was healthy throughout its life and died of injuries incurred in an accident with another animal in its enclosure, not uncommon for individuals in multi-individual enclosures. | [77] | Author Oliver Ryder, collaborator and co-author on previous peer-reviewed publications regarding this program, provided detailed information. |
| White-tailed Deer | Odocoileus virginianis | Research: first clone was produced for conservation cloning research; subsequent efforts for private enterprise (see Figure 1) | Conservation cloning research achieved its end-goal. Cloning of these taxa continues by commercial efforts. | First cloned deer lived 15 years, many individuals are currently alive. | 6–14 years | The first cloned deer, Dewey, died of natural aging, having exceeded typical life expectancy. He was reported as healthy throughout his life. | [79,80] | Reported in popular press, additional information provided by Dr. Alice Blue-McClendon. |
| Red deer | Cervus elaphus | Research: unrelated to conservation | New Zealand Deer Industry stakeholders had no further interest in cloning deer. | Two lived < 1 week, one lived 5 years, one lived 7 years, two terminated at 8 years, three terminated at 10 years of age. | 10–13 years | One calf died likely due to several observed pathologies (contracted flexor tendons in all legs, enlarged fatty liver, and incomplete lung inflation-these issues are observed in naturally reproduced individuals but expected at higher frequency in clones). One calf died due to parental neglect. One adult died from injury, breaking his neck on a fence, and another died of injuries incurred while fighting another clone while rutting-both causes of death not infrequent for males of all deer species. The remaining clones, born in 2003 and 2005 were euthanized together, at ages 8 and 10, due to lack of funding to continue animal care. | [81] | Peer-review publication, additional information provided by Dr. Debbie Berg. |
| Sandcat | Felis margarita | Research: to advance conservation cloning science | No response to inquiry; assumption that live births satisfied research end-goals | Nine clones lived < 36 h, three lived < 30 days, one lived 30 days, one lived 60 days. | Up to 13 years (typical range unknown) | Deaths were due to respiratory failure, possibly due to underdeveloped lungs. Four died of acute pneumonia resulting from aspiration during bottle-feeding. | [84] | Peer-reviewed publication. |
| Gray Wolf | Canis lupus | Research: to advance conservation cloning science | Obtaining live birth satisfied end goal. | 4 years and 11 years | 10–18 years | Exact causes of death for clones born in 2005 are unknown, assumed to be heat exposure and age related. Data for clones born in 2007 was not obtained. | [86,87,142] | Information only obtained for the first 2 cloned wolves produced by [86]. First death reported in popular press, additional information provided by Dr. Hwang Woo-Suk. |
| Esfahan Mouflon | Ovis gmelini isphahanica | Application: To increase population size of ex situ back up populations | No response to inquiry; assumption that live births satisfied research end-goals | <1 day, <14 days | 8–12 years | Clones born in 2010 may have died due to organ abnormalities, potentially indicative of prematurity, though normal phenotypes of newborns for this species are unknown, so cause of death is inconclusive. The clone born in 2015 was reported healthy at 14 days of age. The current status of this clone could not be obtained. | [95,96] | Peer-reviewed publicaton and popular press. |
| Rocky Mountain Bighorn Sheep | Ovis canadensis | Research: to advance conservation cloning science | Limited funding. | NA | 6–15 years | NA | Personal communication. | Information provided by Dr. Mike Kjelland. |
| Bactrian Camel | Camelus bactrianus | Research: proof-of-concept for conservation cloning of wild endangered Bactrian camels (Camelus ferus) | Wild Bactrian camel conservation groups were disinterested in pursuing conservation cloning; high demand for cloning dromedary camels for athletic and aesthetic competition has precluded further bactrian camel work. | <7 days | 20–40 years | Clone died of acute septicemia. | [97] | Death reported in peer-reviewed publication. Reason for termination provided by Dr. Nisar Ahmed Wani. |
| Sterlet | Acipenser ruthenus | Research: to advance conservation cloning science | The primary scientist conducting cloning experiments performed this work to earn her doctorate degree. Once graduated, the program ended. Due to the low efficiency and difficulty of obtaining success in cloning, the laboratory group has shifted focus to other germ-cell based advanced reproductive techniques, including the more promising process of culturing, preserving, and transplanting of primordial germ cells to breeding surrogates. | NA | 22–25 years | NA | [117] | Peer-reviewed publication. Reason for termination provided by Dr. Effrosyni Fatira and Dr. Martin Pšenička. |
| Russian Sturgeon | Acipenser gueldenstaedtii | NA | 38 years | NA | [117,118] | |||
| Beluga | Huso huso | NA | >100 years possible | NA | [117,118] | |||
| Przewalksi’s Horse | Equus przewalskii | Application: genetic rescue of under-represented individual to increase adaptive diversity of ex situ and reintroduced in situ populations | NA—program is ongoing | Clones are alive at 1 and 4 years of age at time of manuscript submission | 20–25 years | NA | [102] | Peer reviewed publication. |
| Black-footed ferret | Mustela nigripes | Application: genetic rescue to of an unrepresented individual (establishing a new founder) to increase adaptive diversity of ex situ and reintroduced in situ populations | NA—program is ongoing | Clones are alive at 1 and 4 years of age at time of manuscript submission | 4–6 years | NA | [4,5] | Preprint and USFWS press release. |
| Arctic wolf | Canis lupus arctos | Research: to advance conservation cloning science | No response to inquiry; assumption that live births satisfied research end-goals | Clone is alive at over 2 years of age at time of submission | 7-–10 years | NA | [103] | Press Release Announcement by SinoGene, China. |
| Higher Classicfication | Somatic Cell Donor | Oocyte Donor | Somatic/Oocyte Species Evolutionary Divergence in MYA * | Note | Reference(s) | Nature of Reference |
|---|---|---|---|---|---|---|
| Fish | Rhodeus sinensis | Carassius auratus | 106 (68–102) | reciprocal transfers also produced live hatching | [49] | Peer-reviewed publication. |
| Fish | Cyprinus carpio | Crassius auratus, Carassius carassius | 34 (21–46) | reciprocal transfers with C. auratus also produced live hatching, no reciprocal transfers with C. carassius performed | [49] | Peer-reviewed publication. |
| Fish | Ctenopharyngoden idellus | Megalobrama amblycaephala | 12.1 (9.3–21.4) | reciprocal transfers also produced live hatching | [54] | Peer-reviewed publication. |
| Amphibian | Pelophylax nigromaculata | Pelophylax porosus brevipoda | 9.92 (4.09–17.22) | reciprocal transfers also produced live hatching | [39] | Peer-reviewed publication. |
| Amphibian | Xenopus victorianus | Xenopus laevis | 11.05 (7.89–14.40) | reciprocal transfers also produced live hatching | [44] | Peer-reviewed publication. |
| Amphibian | Rana ornativentris | Rana japonica | 21.4 (13.7–24.4) | reciprocal transfers also produced live hatching | [46,48] | Peer-reviewed publication. |
| Amphibian | Pleurodeles waltl | Pleurodeles poireti | 17.3 (10.1–19.3) | reciprocal transfers also produced live hatching | [51] | Peer-reviewed publication. |
| Amphibian | Pelophylax esculenta | Pelophylax porosus brevipoda | 33 (21–42) | reciprocal transfers also produced live hatching | [46] | Peer-reviewed publication. |
| Amphibian | Pelophylax chosenicus | Pelophylax porosus brevipoda | 9.92 (4.09–17.22) | reciprocal transfers also produced live hatching | [48] | Peer-reviewed publication. |
| Amphibian | Rana temporaria | Rana japonica | 19.4 (11.4–24.4) | reciprocal transfers also produced live hatching | [47] | Peer-reviewed publication. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novak, B.J.; Brand, S.; Phelan, R.; Plichta, S.; Ryder, O.A.; Wiese, R.J. Towards Practical Conservation Cloning: Understanding the Dichotomy Between the Histories of Commercial and Conservation Cloning. Animals 2025, 15, 989. https://doi.org/10.3390/ani15070989
Novak BJ, Brand S, Phelan R, Plichta S, Ryder OA, Wiese RJ. Towards Practical Conservation Cloning: Understanding the Dichotomy Between the Histories of Commercial and Conservation Cloning. Animals. 2025; 15(7):989. https://doi.org/10.3390/ani15070989
Chicago/Turabian StyleNovak, Ben J., Stewart Brand, Ryan Phelan, Sasha Plichta, Oliver A. Ryder, and Robert J. Wiese. 2025. "Towards Practical Conservation Cloning: Understanding the Dichotomy Between the Histories of Commercial and Conservation Cloning" Animals 15, no. 7: 989. https://doi.org/10.3390/ani15070989
APA StyleNovak, B. J., Brand, S., Phelan, R., Plichta, S., Ryder, O. A., & Wiese, R. J. (2025). Towards Practical Conservation Cloning: Understanding the Dichotomy Between the Histories of Commercial and Conservation Cloning. Animals, 15(7), 989. https://doi.org/10.3390/ani15070989







