Blood Biochemical Biomarkers in Fish Toxicology—A Review
Simple Summary
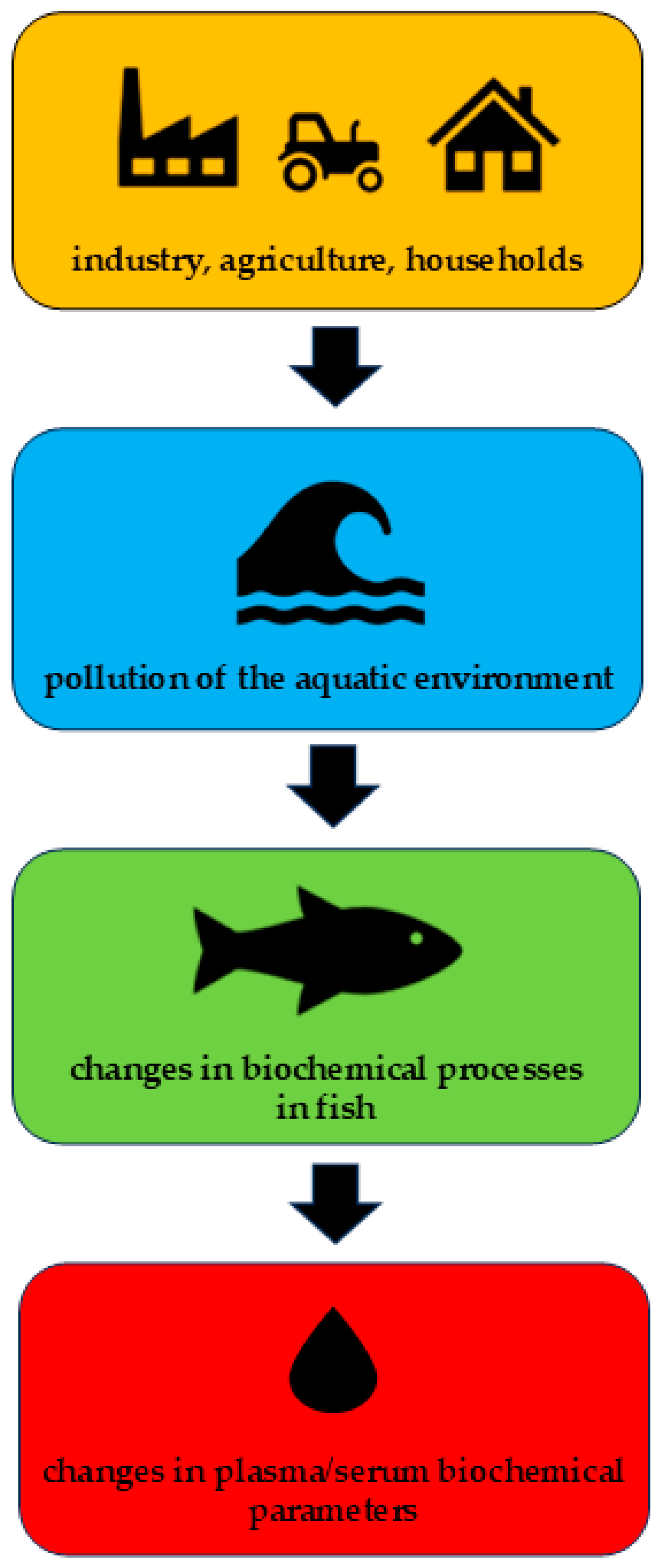
Abstract
1. The Effects of Toxic Agents on Fish Metabolic Biomarkers
| GCS | Fish Species | Toxic Agent | Exp. dur. [d] | Concentration [mg/L] | No Change | Decrease | Increase | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| Em | C. idella | Selenium (as Na2SeO4) | 10–30 | 0.798 | – | TP, Glb | Glu, Alb, Tg, TC | Saha et al. [20] | |
| 1.596 | – | TP, Glb | Glu, Alb, Tg, TC | ||||||
| Em | C. punctata | Selenium (as Na2SeO4) | 10–30 | 1.466 | Glu, Alb, TC | TP, Glb | Glu, Alb, Tg, TC | Saha et al. [20] | |
| 2.932 | – | TP, Glb | Glu, Alb, Tg, TC | ||||||
| Em | L. rohita | Pb(NO3)2 | 4 | 6.80 | Glu, Glb, Tg, TC, Lct | Alb | UAc, TB | Latif et al. [21] | |
| Em | O. mykiss | Cadmium (as CdCl2 · H2O) | 1–30 | 1 * | Glu, TP, TC | Tg, TC | Glu, TP | Heydarnejad et al. [22] | |
| 3 * | Glu | Tg, TC | Glu, TP, Tg, TC | ||||||
| Em | O. niloticus | Copper (as CuSO4 · 5H2O) | 4–21 | 0.05 | Glu, TP, TC | – | Glu | Firat et al. [2] | |
| Em | O. niloticus | Lead [as Pb(NO3)2] | 4–21 | 0.05 | Glu, TP, TC | – | Glu | Firat et al. [2] | |
| Em | P. hypophthalmus | Chromium (as K2Cr2O7) | 4 | 10 | Glu | – | – | Islam et al. [23] | |
| 20 | – | – | Glu | ||||||
| 30 | – | – | Glu | ||||||
| 40 | – | – | Glu | ||||||
| F | O. mykiss | Propiconazole | 7–30 | 0.2 * | Glu, TP, NH3 | – | – | Li et al. [24] | |
| 50 * | Glu, TP, NH3 | – | Glu, NH3 | ||||||
| 500 * | Glu, TP, NH3 | – | Glu, NH3 | ||||||
| H | C. carpio | Chwastox | 1–10 | 1 | Glu, TP, Tg, TC | – | – | Bojarski et al. [25] | |
| 5 | Glu, TP, Tg, TC | TP | – | ||||||
| H | C. carpio | Glyphosate (as Roundup) | 7 | 0.1 | Glu | – | Tg, TC | Kondera et al. [26] | |
| 0.5 | Glu, Tg, TC | – | – | ||||||
| 5.0 | Glu, Tg, TC | – | – | ||||||
| H | C. carpio | Roundup | 1–10 | 1 | Glu, TP, Tg, TC | – | TC | Bojarski et al. [27] | |
| 1–3 | 5 | Glu, TP, Tg, TC | – | Glu | |||||
| H | C. gariepinus | Oxyfluorfen | 60 | 0.58 | – | TP, Alb, Glb | Glu, Ur, Cr | Mansour et al. [28] | |
| H | L. rohita | Glyphosate | 4–12 | 0.5 | Cr, Ur | – | – | Ghaffar et al. [29] | |
| 0.6 | Cr, Ur | – | – | ||||||
| 0.7 | Cr, Ur | – | Cr, Ur | ||||||
| 0.8 | – | – | Cr, Ur | ||||||
| H | O. niloticus | Acetochlor | 4 | 2.625 | TP | – | Glu, Alb, Glb, TC, Cr, UAc | Fathy et al. [30] | |
| H | O. niloticus | Bensulfuron methyl | 4 | 2.50 | Glu, TP, Glb, Cr | – | Alb, TC, UAc | Fathy et al. [30] | |
| H | O. niloticus | Bentazon | 4 | 36.00 | Glu, Glb, Cr, UAc | TP | Alb, TC | Fathy et al. [30] | |
| H | O. niloticus | Bispyribac sodium | 4 | 0.800 | Glu, TP, Cr, UAc | – | Alb, Glb, TC | Fathy et al. [30] | |
| H | O. niloticus | Glyphosate (as Roundup) | 14–28 | 0.6 | – | TP, Alb, Glb | Glu, Cr, Ur | Abdelmagid et al. [31] | |
| H | O. niloticus | Halosulfuron methyl | 4 | 1.275 | Glu, Glb, Cr, UAc | TP | Alb, TC | Fathy et al. [30] | |
| H | O. niloticus | Propanil | 14–56 | 0.22 | Glu, TP, Tg, TC | – | TP | Yaji et al. [32] | |
| 0.44 | Glu, Tg, TC | TC | TP | ||||||
| 0.87 | Glu, Tg, TC | TC | Glu, TP | ||||||
| H | O. niloticus | Quinclorac | 4 | 11.250 | Glu, TP, TC, Cr, UAc | – | Alb, Glb | Fathy et al. [30] | |
| I | C. carpio | Chlorpyrifos | 10–30 | 20 * | Glu, TP, Alb, | TP, Alb, Glb | Glu, Tg | Banaee et al. [3] | |
| Glb, Tg, TC | |||||||||
| 40 * | TP, Alb, Glb, TC | TP, Glb | Glu, Tg, TC | ||||||
| I | C. carpio | Lindane | 1 | 0.38 | – | – | Glu, TP | Saravanan et al. [33] | |
| 5–25 | 0.038 | – | TP | Glu | |||||
| I | C. carpio | Profenofos | 60 | 4.74 * | Alb | Glu, TP, Glb | Tg, TC, Cr, Ur | Abdel Rahman et al. [34] | |
| I | C. gariepinus | Cypermethrin | 4 | 0.025 | TP, Tg | Glu, TC | – | Ojutiku et al. [35] | |
| 0.050 | Tg | Glu | TP, TC | ||||||
| 0.075 | Tg | Glu, TP | TC | ||||||
| 0.100 | Tg | Glu | TP, TC | ||||||
| 0.125 | – | Glu | TP, Tg, TC | ||||||
| I | C. gariepinus | Imidacloprid (as Sunclopride 35% SC) | 60 | 2.03 * | – | TP, Alb, Glb | Cr, Ur | Abdel Rahman et al. [36] | |
| I | C. idella | Endosulfan (as Endosulfan 35 EC) | 15–45 | 0.75 * | – | Glu, TP | TC | Bala [37] | |
| 1 * | Glu | Glu, TP | TC | ||||||
| I | C. mrigala | Diazinon (as Basudin 60 EC) | 10–30 | 0.815 | – | TP, Alb, Glb | Glu | Haider and Rauf [38] | |
| 1.63 | – | TP, Alb, Glb | Glu | ||||||
| I | C. punctata | Malathion (commercial grade) | 4–12 | 0.4 | TC, Alb, Cr | Glu, Ur, UrN, TB | TP, Cr, Ur, UrN | Bharti and Rasool [39] | |
| I | L. rohita | Pyriproxyfen | 10–30 | 0.3 | Glu, TP, Alb, Tg, TC, Cr, Ur | – | – | Naseem et al. [40] | |
| 0.6 | Glu, TP, Alb, Tg, TC, Cr, Ur | TP, Alb | Glu, Tg, TC, Cr, Ur | ||||||
| 0.9 | Glu, TP, Alb, | TP, Alb | Glu, Tg, TC, Cr, Ur | ||||||
| TC, Cr, Ur | |||||||||
| I | L. rohita | Thiamethoxam | 3–5 | 0.5 | Glu, TP, Glb, Tg, TC, Cr, Ur | – | – | Hussain et al. [41] | |
| 1 | Glu, TP, Glb, Tg, TC, Cr, Ur | – | – | ||||||
| 1.5 | Glu, TP, Glb, TC | TP, Glb | Glu, Tg, TC, Cr, Ur | ||||||
| 2 | – | TP, Glb | Glu, Tg, TC, Cr, Ur | ||||||
| I | O. mykiss | Diazinon | 60 | 0.287 | Glu | – | – | Hajirezaee et al. [42] | |
| I | O. niloticus | Cypermethrin | 4–21 | 0.05 * | TP | – | Glu, TC | Firat et al. [2] | |
| I | R. quelen | Fipronil | 4 | 0.3 | TP, Alb | – | – | Fredianelli et al. [43] | |
| 0.4 | TP, Alb | – | – | ||||||
| N | O. niloticus | Al2O3-NPs | 14 | 1 | TC, Cr, UrN | Glu, Tg | – | Canli et al. [44] | |
| 5 | Tg, TC, Cr, UrN | Glu | – | ||||||
| 25 | TC | Glu, Tg | Cr, UrN | ||||||
| N | O. niloticus | CuO-NPs | 14 | 1 | Tg, TC, Cr, UrN | Glu | – | Canli et al. [44] | |
| 5 | Tg, TC, Cr, UrN | Glu | – | ||||||
| 25 | Tg, TC, Cr, UrN | Glu | – | ||||||
| N | O. niloticus | CuO-NPs | 25 | 10 | Ur | – | Cr | Abdel-Latif et al. [45] | |
| 20 | – | – | Ur, Cr | ||||||
| 50 | – | – | Ur, Cr | ||||||
| O | C. carpio | Pyrene | 35 | 10 * | TP, Alb | TC | Tg | Shirdel et al. [46] | |
| 50 * | TP, Alb | TC | Tg | ||||||
| 100 * | – | TP, TC, Alb | Tg | ||||||
| O | C. idella | Ammonium acetate | n/a | 9 ** | Glu, TP, Alb, Glb, Tg, TC | – | – | Xing et al. [47] | |
| O | O. niloticus | Methyl tert-butyl ether | 27 | 2.5 **** | Alb, Tg | TP, Glb | Glu, TC, Cr | Banaee et al. [48] | |
| 5 **** | Alb, Tg | TP, Glb | Glu, TC, Cr | ||||||
| O | P. fulvidraco | Ammonium acetate | n/a | 8 ** | Alb, Tg, TC | Glu, TP, Glb | – | Zhang et al. [49] | |
| Pd | C. carpio | Malachite green | 7–14 | 0.2 | Glu, TP, Tg, TC | – | – | Bojarski et al. [50] | |
| Pd | C. carpio | Methylene blue | 7–14 | 2 | Glu, TP, Tg, TC | – | TP | Bojarski et al. [50] | |
| Pd | C. gariepinus | Clotrimazole (commercial formulation) | 1–21 | 1.94 | Glu, TP | TP | Glu | Melefa et al. [51] | |
| 3.89 | – | Glu, TP | Glu | ||||||
| 7.76 | Glu | Glu, TP | Glu | ||||||
| Pd | H. nobilis | Triclosan | 5–15 | 1 | TP, Tg, TC, Cr, Ur | – | – | Akram et al. [52] | |
| 1.5 | TP, Tg, TC, Cr, Ur | TP | Tg, TC, Cr, Ur | ||||||
| 2.5 | TP, Tg, TC, Cr, Ur | TP | Tg, TC, Cr, Ur | ||||||
| Pd | L. rohita | CuSO4 · 5H2O | 4 | 3.15 | Glu, Glb, Lct | Alb | Tg, TC, UAc, TB | Latif et al. [21] | |
| Pd | P. fluviatilis | Propiscin (etomidate) | 3 min | 1 *** | TP, Alb, Glb, Cr, NH3, TB | – | Glu, Lct | Rożyński et al. [53] | |
| 2 *** | TP, Alb, Glb, Cr, TB | – | Glu, NH3, | ||||||
| Lct | |||||||||
| 10 min | 1 *** | Glu, TP, Alb, | – | Lct | |||||
| Glb, Cr, NH3, TB | |||||||||
| 2 *** | Glu, TP, Alb, | – | – | ||||||
| Glb, Cr, NH3, | |||||||||
| TB, Lct | |||||||||
| Pd | V. vimba | 2-phenoxy- ethanol | 10 min | 0.4 *** | TP, Alb, Glb, Tg, Lct | – | Glu, NH3 | Lepic et al. [54] | |
| Pd | V. vimba | MS 222 | 10 min | 100 | Glu, TP, Alb, Glb, Tg, NH3, Lct | – | – | Lepic et al. [54] | |
| Pd | V. vimba | Propiscin (etomidate) | 10 min | 1 *** | TP, Alb, Glb, Tg, NH3, Lct | – | Glu | Lepic et al. [54] | |
2. The Effects of Toxic Agents on Fish Tissue Damage Biomarkers
| GCS | Fish Species | Toxic Agent | Exp. dur. [d] | Concentration [mg/L] | No Change | Decrease | Increase | Reference |
|---|---|---|---|---|---|---|---|---|
| Em | L. rohita | Pb(NO3)2 | 4 | 6.80 | ALT, AST, LDH | – | ALP | Latif et al. [21] |
| Em | M. cephalus | CuO | 21 | 0.79 1.57 | – – | ALP ALP | ALT, AST, LDH ALT, AST, LDH | Akbary et al. [81] |
| Em | O. mykiss | Cadmium (as CdCl2·H2O) | 1–30 | 1 * 3 * | ALT, ALP ALT, AST, ALP | AST – | ALT, AST, ALP ALT, AST, ALP | Heydarnejad et al. [22] |
| Em | O. niloticus | Copper (as CuSO4·5H2O) | 4–21 | 0.05 | ALP, LDH | – | ALT, AST, ALP, LDH | Firat et al. [2] |
| Em | O. niloticus | Lead [as Pb(NO3)2] | 4–21 | 0.05 | ALP, LDH | – | ALT, AST, ALP, LDH | Firat et al. [2] |
| F | O. mykiss | Propiconazole | 7–30 | 0.2 * 50 * 500 * | ALT, AST, LDH ALT, AST, LDH ALT, AST, LDH | – – – | – LDH ALT, AST, LDH | Li et al. [24] |
| H | C. carpio | Chwastox | 1–10 | 1 5 | ALT ALT | – ALT | – – | Bojarski et al. [25] |
| H | C. carpio | Roundup | 1–10 1–3 | 1 5 | ALT ALT | – – | – – | Bojarski et al. [27] |
| H | C. carpio | Paraquat (as Gramoxone) | 21 | 0.2 0.4 | AST – | – – | ALT, ALP, LDH ALT, AST, ALP, LDH | Nematdoost Haghi and Banaee [82] |
| H | C. gariepinus | Oxyfluorfen | 60 | 0.58 | – | – | ALT, AST, ALP | Mansour et al. [28] |
| H | H. bidorsalis | Glyphosate | 30 | 0.16 0.32 0.48 | ALP ALP – | ALT, AST ALT, AST ALT, AST, ALP | – – – | Inyang et al. [83] |
| H | L. rohita | Glyphosate | 4–12 | 0.5 0.6 0.7 0.8 | ALT, AST, ALP ALT, AST, ALP – – | – – – – | – – ALT, AST, ALP ALT, AST, ALP | Ghaffar et al. [29] |
| H | O. niloticus | Acetochlor | 4 | 2.625 | ALT | – | AST, ALP | Fathy et al. [30] |
| H | O. niloticus | Bensulfuron methyl | 4 | 2.50 | ALT | – | AST, ALP | Fathy et al. [30] |
| H | O. niloticus | Bentazon | 4 | 36.00 | ALT, ALP | – | AST | Fathy et al. [30] |
| H | O. niloticus | Bispyribac sodium | 4 | 0.800 | ALT | – | AST, ALP | Fathy et al. [30] |
| H | O. niloticus | Glyphosate (as Roundup) | 14–28 | 0.6 | – | – | ALT, AST | Abdelmagid et al. [31] |
| H | O. niloticus | Halosulfuron methyl | 4 | 1.275 | ALT, ALP | – | AST | Fathy et al. [30] |
| H | O. niloticus | Propanil | 14–56 | 0.22 0.44 0.87 | ALT, AST ALT ALT | – – – | AST ALT, AST ALT, AST | Yaji et al. [32] |
| H | O. niloticus | Quinclorac | 4 | 11.250 | ALT, AST | – | ALP | Fathy et al. [30] |
| I | C. carpio | Chlorpyrifos | 10–30 | 20 * 40 * | ALT, AST, ALP, LDH ALT, ALP, LDH | – – | AST, LDH ALT, AST, LDH | Banaee et al. [3] |
| I | C. carpio | Profenofos | 60 | 4.74 * | – | – | ALT, AST, ALP, LDH | Abdel Rahman et al. [34] |
| I | C. gariepinus | Cypermethrin | 4 | 0.025 0.050 0.075 0.100 0.125 | ALT, ALP ALP ALT, ALP ALP ALP | AST AST AST AST – | – ALT – ALT ALT, AST | Ojutiku et al. [35] |
| I | C. gariepinus | Deltamethrin (as Butox) | 2 | 0.75 * | – | – | ALT, AST | Amin and Hashem [84] |
| I | C. gariepinus | Imidacloprid (as Sunclopride 35% SC) | 60 | 2.03 * | – | – | ALT, AST | Abdel Rahman et al. [36] |
| I | C. mrigala | Diazinon (as Basudin 60 EC) | 10–30 | 0.815 1.63 | ALT, AST, LDH – | LDH LDH | ALT, AST ALT, AST | Haider and Rauf [38] |
| I | C. punctata | Malathion (commercial grade) | 4–12 | 0.4 | – | ALT, AST, ALP | – | Bharti and Rasool [39] |
| I | L. rohita | Pyriproxyfen | 10–30 | 0.3 0.6 0.9 | ALT, AST, ALP, LDH ALT, AST, ALP, LDH – | – – – | – ALT, AST, ALP, LDH ALT, AST, ALP, LDH | Naseem et al. [40] |
| I | L. rohita | Thiamethoxam | 3–5 | 0.5 1 1.5 2 | ALT, AST, ALP, LDH ALT, AST, ALP, LDH – – | – – – – | – – ALT, AST, ALP, LDH ALT, AST, ALP, LDH | Hussain et al. [41] |
| I | O. niloticus | Cypermethrin | 4–21 | 0.05 * | – | – | ALT, AST, ALP, LDH | Firat et al. [2] |
| I | R. quelen | Fipronil | 4 | 0.3 0.4 | ALP – | – – | ALT, AST ALT, AST, ALP | Fredianelli et al. [43] |
| N | O. niloticus | Ag-NPs | 60 | 1.98 | – | – | ALT, AST | Farag et al. [85] |
| N | O. niloticus | Al2O3-NPs | 14 | 1 5 25 | ALT, AST, ALP ALT, AST, ALP ALT, AST, ALP | – – – | – – – | Canli et al. [44] |
| N | O. niloticus | CuO-NPs | 14 | 1 5 25 | ALT, AST, ALP ALT, AST, ALP ALT, AST, ALP | – – – | – – – | Canli et al. [44] |
| N | O. niloticus | CuO-NPs | 25 | 10 20 50 | – – – | – – – | ALT, AST, ALP ALT, AST, ALP ALT, AST, ALP | Abdel-Latif et al. [45] |
| N | O. niloticus | TiO2-NPs | 14 | 1 5 25 | ALT, AST, ALP ALT, AST, ALP ALT, AST, ALP | – – – | – – – | Canli et al. [44] |
| O | C. carpio | Pyrene | 35 | 10 * 50 * 100 * | ALT, AST, ALP ALT, AST – | – – ALT | – ALP AST, ALP | Shirdel et al. [46] |
| O | C. idella | Ammonium acetate | n/a | 9 ** | – | – | ALP | Xing et al. [47] |
| O | O. niloticus | Methyl tert-butyl ether | 27 | 2.5 **** 5 **** | ALT – | – – | AST, ALP, LDH ALT, AST, ALP, LDH | Banaee et al. [48] |
| O | P. fulvidraco | Ammonium acetate | n/a | 8 ** | – | ALP | – | Zhang et al. [49] |
| Pd | C. carpio | Malachite green | 7–14 | 0.2 | ALT | – | – | Bojarski et al. [50] |
| Pd | C. carpio | Methylene blue | 7–14 | 2 | ALT | – | – | Bojarski et al. [50] |
| Pd | C. gariepinus | Clotrimazole (commercial formulation) | 1–21 | 1.94 3.89 7.76 | ALT, AST, ALP ALT, AST – | – – – | ALT, AST, ALP ALT, AST, ALP ALT, AST, ALP | Melefa et al. [51] |
| Pd | C. gariepinus | Diclofenac | 42 | 1.57 3.14 6.28 | – – – | – – – | ALT, AST, LDH ALT, AST, LDH ALT, AST, LDH | Ajima et al. [86] |
| Pd | H. nobilis | Triclosan | 5–15 | 1 1.5 2.5 | ALT, AST ALT, AST ALT, AST | – – – | – ALT, AST ALT, AST | Akram et al. [52] |
| Pd | L. rohita | CuSO4·5H2O | 4 | 3.15 | ALT, AST, ALP | – | LDH | Latif et al. [21] |
| Pd | P. fluviatilis | Propiscin (etomidate) | 3 min 10 min | 1 *** 2 *** 1 *** 2 *** | ALT, AST, ALP ALT, AST, ALP ALT, AST, ALP ALT, AST, ALP | – – – – | – – – – | Rożyński et al. [53] |
| Pd | V. vimba | 2-phenoxy- ethanol | 10 min | 0.4 *** | ALT, ALP, LDH | – | AST | Lepic et al. [54] |
| Pd | V. vimba | MS 222 | 10 min | 100 | ALT, AST, ALP | LDH | – | Lepic et al. [54] |
| Pd | V. vimba | Propiscin (etomidate) | 10 min | 1 *** | ALT, ALP, LDH | AST | – | Lepic et al. [54] |
3. The Effects of Toxic Agents on Hormone Levels in Fish
| GCS | Fish Species | Toxic Agent | Concentration [mg/L] | Exp. dur. [d] | Hormone Activity | Reference |
|---|---|---|---|---|---|---|
| Em | D. rerio embryos/larvae | Pb(CH3CO2)2·3H2O | 5–10 20 | 5 | GH– IGF↓ GH↑ IGF↓ | Yan et al. [104] |
| Em | D. rerio embryos/larvae | UO22+ (as UO2(NO3)2·6H2O | 2 * 20 * 100 * | 5 | T3– T4– T3↓ T4↑ T3↓ T4– | Xu et al. [105] |
| Em | D. rerio embryos/larvae | Cadmium (as CdCl2·2.5H2O) | 10 * 100 * 1000 * | 5 | T3– T4– T3↓ T4↑ T3↓ T4↑ | Zhong et al. [106] |
| Em | D. rerio embryos/larvae | Mercury (as HgCl2) | 0.1 * 1 * 10 * | 5 | T3– T4↑ T3– T4↑ T3↑ T4↑ | Zhong et al. [106] |
| Em | D. rerio embryos/larvae | Copper (as CuSO4·5H2O) | 1.5 * 15–150 * | 5 | T3– T4– T3↓ T4↑ | Zhong et al. [107] |
| Em | D. rerio embryos/larvae | Zinc (as ZnSO4·7H2O) | 20 * 200 * 2000 * | 5 | T3– T4– T3– T4↑ T3↓ T4↑ | Zhong et al. [107] |
| Em | D. rerio embryos/larvae | Cadmium (as CdCl2) | 0.05–0.5 *** 1 *** | 4 | T3– T4– T3↑ T4↓ | Di Paola et al. [108] |
| Em | D. rerio embryos/larvae | ZnCl2 | 0.1 | 5 | GH– FSH– LH↓ E– | Liu et al. [109] |
| Em | H. molitrix | Mercury (as HgCl2) | 0.05–0.5 * 5 * 50 * | 7–28 7 14 28 7 14 28 | GH– IGF– T3– T4– GH– IGF– T3– T4↓ GH– IGF– T3↓ T4↓ GH– IGF– T3↓ T4– GH↓ IGF↓ T3– T4↓ GH↓ IGF– T3↓ T4↓ GH↓ IGF– T3↓ T4– | Pu et al. [110] |
| Em | M. cephalus | NiCl2 | 5 * 10 * 15 * | 4 | C↑ T3↓ T4↑ C↑ T3↓ T4↓ C↑ T3↓ T4↓ | Jasim et al. [111] |
| Em | P. hypophthalmus | Manganese (as Mn(CH3CO2)2 | 110–114 | 4 | C↑ | Kumar et al. [112] |
| F | D. rerio | Difenoconazole | 5–500 * | 7 | ♀ GH↑ IGF↑ ♂ GH↑ IGF↑ | Teng et al. [113] |
| F | D. rerio | Tebuconazole | 0.4 0.8 1.6 | 21 | ♀♂ E– T– ♀ E– T– ♂ E– T↓ ♀ E↓ T↓ ♂ E– T↓ | Yan et al. [114] |
| H | C. gariepinus | Oxyfluorfen | 0.58 | 60 | ♂ E↑ T↓ FSH↑ LH↓ | Mansour et al. [28] |
| H | D. rerio embryos/larvae | Glyphosate | 0.7–7 35 | 5 | GH– IGF– GH↓ IGF↓ | Liu et al. [115] |
| H | L. rohita | Butachlor (as Shaktiman®) | 12.42–62.1 * | 1 2–3 | C– C↑ | Kumar et al. [116] |
| H | M. salmoides | Glyphosate | 0.5–10 | 21 | C– T↓ E↓ | De Maria et al. [117] |
| H | M. salmoides | Glyphosate (as Rodeo®) | 0.5–10 | 21 | C– T↓ E↓ | De Maria et al. [117] |
| H | P. major | Diuron | 0.1 * | 30–60 | C– E– T– | Nam et al. [118] |
| 1 * | 30 | C↑ E– T– | ||||
| 60 | C– E– T– | |||||
| 10 * | 30 | C– E– T– | ||||
| 60 | C↑ E↓ T↓ | |||||
| H | S. schlegelii | Diuron | 0.1 * | 30–60 | C– E– T– | Nam et al. [118] |
| 1 * | 30 | C– E– T– | ||||
| 60 | C↑ E↓ T– | |||||
| 10 * | 30 | C↑ E↓ T↓ | ||||
| 60 | C↑ E– T↓ | |||||
| I | L. catla | Cypermethrin | 0.14–0.7 * | 30 | E↓ T↓ | Ganguly et al. [119] |
| I | O. mykiss | Diazinon | 0.287 | 60 | T3– T4↓ C↑ | Hajirezaee et al. [42] |
| N | O. niloticus | ZnO-NPs | 1.14 | 28 | T3↓ T4↓ | Abou-Zeid et al. [120] |
| N | O. niloticus | Ag-NPs | 1.98 | 60 | GH↓ T3– T4– G– | Farag et al. [85] |
| N | O. niloticus | Al2O3-NPs | 1 5 25 | 14 | C– T3– T4– C– T3– T4↓ C↑ T3– T4– | Canli et al. [44] |
| N | O. niloticus | CuO-NPs | 1 5 25 | 14 | C– T3– T4– C– T3– T4– C– T3– T4– | Canli et al. [44] |
| N | O. niloticus | TiO2-NPs | 1 5 25 | 14 | C– T3– T4– C– T3– T4↓ C– T3– T4– | Canli et al. [44] |
| N | P. hypophthalmus | Mn-NPs | 91–95 | 4 | C↑ | Kumar et al. [112] |
| O | C. carpio | Pyrene | 10–50 * 100 * | 35 | T3– T4– T3↓ T4↓ | Shirdel et al. [46] |
| O | C. gariepinus | Bisphenol A | 1.43 * | 30 | ♀ FSH↓ LH↑ T↑ E↓ | Hamed et al. [121] |
| O | C. gariepinus | 4-nonylphenol | 0.1 0.2 0.3 | 15 | FSH– LH– T– E↑ FSH– LH– T↓ E↑ FSH↓ LH↑ T↓ E↑ | Sayed et al. [122] |
| O | D. rerio | Tris(2-chloroethyl) phosphate | 0.5 * 5 * | 28 | T3– T4↑ T3↓ T4↑ | Tian et al. [123] |
| O | D. rerio | Tris(2-chloroethyl) phosphate | 0.1 1.5 | 14 28 14 28 | ♀ E– T– ♂ E– T– ♀ E↓ T↓ ♂ E↓ T↓ ♀ E↓ T↓ ♂ E↓ T↓ ♀ E↓ T↓ ♂ E↓ T↓ | Sutha et al. [124] |
| O | D. rerio | 2-ethylhexyl diphenyl phosphate | 2.5 * 50 * 250 * | 21 | ♀ E– T↓ ♂ E– T– ♀ E– T↓ ♂ E↑ T↑ ♀ E↑ T– ♂ E↑ T↑ | Yang et al. [125] |
| O | D. rerio embryos/larvae | KClO4 | 0.5–1 **** 1.5 **** | 4 | T3– T4– T3↑ T4↓ | Di Paola et al. [108] |
| O | D. rerio embryos/larvae | N-(1,3-dimethylbutyl)-N′ -phenyl-p-phenylenediamine | 2.2 * 22 * 220 * | 5 | GH– IGF↓ T3↓ T4– GH– IGF– T3↓ T4↑ GH↓ IGF↓ T3↓ T4↑ | Peng et al. [126] |
| O | D. rerio embryos/larvae | Perfluorohexanoic acid | 0.48 2.4 12 | 4 | T3– T4↓ T3– T4↓ T3↑ T4↑ | Zhang et al. [127] |
| O | D. rerio embryos/larvae | Tetrabromobisphenol A | 50–100 * 200 * | 5 | T3– T4– T3↓ T4↑ | Zhu et al. [128] |
| O | D. rerio embryos/larvae | Dichlorooctylisothiazolinone | 0.003–0.3 * 3–30 * | 4 | T3– T4– T3↓ T4↓ | Lee and Ji [129] |
| O | D. rerio embryos/larvae | Avobenzone | 0.3–3 *** 10 *** 30 *** | 5 | T3– T4– T3– T4↓ T3↑ T4↓ | Ka and Ji [130] |
| O | D. rerio embryos/larvae | Octinoxate | 0.3–10 *** 30 *** | 5 | T3– T4– T3↓ T4↓ | Ka and Ji [130] |
| O | O. niloticus | Ammonium chloride (NH4Cl) | 3 | 4 | ♀ C↑ ♂ C↑ | Zeitoun et al. [131] |
| O | S. maximus | Nitrate nitrogen (NO3-N) | 50 200 400 | 60 | T3↓ T4– GH– IGF– CRH– C– T3↓ T4– GH↓ IGF– CRH↑ C– T3↓ T4↓ GH↓ IGF↓ CRH↑ C↑ | Yu et al. [132] |
| O | T. rubripes | Ammonia (NH3) | 5 | 0.5–4 | T3– T4– CRH– ACTH– C– | Gao et al. [133] |
| 50 | 0.5 | T3– T4– CRH– ACTH– C↑ | ||||
| 1–4 | T3↑ T4↓ CRH↑ ACTH↑ C↑ | |||||
| 100–150 | 0.5–4 | T3↑ T4↓ CRH↑ ACTH↑ C↑ | ||||
| Pd | D. rerio embryos | Pazopanib | 10 ** 50 ** 100 ** | 4 | T3– T4– TSH– T3– T4– TSH↑ T3↓ T4↓ TSH↑ | Yang et al. [134] |
| Pd | D. rerio embryos | Axitinib | 10 ** 50 ** 100 ** | 4 | T3– T4– TSH↑ T3– T4– TSH↑ T3↓ T4↓ TSH↑ | Yang et al. [134] |
| Pd | D. rerio embryos/larvae | Diclofenac | 15.5–139.5 * | 5 | T3– T4↓ | Wang et al. [135] |
| Pd | D. rerio embryos/larvae | Polyhexamethylene guanidine hydrochloride | 4–40 * 400 * | 7 | T3– T4– T3↑ T4↓ | Park et al. [136] |
| Pd | D. rerio embryos/larvae | Chlortetracycline hydrochloride | 2 | 5 | GH– FSH– LH– E– | Liu et al. [109] |
| Pd | D. rerio embryos/larvae | Oxytetracycline | 2 | 5 | GH– FSH– LH– E↑ | Liu et al. [109] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACTH | adrenocorticotropic hormone |
| Alb | albumins |
| ALP | alkaline phosphatase |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| C | cortisol |
| C. carpio | Cyprinus carpio |
| C. gariepinus | Clarias gariepinus |
| C. idella | Ctenopharyngodon idella |
| C. mrigala | Cirrhinus mrigala |
| C. punctata | Channa punctata |
| Cr | creatinine |
| CRH | Corticotropin-releasing hormone |
| d | days |
| D. rerio | Danio rerio |
| E | 17β-estradiol or estrone |
| EDs | endocrine disruptors |
| Em | metals and other elements |
| Exp. dur. | exposure duration |
| F | fungicides |
| FSH | follicle-stimulating hormone |
| G | glucagon |
| GCS | group of chemical substance |
| GH | growth hormone |
| Glb | globulins |
| Glu | glucose |
| H | herbicides |
| H. bidorsalis | Heterobranchus bidorsalis |
| H. molitrix | Hypophthalmichthys molitrix |
| H. nobilis | Hypophthalmichthys nobilis |
| I | insecticides |
| IGF | insulin-like growth factor |
| L. catla | Labeo catla |
| L. rohita | Labeo rohita |
| Lct | lactate |
| LDH | lactate dehydrogenase |
| LH | luteinizing hormone |
| M | microplastics |
| M. cephalus | Mugil cephalus |
| M. salmoides | Micropterus salmoides |
| N | nanoparticles |
| n/a | not applicable |
| NH3 | ammonia |
| NPs | nanoparticles |
| O | other substances |
| O. mykiss | Oncorhynchus mykiss |
| O. niloticus | Oreochromis niloticus |
| P. fluviatilis | Perca fluviatilis |
| P. fulvidraco | Pelteobagrus fulvidraco |
| P. hypophthalmus | Pangasianodon hypophthalmus |
| P. major | Pagrus major |
| Pd | pharmaceuticals and disinfectants |
| R. quelen | Rhamdia quelen |
| S. maximus | Scophthalmus maximus |
| S. schlegelii | Sebastes schlegelii |
| T | testosterone or 11-ketotestosterone |
| T. rubripes | Takifugu rubripes |
| T3 | triiodothyronine |
| T4 | thyroxine |
| TB | total bilirubin |
| TC | total cholesterol |
| Tg | triglycerides |
| TP | total protein |
| TSH | thyrotropin |
| UAc | uric acid |
| Ur | urea |
| UrN | urea nitrogen |
| V. vimba | Vimba vimba |
References
- Barton, B.A.; Iwama, G.K. Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Ann. Rev. Fish Dis. 1991, 1, 3–26. [Google Scholar] [CrossRef]
- Firat, O.; Cogun, H.Y.; Yüzereroglu, T.A.; Gök, G.; Fırat, O.; Kargin, F.; Kötemen, Y. A comparative study on the effects of a pesticide (cypermethrin) and two metals (copper, lead) to serum biochemistry of Nile tilapia, Oreochromis niloticus. Fish Physiol. Biochem. 2011, 37, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Banaee, M.; Haghi, B.N.; Ibrahim, A.T.A. Sub-lethal toxicity of chlorpyrifos on common carp, Cyprinus carpio (Linnaeus, 1758): Biochemical response. Int. J. Aquat. Biol. 2013, 1, 281–288. [Google Scholar] [CrossRef]
- Mehrabi, Z.; Firouzbakhsh, F.; Jafarpour, A. Effects of dietary supplementation of synbiotic on growth performance, serum biochemical parameters and carcass composition in rainbow trout (Oncorhynchus mykiss) fingerlings. J. Anim. Physiol. Anim. Nutr. 2012, 96, 474–481. [Google Scholar] [CrossRef]
- Yarahmadi, P.; Kolangi Miandare, H.; Hoseinifar, S.H. Haemato-immunological and serum biochemical parameters, intestinal histomorphology and growth performance of rainbow trout (Oncorhynchus mykiss) fed dietary fermentable fibre (Vitacel®). Aquac. Nutr. 2016, 22, 1134–1142. [Google Scholar] [CrossRef]
- Wnęk-Auguścik, K.; Witeska, M.; Niemiec, T.; Piotrowska, I.; Fajkowska, M.; Gomułka, P.; Kondera, E.; Łozicki, A.; Zglińska, K.; Rzepkowska, M. The effects of diets containing rapeseed meal on Siberian sturgeon (Acipenser baerii) growth, muscle composition, and physiological performance. Aquac. Rep. 2024, 34, 101891. [Google Scholar] [CrossRef]
- Srivastava, S.J.; Singh, N.D.; Srivastava, A.K.; Sinha, R. Acute toxicity of malachite green and its effects on certain blood parameters of a catfish, Heteropneustes fossilis. Aquat. Toxicol. 1995, 31, 241–247. [Google Scholar] [CrossRef]
- Velisek, J.; Svobodova, Z.; Piackova, V.; Sudova, E. Effects of acute exposure to metribuzin on some hematological, biochemical and histopathological parameters of common carp (Cyprinus carpio L.). Bull. Environ. Contam. Toxicol. 2009, 82, 492–495. [Google Scholar] [CrossRef]
- Lemaire, P.; Drai, P.; Mathieu, A.; Lemaire, S.; Carriere, S.; Giudicelli, J.; Lafaurie, M. Changes with different diets in plasma enzymes (GOT, GPT, LDH, ALP) and plasma lipids (cholesterol, triglycerides) of sea-bass (Dicentrarchus labrax). Aquaculture 1991, 93, 63–75. [Google Scholar] [CrossRef]
- Congleton, J.L.; Wagner, T. Blood-chemistry indicators of nutritional status in juvenile salmonids. J. Fish Biol. 2006, 69, 473–490. [Google Scholar] [CrossRef]
- Georgieva, E.; Kovacheva, E.; Yancheva, V.; Velcheva, I.; Hrischev, P.; Atanassova, P.; Tomov, S.; Stoyanova, S. Pesticides induce fatty degeneration in liver of Cyprinus carpio (Linnaeus 1758) after acute exposure. Ecol. Balk. 2023, 15, 77–82. [Google Scholar]
- Zhu, T.; Corraze, G.; Plagnes-Juan, E.; Skiba-Cassy, S. Cholesterol metabolism regulation mediated by SREBP-2, LXRα and miR-33a in rainbow trout (Oncorhynchus mykiss) both in vivo and in vitro. PLoS ONE 2020, 15, e0223813. [Google Scholar] [CrossRef]
- Borchel, A.; Verleih, M.; Kühn, C.; Rebl, A.; Goldammer, T. Evolutionary expression differences of creatine synthesis-related genes: Implications for skeletal muscle metabolism in fish. Sci. Rep. 2019, 9, 5429. [Google Scholar] [CrossRef]
- Ip, Y.K.; Chew, S.F. Ammonia production, excretion, toxicity, and defense in fish: A review. Front. Physiol. 2010, 1, 134. [Google Scholar] [CrossRef]
- Mathai, J.C. Ammonotelic teleosts and urea transporters. Am. J. Physiol. Renal Physiol. 2005, 288, F453–F454. [Google Scholar] [CrossRef]
- Wright, P.A.; Land, M.D. Urea production and transport in teleost fishes. Comp. Biochem. Physiol. Part A Molec. Integr. Physiol. 1998, 119, 47–54. [Google Scholar] [CrossRef]
- Napolitano, G.; Fasciolo, G.; Agnisola, C.; Venditti, P. Urea excretion and arginase activity as new biomarkers for nitrite stress in freshwater aquatic animals. Water 2021, 13, 3521. [Google Scholar] [CrossRef]
- Omlin, T.; Weber, J.M. Hypoxia stimulates lactate disposal in rainbow trout. J. Exp. Biol. 2010, 213, 3802–3809. [Google Scholar] [CrossRef]
- Kalakonda, A.; Jenkins, B.A.; John, S. Physiology, Bilirubin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Saha, S.; Dhara, K.; Pal, P.; Saha, N.C.; Faggio, C.; Chukwuka, A.V. Longer-term adverse effects of selenate exposures on hematological and serum biochemical variables in air-breathing fish Channa punctata (Bloch, 1973) and non-air breathing fish Ctenopharyngodon idella (Cuvier, 1844): An integrated biomarker response approach. Biol. Trace Elem. Res. 2023, 201, 3497–3512. [Google Scholar] [CrossRef]
- Latif, A.; Khalid, M.; Ali, M. Evaluation of toxic stress of copper sulphate and lead nitrate on hematological and serum biochemical characteristics of freshwater cyprinid (Labeo rohita). Int. J. Curr. Eng. Technol. 2014, 4, 366–372. [Google Scholar]
- Heydarnejad, M.S.; Khosravian-Hemamai, M.; Nematollahi, A. Effects of cadmium at sub-lethal concentration on growth and biochemical parameters in rainbow trout (Oncorhynchus mykiss). Irish Vet. J. 2013, 66, 11. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.M.; Rohani, M.F.; Zabed, S.A.; Islam, M.T.; Jannat, R.; Akter, Y.; Shahjahan, M. Acute effects of chromium on hemato-biochemical parameters and morphology of erythrocytes in striped catfish Pangasianodon hypophthalmus. Toxicol. Rep. 2020, 7, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Velisek, J.; Grabic, R.; Li, P.; Kolarova, J.; Randak, T. Use of hematological and plasma biochemical parameters to assess the chronic effects of a fungicide propiconazole on a freshwater teleost. Chemosphere 2011, 83, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Bojarski, B.; Szała, L.; Osikowski, A.; Hofman, S.; Urbański, K.; Kamińska-Gibas, T.; Rombel-Bryzek, A. Effects of an MCPA-based herbicide formulation on the common carp Cyprinus carpio Linnaeus, 1758—Haematological, biochemical and histological evaluation. Folia Biol. 2023, 71, 146–158. [Google Scholar] [CrossRef]
- Kondera, E.; Teodorczuk, B.; Ługowska, K.; Witeska, M. Effect of glyphosate-based herbicide on hematological and hemopoietic parameters in common carp (Cyprinus carpio L.). Fish Physiol. Biochem. 2018, 44, 1011–1018. [Google Scholar] [CrossRef]
- Bojarski, B.; Osikowski, A.; Hofman, S.; Szała, L.; Szczygieł, J.; Rombel-Bryzek, A. Effects of exposure to a glyphosate-based herbicide on haematological parameters, plasma biochemical indices and the microstructure of selected organs of the common carp (Cyprinus carpio Linnaeus, 1758). Folia Biol. 2022, 70, 213–229. [Google Scholar] [CrossRef]
- Mansour, A.T.; Orabi, S.H.; Ramah, A.; Amen, R.M.; Mahboub, H.H.; Hamed, H.S. Exposure to oxyfluorfen-induced hematobiochemical alterations, oxidative stress, genotoxicity, and disruption of sex hormones in male African catfish and the potential to confront by Chlorella vulgaris. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 267, 109583. [Google Scholar] [CrossRef]
- Ghaffar, A.; Hussain, R.; Ahmad, N.; Ghafoor, R.; Akram, M.W.; Khan, I.; Khan, A. Evaluation of hemato-biochemical, antioxidant enzymes as biochemical biomarkers and genotoxic potential of glyphosate in freshwater fish (Labeo rohita). Chem. Ecol. 2021, 37, 646–667. [Google Scholar] [CrossRef]
- Fathy, M.; Mohamed, I.A.; Farghal, A.I.; Temerak, S.A.; Sayed, A.E.D.H. Hemotoxic effects of some herbicides on juvenile of Nile tilapia Oreochromis niloticus. Environ. Sci. Pollut. Res. 2019, 26, 30857–30865. [Google Scholar] [CrossRef]
- Abdelmagid, A.D.; Said, A.M.; Gawad, E.A.A.; Shalaby, S.A.; Dawood, M.A. Propolis nanoparticles relieved the impacts of glyphosate-induced oxidative stress and immunosuppression in Nile tilapia. Environ. Sci. Pollut. Res. 2022, 29, 19778–19789. [Google Scholar] [CrossRef]
- Yaji, A.; Iheanacho, S.; Ogueji, E. Sublethal exposure and toxicity effect of propanil on hematology and serum biochemistry in Oreochromis niloticus in a static bioassay. Gazi Univ. J. Sci. 2018, 31, 1048–1062. [Google Scholar]
- Saravanan, M.; Kumar, K.P.; Ramesh, M. Haematological and biochemical responses of freshwater teleost fish Cyprinus carpio (Actinopterygii: Cypriniformes) during acute and chronic sublethal exposure to lindane. Pestic. Biochem. Physiol. 2011, 100, 206–211. [Google Scholar] [CrossRef]
- Abdel Rahman, A.N.; Mohamed, A.A.R.; Mohammed, H.H.; Elseddawy, N.M.; Salem, G.A.; El-Ghareeb, W.R. The ameliorative role of geranium (Pelargonium graveolens) essential oil against hepato-renal toxicity, immunosuppression, and oxidative stress of profenofos in common carp, Cyprinus carpio (L.). Aquaculture 2020, 517, 734777. [Google Scholar] [CrossRef]
- Ojutiku, R.O.; Asuwaju, F.P.; Ayanda, I.O.; Obande, R.A.; Agbelege, O.O. Effect of acute toxicity of cypermethrin on some biochemical parameters of juveniles of Clarias gariepinus (Burchell, 1822). Int. J. Eng. Sci. Invent. 2013, 2, 01–07. [Google Scholar]
- Abdel Rahman, A.N.; Mansour, D.A.; Abd El-Rahman, G.I.; Elseddawy, N.M.; Zaglool, A.W.; Khamis, T.; Mahmoud, S.F.; Mahboub, H.H. Imidacloprid toxicity in Clarias gariepinus: Protective role of dietary Hyphaene thebaica against biochemical and histopathological disruption, oxidative stress, immune genes expressions, and Aeromonas sobria infection. Aquaculture 2022, 555, 738170. [Google Scholar] [CrossRef]
- Bala, R. Toxic effect of endosulfan on certain serum biochemical parameters in exotic freshwater fish, Ctenopharyngodon idella (Cuv. and Val.). Trends Life Sci. 2014, 3, 27–30. [Google Scholar]
- Haider, M.J.; Rauf, A. Sub-lethal effects of diazinon on hematological indices and blood biochemical parameters in Indian carp, Cirrhinus mrigala (Hamilton). Braz. Arch. Biol. Technol. 2014, 57, 947–953. [Google Scholar] [CrossRef]
- Bharti, S.; Rasool, F. Analysis of the biochemical and histopathological impact of a mild dose of commercial malathion on Channa punctatus (Bloch) fish. Toxicol. Rep. 2021, 8, 443–455. [Google Scholar] [CrossRef]
- Naseem, S.; Ghaffar, A.; Hussain, R.; Khan, A. Inquisition of toxic effects of pyriproxyfen on physical, hemato-biochemical and histopathological parameters in Labeo rohita Fish. Pak. Vet. J. 2022, 42, 308–315. [Google Scholar]
- Hussain, R.; Ghaffar, A.; Abbas, G.; Jabeen, G.; Khan, I.; Abbas, R.Z.; Noreen, S.; Iqubal, Z.; Chaudhary, I.R.; Ishaq, H.M.; et al. Thiamethoxam at sublethal concentrations induces histopathological, serum biochemical alterations and DNA damage in fish (Labeo rohita). Toxin Rev. 2022, 41, 154–164. [Google Scholar] [CrossRef]
- Hajirezaee, S.; Rafieepour, A.; Rahini, R.; Shafiei, S. Effects of gingko, Ginkgo biloba extract on metabolic hormones, liver histology, and growth parameters of rainbow trout, Oncorhynchus mykiss exposed to diazinon. Toxin Rev. 2019, 40, 632–644. [Google Scholar] [CrossRef]
- Fredianelli, A.C.; Pierin, V.H.; Uhlig, S.C.; Galeb, L.; Rocha, D.C.C.; Ribeiro, D.R.; Anater, A.; Pimpao, C.T. Hematologic, biochemical, genetic, and histological biomarkers for the evaluation of the toxic effects of fipronil for Rhamdia quelen. Turk. J. Vet. Anim. Sci. 2019, 43, 54–59. [Google Scholar] [CrossRef]
- Canli, E.G.; Dogan, A.; Canli, M. Serum biomarker levels alter following nanoparticle (Al2O3, CuO, TiO2) exposures in freshwater fish (Oreochromis niloticus). Environ. Toxicol. Pharmacol. 2018, 62, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Latif, H.M.R.; Dawood, M.A.O.; Mahmoud, S.F.; Shukry, M.; Noreldin, A.E.; Ghetas, H.A.; Khallaf, M.A. Copper oxide nanoparticles alter serum biochemical indices, induce histopathological alterations, and modulate transcription of cytokines, HSP70, and oxidative stress genes in Oreochromis niloticus. Animals 2021, 11, 652. [Google Scholar] [CrossRef]
- Shirdel, I.; Kalbassi, M.R.; Shokri, M.; Olyaei, R.; Sharifpour, I. The response of thyroid hormones, biochemical and enzymological biomarkers to pyrene exposure in common carp (Cyprinus carpio). Ecotoxicol. Environ. Saf. 2016, 130, 207–213. [Google Scholar] [CrossRef]
- Xing, X.; Li, M.; Yuan, L.; Song, M.; Ren, Q.; Shi, G.; Meng, F.; Wang, R. The protective effects of taurine on acute ammonia toxicity in grass carp Ctenopharynodon idellus. Fish Shellfish Immunol. 2016, 56, 517–522. [Google Scholar] [CrossRef]
- Banaee, M.; Badr, A.A.; Multisanti, C.R.; Haghi, B.N.; Faggio, C. The toxicity effects of the individual and combined exposure of methyl tert-butyl ether (MTBE) and tire rubber powder (RP) on Nile tilapia fish (Oreochromis niloticus). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 274, 109759. [Google Scholar] [CrossRef]
- Zhang, M.; Li, M.; Wang, R.; Qian, Y. Effects of acute ammonia toxicity on oxidative stress, immune response and apoptosis of juvenile yellow catfish Pelteobagrus fulvidraco and the mitigation of exogenous taurine. Fish Shellfish Immunol. 2018, 79, 313–320. [Google Scholar] [CrossRef]
- Bojarski, B.; Jurecka, P.; Szala, L.; Kondera, E.; Gaj-Chucher, C.; Stonawski, B.; Rombel-Bryzek, A. The influence of methylene blue and malachite green on common carp (Cyprinus carpio) blood parameters. Anim. Sci. Genet. 2023, 19, 27–41. [Google Scholar] [CrossRef]
- Melefa, T.D.; Mgbenka, B.O.; Aguzie, I.O.; Andong, F.A.; Nwakor, U.; Nwani, C.D. Morphological, haematological and biochemical changes in African catfish Clarias gariepinus (Burchell 1822) juveniles exposed to clotrimazole. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 236, 108815. [Google Scholar] [CrossRef]
- Akram, R.; Ghaffar, A.; Hussain, R.; Khan, I.; de Assis Santana, V.L.; Mehmood, K.; Naz, S.; Iqbal, R.; Imran, H.M.; Qamar, M.R.; et al. Hematological, serum biochemistry, histopathological and mutagenic impacts of triclosan on fish (bighead carp). Agrobiol. Rec. 2022, 7, 18–28. [Google Scholar] [CrossRef]
- Rożyński, M.; Demska-Zakęś, K.; Sikora, A.; Zakęś, Z. Impact of inducing general anesthesia with Propiscin (etomidate) on the physiology and health of European perch (Perca fluviatilis L.). Fish Physiol. Biochem. 2018, 44, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Lepic, P.; Stara, A.; Turek, J.; Kozak, P.; Velisek, J. The effects of four anaesthetics on haematological and blood biochemical profiles in vimba bream, Vimba vimba. Vet. Med. 2014, 59, 81–87. [Google Scholar] [CrossRef]
- Oliveira, J.; Oliva-Teles, A.; Couto, A. Tracking Biomarkers for the Health and Welfare of Aquaculture Fish. Fishes 2024, 9, 289. [Google Scholar] [CrossRef]
- Georgieva, E.; Velcheva, I.; Yancheva, V.; Stoyanova, S.; Vasileva, T.; Bivolarski, V.; Todorova, B.; Iliev, I. A Review on Multi-Biomarkers in Fish for the Assessment of Aquatic Ecosystem Contamination with Organic Pollutants. Ecol. Balk. 2021, 13, 321–330. [Google Scholar]
- Shahsavani, D.; Mohri, M.; Gholipour Kanani, H. Determination of normal values of some blood serum enzymes in Acipenser stellatus Pallas. Fish Physiol. Biochem. 2010, 36, 39–43. [Google Scholar] [CrossRef]
- Ozer, J.; Ratner, M.; Shaw, M.; Bailey, W.; Schomaker, S. The current state of serum biomarkers of hepatotoxicity. Toxicology 2008, 245, 194–205. [Google Scholar] [CrossRef]
- Savari, A.; Hedayati, A.; Safahieh, A.; Movahedinia, A. Determination of some enzymatic indices of yellowfin sea bream (Acanthopagrus latus) in Mahshahr Creeks (North West of Persian Gulf). World 2010, 2, 475–480. [Google Scholar]
- Klykken, C.; Reed, A.K.; Dalum, A.S.; Olsen, R.E.; Moe, M.K.; Attramadal, K.J.K.; Boissonnot, L. Physiological changes observed in farmed Atlantic salmon (Salmo salar L.) with nephrocalcinosis. Aquaculture 2022, 554, 738104. [Google Scholar] [CrossRef]
- Matsche, M.A.; Gibbons, J. Annual variation of hematology and plasma chemistry in shortnose sturgeon, Acipenser brevirostrum, during a dam-impeded spawning run. Fish Physiol. Biochem. 2012, 38, 1679–1696. [Google Scholar] [CrossRef]
- Li, P.; Mai, K.; Trushenski, J.; Wu, G. New developments in fish amino acid nutrition: Towards functional and environmentally oriented aquafeeds. Amino Acids 2009, 37, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Aramli, M.S.; Kalbassi, M.R.; Nazari, R.M. Blood and seminal plasma enzyme values of Persian sturgeon, Acipenser persicus (Chordata: Osteichthyes). Ital. J. Zool. 2013, 80, 490–493. [Google Scholar] [CrossRef]
- Tripathi, N.K.; Latimer, K.S.; Lewis, T.L.; Burnley, V.V. Biochemical reference intervals for koi (Cyprinus carpio). Comp. Cli. Pathol. 2003, 12, 160–165. [Google Scholar] [CrossRef]
- Kondera, E.; Bojarski, B.; Ługowska, K.; Kot, B.; Witeska, M. Effects of oxytetracycline and gentamicin therapeutic doses on hematological, biochemical and hematopoietic parameters in Cyprinus carpio juveniles. Animals 2020, 10, 2278. [Google Scholar] [CrossRef]
- Oliveira, H.H.; Liebel, S.; Rossi, S.C.; Azevedo, A.C.; Barrera, E.A.; Garcia, J.R.E.; Grötzner, S.R.; Neto, F.F.; Randi, M.A.F.; Ribeiro, C.A.O. Mixtures of benzo(a)pyrene, dichlorodiphenyltrichloroethane and tributyltin are more toxic to neotropical fish Rhamdia quelen than isolated exposures. Ecotoxicol. Environ. Saf. 2015, 122, 106–115. [Google Scholar] [CrossRef]
- Mahamood, M.; Javed, M.; Alhewairini, S.S.; Zahir, F.; Sah, A.K.; Ahmad, M.I. Labeo rohita, a bioindicator for water quality and associated biomarkers of heavy metal toxicity. NPJ Clean Water 2021, 4, 17. [Google Scholar] [CrossRef]
- Giannini, E.G.; Testa, R.; Savarino, V. Liver enzyme alteration: A guide for clinicians. CMAJ 2005, 172, 367–379. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Li, J.; Chen, Y.; Han, L.; He, Q.; Chu, J.; Liu, K. The role of hepatic antioxidant capacity and hepatobiliary transporter in liver injury induced by isopsoralen in zebrafish larvae. Hum. Exp. Toxicol. 2019, 38, 36–44. [Google Scholar] [CrossRef]
- Martins, R.X.; Vieira, L.; Souza, J.A.C.R.; Silva, M.G.F.; Muniz, M.S.; Souza, T.; Queiroga, F.R.; Machado, M.R.F.; da Silva, P.M.; Farias, D. Exposure to 2,4-D herbicide induces hepatotoxicity in zebrafish larvae. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 248, 109110. [Google Scholar] [CrossRef]
- Banaee, M. Alkaline phosphatase activity as a biochemical biomarker in aqua-toxicological studies. Int. J. Aquat. Biol. 2020, 8, 143–147. [Google Scholar] [CrossRef]
- Kulkarni, R.S. Sex differences in the blood biochemical parameters of the fresh water fish, Notopterus notopterus (Pallas, 1789). World News Nat. Sci. 2017, 6, 36–43. [Google Scholar]
- Peres, H.; Santos, S.; Oliva-Teles, A. Blood chemistry profile as indicator of nutritional status in European seabass (Dicentrarchus labrax). Fish Physiol. Biochem. 2014, 40, 1339–1347. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sahu, N.P.; Pal, A.K.; Kumar, S.; Sinha, A.K.; Ranjan, J.; Baruah, K. Modulation of key enzymes of glycolysis, gluconeogenesis, amino acid catabolism, and TCA cycle of the tropical freshwater fish Labeo rohita fed gelatinized and non-gelatinized starch diet. Fish Physiol. Biochem. 2010, 36, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A. An evaluation of lactate dehydrogenase in the lindane exposed fresh water fish, Channa punctatus. Int. J. Anim. Biotechnol. Appl. 2021, 7, 9–19. [Google Scholar]
- Diamantino, T.C.; Almeida, E.; Soares, A.M.; Guilhermino, L. Lactate dehydrogenase activity as an effect criterion in toxicity tests with Daphnia magna straus. Chemosphere 2001, 45, 553–560. [Google Scholar] [CrossRef]
- Abhijith, B.D.; Ramesh, M.; Poopal, R.K. Responses of metabolic and antioxidant enzymatic activities in gill, liver and plasma of Catla catla during methyl parathion exposure. J. Basic Appl. Zool. 2016, 77, 31–40. [Google Scholar] [CrossRef]
- Parveen, S.; Bharose, R.; Singh, D. Effect of tannery waste water on lactate dehydrogenase (LDH) enzyme activity of fresh water fish, Channa punctatus. J. Entomol. Zool. Stud. 2017, 5, 643–647. [Google Scholar]
- Bury, N.R.; McGeer, J.C.; Eddy, F.B.; Codd, G.A. Liver damage in brown trout, Salmo trutta L., and rainbow trout, Oncorhynchus mykiss (Walbaum), following administration of the cyanobacterial hepatotoxin microcystin-LR via the dorsal aorta. J. Fish Dis. 1997, 20, 209–215. [Google Scholar] [CrossRef]
- Das, P.C.; Ayyappan, S.; Das, B.K.; Jena, J.K. Nitrite toxicity in Indian major carps: Sublethal effect on selected enzymes in fingerlings of Catla catla, Labeo rohita and Cirrhinus mrigala. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2004, 138, 3–10. [Google Scholar] [CrossRef]
- Akbary, P.; Sartipi Yarahmadi, S.; Jahanbakhshi, A. Hematological, hepatic enzymes’ activity and oxidative stress responses of gray mullet (Mugil cephalus) after sub-acute exposure to copper oxide. Environ. Sci. Pollut. Res. 2018, 25, 1800–1808. [Google Scholar] [CrossRef]
- Nematdoost Haghi, B.; Banaee, M. Effects of micro-plastic particles on paraquat toxicity to common carp (Cyprinus carpio): Biochemical changes. Int. J. Environ. Sci. Technol. 2017, 14, 521–530. [Google Scholar] [CrossRef]
- Inyang, I.R.; Okon, N.C.; Izah, S.C. Effect of glyphosate on some enzymes and electrolytes in Heterobranchus bidorsalis (a common African catfish). Biotechnol. Res. 2016, 2, 161–165. [Google Scholar]
- Amin, K.A.; Hashem, K.S. Deltamethrin-induced oxidative stress and biochemical changes in tissues and blood of catfish (Clarias gariepinus): Antioxidant defense and role of alpha-tocopherol. BMC Vet. Res. 2012, 8, 45. [Google Scholar] [CrossRef]
- Farag, M.R.; Abo-Al-Ela, H.G.; Alagawany, M.; Azzam, M.M.; El-Saadony, M.T.; Rea, S.; Cerbo, A.D.; Nouh, D.S. Effect of quercetin nanoparticles on hepatic and intestinal enzymes and stress-related genes in Nile tilapia fish exposed to silver nanoparticles. Biomedicines 2023, 11, 663. [Google Scholar] [CrossRef] [PubMed]
- Ajima, M.N.; Ogo, O.A.; Audu, B.S.; Ugwoegbu, K.C. Chronic diclofenac (DCF) exposure alters both enzymatic and haematological profile of African catfish, Clarias gariepinus. Drug Chem. Toxicol. 2015, 38, 383–390. [Google Scholar] [CrossRef]
- WHO/IPCS. Global Assessment of the State-of-the-Science of Endocrine Disruptors. 2002. Available online: http://www.who.int/ (accessed on 10 August 2024).
- Brehm, E.; Flaws, J.A. Transgenerational effects of endocrine-disrupting chemicals on male and female reproduction. Endocrinology 2019, 160, 1421–1435. [Google Scholar] [CrossRef]
- Power, D.M.; Llewellyn, L.; Faustino, M.; Nowell, M.A.; Bjornsson, B.T.; Einarsdottir, I.E.; Canario, A.V.M.; Sweeney, G.E. Thyroid hormones in growth and development of fish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 130, 447–459. [Google Scholar] [CrossRef]
- Robaire, B.; Delbes, G.; Head, J.A.; Marlatt, V.L.; Martyniuk, C.J.; Reynaud, S.; Trudeau, V.L.; Mennigen, J.A. A cross-species comparative approach to assessing multi- and transgenerational effects of endocrine disrupting chemicals. Environ. Res. 2022, 204, 112063. [Google Scholar] [CrossRef]
- Deal, C.K.; Volkoff, H. The role of the thyroid axis in fish. Front. Endocrinol. 2020, 11, 596585. [Google Scholar] [CrossRef]
- Jain, A.; Singh, S.; Yadav, S.; Dubey, A.; Awasthi, Y.; Tiwari, V.; Kumar, V.; Dubey, I.; Trivedi, S.P.; Kumar, M. Oxidative toxicity mediated oophoritis alters ovarian growth in Channa punctatus under prolonged exposure to herbicide, paraquat dichloride. Sci. Rep. 2025, 15, 1304. [Google Scholar] [CrossRef]
- Santhi, J.J.; Kumar Issac, P.; Velayutham, M.; Hussain, S.A.; Rafi Shaik, M.; Shaik, B.; Guru, A. Reproductive toxicity of perfluorobutane sulfonate in zebrafish (Danio rerio): Impacts on oxidative stress, hormone disruption and HPGL axis dysregulation. Comp. Biochem. Physiol. Part C 2025, 289, 110122. [Google Scholar] [CrossRef] [PubMed]
- Lemos, L.S.; Angarica, L.M.; Hauser-Davis, R.A.; Quinete, N. Cortisol as a stress indicator in fish: Sampling methods, analytical techniques, and organic pollutant exposure assessments. Int. J. Environ. Res. Public Health 2023, 20, 6237. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.N.; Valdés, J.A.; Molina, A.; Björnsson, B.T. Regulation of skeletal muscle growth in fish by the growth hormone—Insulin-like growth factor system. Gen. Comp. Endocrinol. 2013, 192, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Reinecke, M.; Björnsson, B.T.; Dickho, W.W.; McCormick, S.D.; Navarro, I.; Power, D.M.; Gutiérrez, J. Growth hormone and insulin-like growth factors in fish: Where we are and where to go. Gen. Comp. Endocrinol. 2005, 142, 20–24. [Google Scholar] [CrossRef]
- Ndandala, C.B.; Liu, J.; Huang, H.; Dai, M.; Li, G.; Mustapha, U.F.; Chen, H. Current research and future perspectives of GH and IGFs family genes in somatic growth and reproduction of teleost fish. Aquac. Rep. 2022, 26, 101289. [Google Scholar] [CrossRef]
- Celino-Brady, F.T.; Lerner, D.T.; Seale, A.P. Experimental approaches for characterizing the endocrine disrupting effects of environmental chemicals in fish. Front. Endocrinol. 2021, 11, 619361. [Google Scholar] [CrossRef]
- Xu, C.; Gong, H.; Sun, X.; Li, L.; Niu, L.; Liu, W. Maternal exposure to dietary uranium causes oxidative stress and thyroid disruption in zebrafish offspring. Ecotoxicol. Environ. Saf. 2023, 265, 115501. [Google Scholar] [CrossRef]
- Teng, M.; Zhao, W.; Chen, X.; Wang, C.; Zhou, L.; Wang, C.; Xu, Y. Parental exposure to propiconazole at environmentally relevant concentrations induces thyroid and metabolism disruption in zebrafish (Danio rerio) offspring: An in vivo, in silico and in vitro study. Ecotoxicol. Environ. Saf. 2022, 242, 113865. [Google Scholar] [CrossRef]
- Wang, H.; Jing, C.; Peng, H.; Liu, S.; Zhao, H.; Zhang, W.; Chen, X.; Hu, F. Parental whole life-cycle exposure to tris (2-chloroethyl) phosphate (TCEP) disrupts embryonic development and thyroid system in zebrafish offspring. Ecotoxicol. Environmen. Saf. 2022, 248, 114313. [Google Scholar] [CrossRef]
- Li, R.; Yang, L.; Zhou, B. Early-life exposure to tris (1,3-dichloro-2-propyl) phosphate caused multigenerational neurodevelopmental toxicity in zebrafish via altering maternal thyroid hormones transfer and epigenetic modifications. Environ. Pollut. 2021, 285, 117471. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, Z.; Li, X.; Zhuang, Z.; Li, Y.; Wang, X.; Liao, C.; Chen, L.; Luo, Q.; Chen, X. Reproductive toxicity and transgenerational effects of co-exposure to polystyrene microplastics and arsenic in zebrafish. Comp. Biochem. Physiol. Part C 2025, 290, 110134. [Google Scholar] [CrossRef]
- Yan, R.; Ding, J.; Yang, Q.; Zhang, X.; Han, J.; Jin, T.; Shi, S.; Wang, X.; Zheng, Y.; Li, H.; et al. Lead acetate induces cartilage defects and bone loss in zebrafish embryos by disrupting the GH/IGF-1 axis. Ecotoxicol. Environ. Saf. 2023, 253, 114666. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Niu, L.; Li, T.; Hu, C.; Guo, H.; Ye, J.; Li, L.; Liu, W. Waterborne uranium causes toxic effect and thyroid disruption in zebrafish larvae. Ecotoxicol. Environ. Saf. 2021, 208, 111585. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Zhang, H.; Li, Y. Thyroid-disrupting effects of cadmium and mercury in zebrafish embryos/larvae. Water 2023, 15, 135. [Google Scholar] [CrossRef]
- Zhong, L.; Zhang, H.; Li, Y. Copper and zinc treatments alter the thyroid endocrine system in zebrafish embryos/larvae. Toxics 2022, 10, 756. [Google Scholar] [CrossRef]
- Di Paola, D.; Natale, S.; Iaria, C.; Crupi, R.; Spanò, N.; Gugliandolo, E.; Cuzzocrea, S.; Peritore, A.F. Environmental co-exposure to potassium perchlorate and Cd caused toxicity and thyroid endocrine disruption in zebrafish embryos and larvae (Danio rerio). Toxics 2022, 10, 198. [Google Scholar] [CrossRef]
- Liu, S.; Tu, X.; Chen, X.; Mo, L.; Liu, Y.; Xu, J.; Deng, M.; Wu, Y. Effects of single and combined exposure to zinc and two tetracycline antibiotics on zebrafish at the early stage. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 264, 109522. [Google Scholar] [CrossRef]
- Pu, Y.; Guo, J.; Yang, H.; Zhong, L.; Tian, H.; Deng, H.; Duan, X.; Liu, S.; Chen, D. Environmentally relevant concentrations of mercury inhibit the growth of juvenile silver carp (Hypophthalmichthys molitrix): Oxidative stress and GH/ IGF axis. Ecotoxicol. Environ. Saf. 2022, 236, 113484. [Google Scholar] [CrossRef]
- Jasim, S.A.; Golgouneh, S.; Jaber, M.M.; Indiaminov, S.I.; Alsaikhan, F.; Hammid, A.T.; Mustafa, J.F.; Karim, Y.S.; Sultan, M.Q.; Norbakhsh, M. Effects of short-term exposure to the heavy metal, nickel chloride (NiCl2) on gill histology and osmoregulation components of the gray mullet, Mugil cephalus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 258, 109361. [Google Scholar] [CrossRef]
- Kumar, N.; Thorat, S.T.; Reddy, K.S. Multi biomarker approach to assess manganese and manganese nanoparticles toxicity in Pangasianodon hypophthalmus. Sci. Rep. 2023, 13, 8505. [Google Scholar] [CrossRef]
- Teng, M.; Qi, S.; Zhu, W.; Wang, Y.; Wang, D.; Yang, Y.; Li, H.; Li, C.; Dong, K.; Wang, C. Sex-specific effects of difenoconazole on the growth hormone endocrine axis in adult zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2017, 144, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Li, G.; Lu, Q.; Hou, J.; Pan, M.; Peng, M.; Peng, X.; Wan, H.; Liu, X.; Wu, Q. Molecular mechanisms of tebuconazole affecting the social behavior and reproduction of zebrafish. Int. J. Environ. Res. Public Health 2023, 20, 3928. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shangguan, Y.; Zhu, P.; Sultan, Y.; Feng, Y.; Li, X.; Ma, J. Developmental toxicity of glyphosate on embryo-larval zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2022, 236, 113493. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Swain, H.S.; Roya, S.; Das, B.K.; Upadhyay, A.; Ramteke, M.K.; Kumar, V.; Kole, R.K.; Banerjee, H. Integrated biomarker approach strongly explaining in vivo sub-lethal acute toxicity of butachlor on Labeo rohita. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 261, 10942. [Google Scholar] [CrossRef]
- De Maria, M.; Kroll, K.J.; Yu, F.; Nouri, M.-Z.; Silva-Sanchez, C.; Guillermo Perez, J.; Moraga Amador, D.A.; Zhang, Y.; Walsh, M.T.; Denslow, N.D. Endocrine, immune and renal toxicity in male largemouth bass after chronic exposure to glyphosate and Rodeo®. Aquat. Toxicol. 2022, 246, 106142. [Google Scholar] [CrossRef]
- Nam, S.-E.; Haque, N.; Do, S.D.; Rhee, J.-S. Chronic effects of environmental concentrations of antifoulant diuron on two marine fish: Assessment of hormone levels, immunity, and antioxidant defense system. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 263, 109510. [Google Scholar] [CrossRef]
- Ganguly, S.; Adhikari, A.; Sadhukhan, D.; Raut, S.S.; Kumar, V.S.; Nag, S.K.; Das, B.K. Endocrine disruptive toxicity of cypermethrin in Labeo catla: Involvement of genes and proteins related to the HPG axis. Sci. Total Environ. 2023, 901, 165958. [Google Scholar] [CrossRef]
- Abou-Zeid, S.M.; Elsabbagh, H.S.; Zheng, C.; Khalil, S.R.; Siddique, M.S.; Farag, M.R.; Mawed, S.A.; Azzam, M.M.; Di Cerbo, A.; Elkhadrawey, B.A. Thymol-enriched diet alleviates the toxic impacts of zinc oxide nanoparticles on growth performance, blood biochemistry, oxidant/ antioxidant status and stress-related genes and histology of liver and gills in Oreochromis niloticus. Aquac. Rep. 2023, 33, 101750. [Google Scholar] [CrossRef]
- Hamed, H.S.; Ali, R.M.; Shaheen, A.A.; Hussein, N.M. Chitosan nanoparticles alleviated endocrine disruption, oxidative damage, and genotoxicity of Bisphenol-A- intoxicated female African catfish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 24, 109104. [Google Scholar] [CrossRef]
- Sayed, A.E.-D.H.; Eid, Z.; Mahmoud, U.M.; Lee, J.-S.; Mekkawy, I.A.A. Reproductive toxicity and recovery associated with 4-nonylphenol exposure in juvenile African catfish (Clarias gariepinus). Front. Physiol. 2022, 13, 851031. [Google Scholar] [CrossRef]
- Tian, D.; Yu, Y.; Yu, Y.; Lu, L.; Tong, D.; Zhang, W.; Zhang, X.; Shi, W.; Liu, G. Tris(2-chloroethyl) phosphate exerts hepatotoxic impacts on zebrafish by disrupting hypothalamic−pituitary−thyroid and gut−liver axes. Environ. Sci. Technol. 2023, 57, 9043–9054. [Google Scholar] [CrossRef] [PubMed]
- Sutha, J.; Anila, P.A.; Gayathri, M.; Ramesh, M. Long term exposure to tris (2-chloroethyl) phosphate (TCEP) causes alterations in reproductive hormones, vitellogenin, antioxidant enzymes, and histology of gonads in zebrafish (Danio rerio): In vivo and computational analysis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 254, 109263. [Google Scholar] [CrossRef]
- Yang, R.; Wang, X.; Wang, J.; Chen, P.; Liu, Q.; Zhong, W.; Zhu, L. Insights into the sex-dependent reproductive toxicity of 2-ethylhexyl diphenyl phosphate on zebrafish (Danio rerio). Environ. Int. 2022, 158, 106928. [Google Scholar] [CrossRef]
- Peng, W.; Liu, C.; Chen, D.; Duan, X.; Zhong, L. Exposure to N-(1,3-dimethylbutyl)-N′-phenyl-p-phenylenediamine (6PPD) affects the growth and development of zebrafish embryos/larvae. Ecotoxicol. Environ. Saf. 2022, 232, 113221. [Google Scholar] [CrossRef]
- Zhang, S.; Han, Z.; Guo, X.; Lu, S.; He, J.; Wu, Q.; Liu, X.; Xie, P. Perfluorohexanoic acid caused disruption of the hypothalamus-pituitary-thyroid axis in zebrafish larvae. Ecotoxicol. Environ. Saf. 2022, 232, 113283. [Google Scholar] [CrossRef]
- Zhu, B.; Han, J.; Lei, L.; Hua, J.; Zuo, Y.; Zhou, B. Effects of SiO2 nanoparticles on the uptake of tetrabromobisphenol A and its impact on the thyroid endocrine system in zebrafish larvae. Ecotoxicol. Environ. Saf. 2021, 209, 111845. [Google Scholar] [CrossRef]
- Lee, S.; Ji, K. Toxicological signature for thyroid endocrine disruption of dichlorooctylisothiazolinone in zebrafish larvae. Ecotoxicology 2023, 32, 38–45. [Google Scholar] [CrossRef]
- Ka, Y.; Ji, K. Waterborne exposure to avobenzone and octinoxate induces thyroid endocrine disruption in wild-type and thrαa−/− zebrafish larvae. Ecotoxicology 2022, 31, 948–955. [Google Scholar] [CrossRef]
- Zeitoun, M.M.; El-Azrak, K.E.M.; Zaki, M.A.; Nemat-Allah, B.R.; Mehana, E.E. Effects of ammonia toxicity on growth performance, cortisol, glucose and hematological response of Nile Tilapia (Oreochromis niloticus). Aceh J. Anim. Sci. 2016, 1, 21–28. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Y.; Xiao, Y.; Li, X.; Zhou, L.; Wang, Y.; Du, T.; Ma, X.; Li, J. Investigating the effect of nitrate on juvenile turbot (Scophthalmus maximus) growth performance, health status, and endocrine function in marine recirculation aquaculture systems. Ecotoxicol. Environ. Saf. 2021, 208, 111617. [Google Scholar] [CrossRef]
- Gao, X.; Wang, X.; Wang, X.; Fang, Y.; Cao, S.; Huang, B.; Chen, H.; Xing, R.; Liu, B. Toxicity in Takifugu rubripes exposed to acute ammonia: Effects on immune responses, brain neurotransmitter levels, and thyroid endocrine hormones. Ecotoxicol. Environ. Saf. 2022, 244, 114050. [Google Scholar] [CrossRef]
- Yang, L.; Tu, P.-H.; Zhang, C.-X.; Xie, R.-R.; Dong, M.; Jing, Y.; Chen, X.; Wei, G.; Song, H.-D. Influence of two anti-tumor drugs, pazopanib, and axitinib, on the development and thyroid-axis of zebrafish (Danio rerio) embryos/larvae. Front. Endocrinol. 2023, 14, 1204678. [Google Scholar] [CrossRef]
- Wang, H.; Dong, F.; Zhao, Y.; Fu, S.; Zhao, H.; Liu, S.; Zhang, W.; Hu, F. Exposure to diclofenac alters thyroid hormone levels and transcription of genes involved in the hypothalamic–pituitary–thyroid axis in zebrafish embryos/larvae. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 257, 109335. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.; Ji, K. Developmental toxicity and thyroid endocrine disruption of polyhexamethylene guanidine hydrochloride and humidifier disinfectant in zebrafish larvae. Appl. Sci. 2021, 11, 4884. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bojarski, B.; Witeska, M.; Kondera, E. Blood Biochemical Biomarkers in Fish Toxicology—A Review. Animals 2025, 15, 965. https://doi.org/10.3390/ani15070965
Bojarski B, Witeska M, Kondera E. Blood Biochemical Biomarkers in Fish Toxicology—A Review. Animals. 2025; 15(7):965. https://doi.org/10.3390/ani15070965
Chicago/Turabian StyleBojarski, Bartosz, Małgorzata Witeska, and Elżbieta Kondera. 2025. "Blood Biochemical Biomarkers in Fish Toxicology—A Review" Animals 15, no. 7: 965. https://doi.org/10.3390/ani15070965
APA StyleBojarski, B., Witeska, M., & Kondera, E. (2025). Blood Biochemical Biomarkers in Fish Toxicology—A Review. Animals, 15(7), 965. https://doi.org/10.3390/ani15070965







