Genetic Diversity of Sodefrin-Variant Pheromones and Pheromone Responsiveness in Subspecies of the Japanese Sword-Tail Newt Cynops ensicauda
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sequence Determination of cDNA Encoding Sodefrin or Its Variant Precursors or SPF Proteins
2.3. Phylogenetic Tree Reconstruction
2.4. Test for Biological Activity of Sodefrin, Sodefrin-Variant Peptides, and Imorin
3. Results
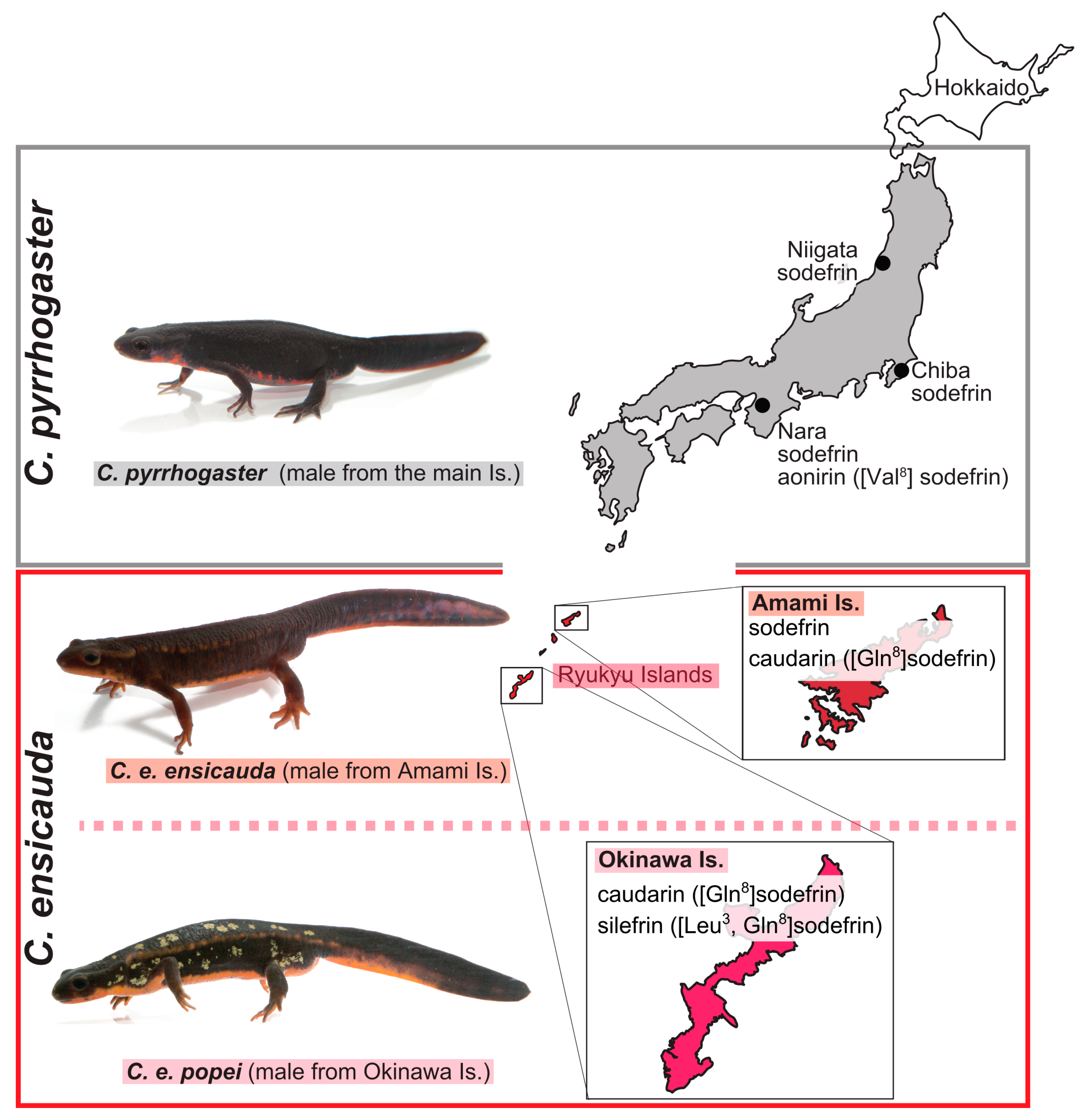
3.1. Analysis of Transcripts Encoding Sodefrin Precursor, Sodefrin-Variant Precursor, and SPF Proteins
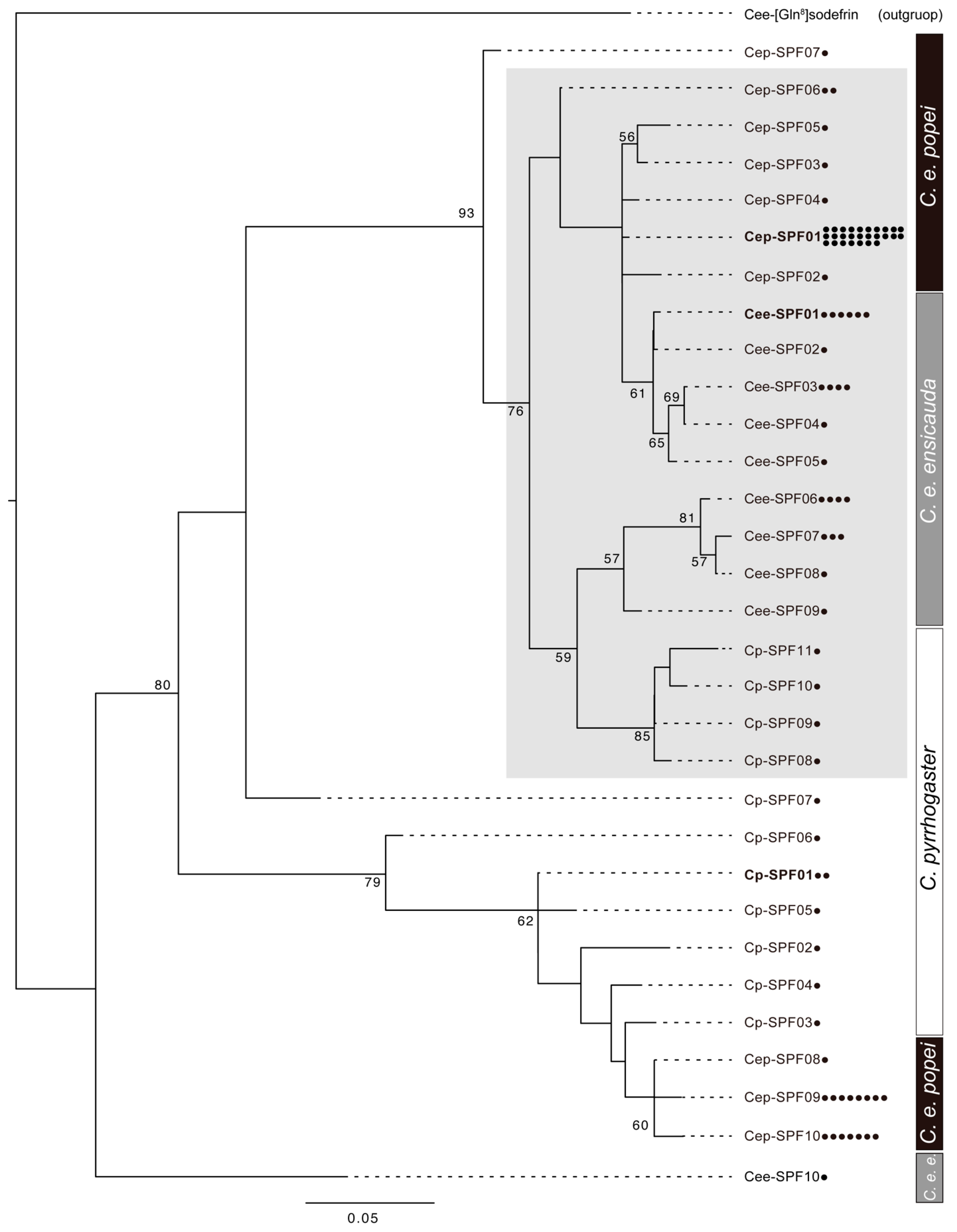
3.2. Phylogenetic Analysis of the Sodefrin Precursor-Related Proteins
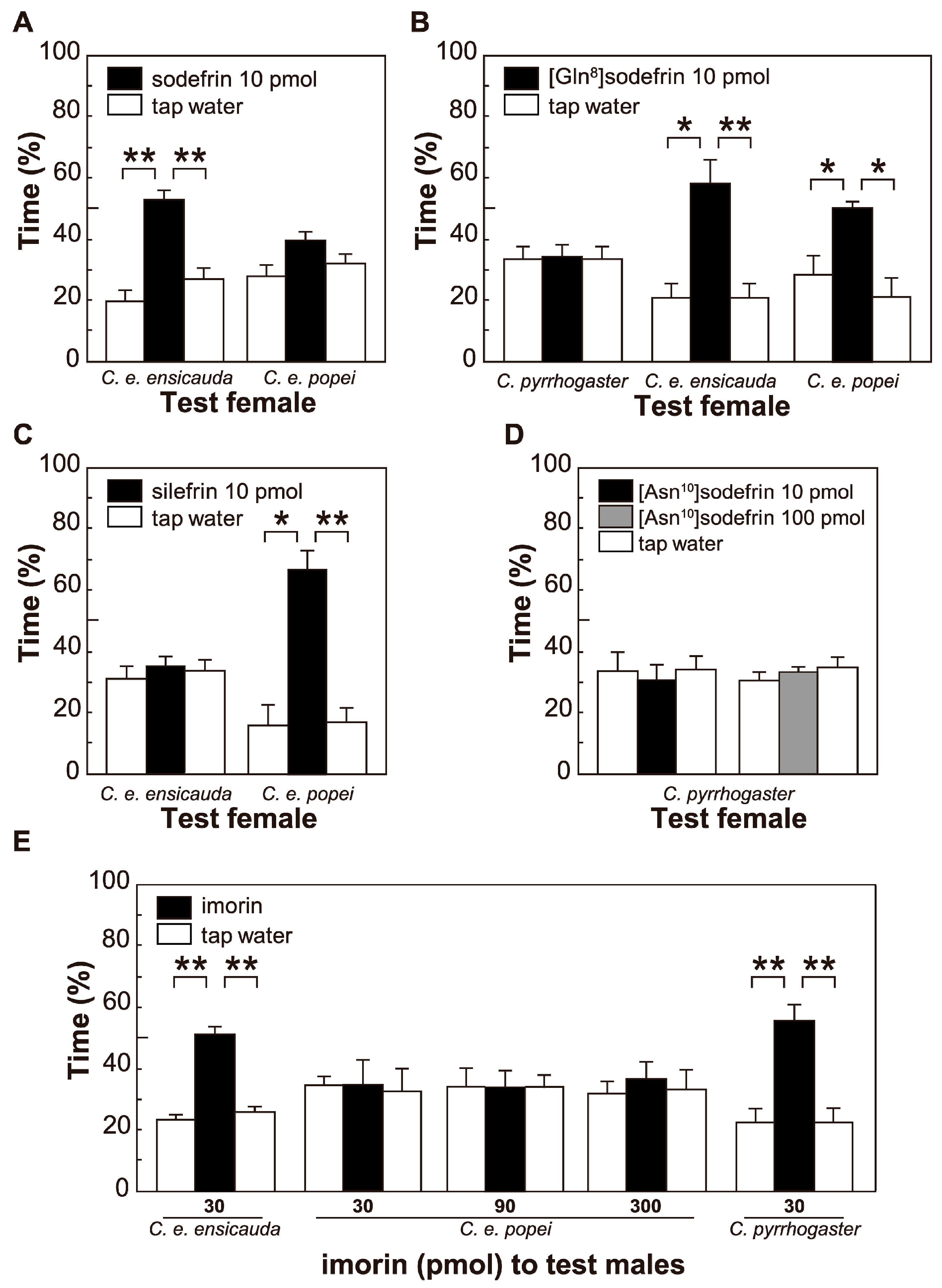
3.3. Evaluation of the Pheromonal Activity of Sodefrin, Sodefrin Variants and Imorin
3.4. Obtaining and Organizing the Amino Acid Sequences of Known Sodefrin or Sodefrin-Variant Precursor and SPF Proteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Group | Abbreviated Transcript Name | Accession | mRNA Length (Base) | Number of Amino Acid Residues | Total Number of Clones | Number of Clones per Individual | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cynops ensicauda ensicauda | C. e. popei | ||||||||||||||||||||
| A | B | C | D | E | F | G | H | A | B | C | D | E | F | G | H | ||||||
| Sodefrin and Sodefrin-variant Precursor (S-SVP) | S-SVP subgroup | 316–625 | 102–205 | 55 | 6 | 6 | 5 | 5 | 6 | 5 | 4 | 4 | 4 | 2 | 1 | 1 | 3 | 1 | 1 | 1 | |
| Sodefrin precursor | 577 | 189 | 11 | 6 | 3 | 1 | 1 | ||||||||||||||
| Cee-sodefrin01-1 | LC861879 | 577 | 189 | 3 | 3 | ||||||||||||||||
| Cee-sodefrin01-2 | LC861880 | 577 | 189 | 1 | 1 | ||||||||||||||||
| Cee-sodefrin02-1 | LC861881 | 577 | 189 | 1 | 1 | ||||||||||||||||
| Cee-sodefrin03-1 | LC861882 | 577 | 189 | 1 | 1 | ||||||||||||||||
| Cee-sodefrin03-2 | LC861883 | 577 | 189 | 1 | 1 | ||||||||||||||||
| Cee-sodefrin04-1 | LC861884 | 577 | 189 | 2 | 2 | ||||||||||||||||
| Cee-sodefrin04-2 | LC861885 | 577 | 189 | 1 | 1 | ||||||||||||||||
| Cee-sodefrin05-1 | LC861886 | 577 | 189 | 1 | 1 | ||||||||||||||||
| [Gln8]sodefrin precursor | 316–625 | 102–205 | 36 | 3 | 4 | 4 | 6 | 5 | 4 | 4 | 3 | 1 | 1 | 1 | |||||||
| Cee-[Gln8]sodefrin01-1 | LC861867 | 586 | 192 | 10 | 3 | 2 | 4 | 1 | |||||||||||||
| Cee-[Gln8]sodefrin01-2 | LC861868 | 586 | 192 | 2 | 2 | ||||||||||||||||
| Cee-[Gln8]sodefrin02-1 | LC861869 | 586 | 192 | 2 | 2 | ||||||||||||||||
| Cee-[Gln8]sodefrin02-2 | LC861870 | 586 | 192 | 1 | 1 | ||||||||||||||||
| Cee-[Gln8]sodefrin03-1 | LC861871 | 586 | 192 | 5 | 1 | 4 | |||||||||||||||
| Cee-[Gln8]sodefrin04-1 | LC861872 | 316 | 102 | 1 | 1 | ||||||||||||||||
| Cee-[Gln8]sodefrin05-1 | LC861873 | 586 | 192 | 1 | 1 | ||||||||||||||||
| Cee-[Gln8]sodefrin05-2 | LC861874 | 586 | 192 | 1 | 1 | ||||||||||||||||
| Cee-[Gln8]sodefrin06-1 | LC861875 | 586 | 192 | 3 | 2 | 1 | |||||||||||||||
| Cee-[Gln8]sodefrin06-2 | LC861876 | 586 | 192 | 2 | 2 | ||||||||||||||||
| Cee-[Gln8]sodefrin07-1 | LC861877 | 586 | 192 | 1 | 1 | ||||||||||||||||
| Cee-[Gln8]sodefrin08-1 | LC861878 | 577 | 189 | 1 | 1 | 3 | 1 | 1 | |||||||||||||
| Cep-[Gln8]sodefrin01-1 | LC861897 | 586 | 192 | 5 | |||||||||||||||||
| Cep-[Gln8]sodefrin02-1 | LC861898 | 625 | 205 | 1 | 1 | ||||||||||||||||
| Silefrin precursor | 529–586 | 173–192 | 8 | 4 | 2 | 1 | 1 | ||||||||||||||
| Cep-silefrin01-1 | LC861899 | 586 | 192 | 4 | 4 | ||||||||||||||||
| Cep-silefrin02-1 | LC861900 | 586 | 192 | 1 | 1 | ||||||||||||||||
| Cep-silefrin03-1 | LC861901 | 586 | 192 | 1 | 1 | ||||||||||||||||
| Cep-silefrin04-1 | LC861902 | 529 | 192 | 1 | 1 | ||||||||||||||||
| Cep-silefrin05-1 | LC861903 | 586 | 173 | 1 | 1 | ||||||||||||||||
| Sodefrin precursor-like factor (SPF) | SPF-ß subgroup | 374–539 | 124–179 | 73 | 2 | 2 | 3 | 3 | 2 | 3 | 4 | 4 | 4 | 6 | 7 | 7 | 5 | 7 | 7 | 7 | |
| Cee-SPF-ß01-1 | LC861887 | 506 | 168 | 6 | 3 | 3 | |||||||||||||||
| Cee-SPF-ß02-1 | LC861888 | 491 | 163 | 1 | 1 | ||||||||||||||||
| Cee-SPF-ß03-1 | LC861889 | 506 | 168 | 4 | |||||||||||||||||
| Cee-SPF-ß04-1 | LC861890 | 491 | 163 | 1 | 1 | 3 | |||||||||||||||
| Cee-SPF-ß05-1 | LC861891 | 506 | 168 | 1 | 1 | 1 | |||||||||||||||
| Cee-SPF-ß06-1 | LC861892 | 506 | 168 | 4 | 1 | 3 | |||||||||||||||
| Cee-SPF-ß07-1 | LC861893 | 506 | 168 | 3 | 1 | 2 | |||||||||||||||
| Cee-SPF-ß08-1 | LC861894 | 506 | 168 | 1 | 1 | ||||||||||||||||
| Cee-SPF-ß09-1 | LC861895 | 506 | 168 | 1 | 1 | ||||||||||||||||
| Cee-SPF-ß10-1 | LC861896 | 539 | 179 | 1 | 1 | ||||||||||||||||
| Cep-SPF-ß01-1 | LC861904 | 506 | 168 | 27 | 3 | 1 | 5 | 6 | 4 | 3 | 5 | ||||||||||
| Cep-SPF-ß02-1 | LC861905 | 389 | 129 | 1 | 1 | ||||||||||||||||
| Cep-SPF-ß03-1 | LC861906 | 506 | 168 | 1 | 1 | ||||||||||||||||
| Cep-SPF-ß04-1 | LC861907 | 506 | 168 | 1 | 1 | ||||||||||||||||
| Cep-SPF-ß05-1 | LC861908 | 506 | 168 | 1 | 1 | ||||||||||||||||
| Cep-SPF-ß06-1 | LC861909 | 506 | 168 | 2 | 2 | ||||||||||||||||
| Cep-SPF-ß07-1 | LC861910 | 506 | 168 | 1 | 1 | ||||||||||||||||
| Cep-SPF-ß08-1 | LC861911 | 389 | 129 | 1 | 1 | ||||||||||||||||
| Cep-SPF-ß09-1 | LC861912 | 539 | 179 | 6 | 3 | 1 | 2 | ||||||||||||||
| Cep-SPF-ß09-2 | LC861913 | 539 | 179 | 1 | 1 | ||||||||||||||||
| Cep-SPF-ß09-3 | LC861914 | 539 | 179 | 1 | 1 | ||||||||||||||||
| Cep-SPF-ß10-1 | LC861915 | 539 | 179 | 4 | 1 | 1 | 1 | 1 | |||||||||||||
| Cep-SPF-ß10-2 | LC861916 | 539 | 179 | 3 | 3 | ||||||||||||||||
| Group | Abbreviated Transcript Name | Accession | mRNA Length (Base) | Number of Amino Acid Residues | Total Number of Clones | Number of Clones per Individual | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cynops pyrrhogaster | |||||||||||||
| A | B | C | D | E | F | G | H | ||||||
| Sodefrin and Sodefrin-variant Precursor (S-SVP) | S-SVP subgroup | 460–577 | 150–189 | 52 | 4 | 8 | 5 | 7 | 8 | 7 | 7 | 6 | |
| Sodefrin precursor | 460–577 | 150–189 | 28 | 4 | 5 | 1 | 3 | 5 | 3 | 4 | 3 | ||
| Cp-sodefrin01-1 | LC861931 | 577 | 189 | 6 | 1 | 3 | 1 | 1 | |||||
| Cp-sodefrin01-2 | LC861932 | 577 | 189 | 1 | 1 | ||||||||
| Cp-sodefrin02-1 | LC861933 | 577 | 189 | 1 | 1 | ||||||||
| Cp-sodefrin03-1 | LC861934 | 460 | 150 | 1 | 1 | ||||||||
| Cp-sodefrin04-1 | LC861935 | 577 | 189 | 1 | 1 | ||||||||
| Cp-sodefrin05-1 | LC861936 | 577 | 189 | 1 | 1 | ||||||||
| Cp-sodefrin06-1 | LC861937 | 529 | 173 | 1 | 1 | ||||||||
| Cp-sodefrin07-1 | LC861938 | 529 | 173 | 1 | 1 | ||||||||
| Cp-sodefrin08-1 | LC861939 | 577 | 189 | 1 | 1 | ||||||||
| Cp-sodefrin09-1 | LC861940 | 577 | 189 | 1 | 1 | ||||||||
| Cp-sodefrin10-1 | LC861941 | 529 | 173 | 4 | 2 | 2 | |||||||
| Cp-sodefrin10-2 | LC861942 | 529 | 173 | 2 | 1 | 1 | |||||||
| Cp-sodefrin11-1 | LC861943 | 514 | 168 | 1 | 1 | ||||||||
| Cp-sodefrin12-1 | LC861944 | 577 | 189 | 1 | 1 | ||||||||
| Cp-sodefrin13-1 | LC861945 | 529 | 173 | 1 | 1 | ||||||||
| Cp-sodefrin14-1 | LC861946 | 529 | 173 | 1 | 1 | ||||||||
| Cp-sodefrin15-1 | LC861947 | 553 | 181 | 1 | 1 | ||||||||
| Cp-sodefrin16-1 | LC861948 | 529 | 173 | 1 | 1 | ||||||||
| Cp-sodefrin17-1 | LC861949 | 529 | 173 | 1 | 1 | ||||||||
| [Asn10]sodefrin precursor | 577 | 189 | 24 | 3 | 4 | 4 | 3 | 4 | 3 | 3 | |||
| Cp-[Asn10]sodefrin01-1 | LC861917 | 577 | 189 | 5 | 3 | 1 | 1 | ||||||
| Cp-[Asn10]sodefrin01-2 | LC861918 | 577 | 189 | 1 | 1 | ||||||||
| Cp-[Asn10]sodefrin02-1 | LC861919 | 577 | 189 | 1 | 1 | ||||||||
| Cp-[Asn10]sodefrin03-1 | LC861920 | 577 | 189 | 1 | 1 | ||||||||
| Cp-[Asn10]sodefrin04-1 | LC861921 | 577 | 189 | 1 | 1 | ||||||||
| Cp-[Asn10]sodefrin05-1 | LC861922 | 577 | 189 | 1 | 1 | ||||||||
| Cp-[Asn10]sodefrin05-2 | LC861923 | 577 | 189 | 1 | 1 | ||||||||
| Cp-[Asn10]sodefrin06-1 | LC861924 | 577 | 189 | 1 | 1 | ||||||||
| Cp-[Asn10]sodefrin07-1 | LC861925 | 577 | 189 | 4 | 2 | 2 | |||||||
| Cp-[Asn10]sodefrin08-1 | LC861926 | 577 | 189 | 1 | 1 | ||||||||
| Cp-[Asn10]sodefrin09-1 | LC861927 | 577 | 189 | 3 | 1 | 2 | |||||||
| Cp-[Asn10]sodefrin09-2 | LC861928 | 577 | 189 | 2 | 2 | ||||||||
| Cp-[Asn10]sodefrin10-1 | LC861929 | 577 | 189 | 1 | 1 | ||||||||
| Cp-[Asn10]sodefrin11-1 | LC861930 | 577 | 189 | 1 | 1 | ||||||||
| Sodefrin precursor-like factor (SPF) | SPF-ß subgroup | 422–539 | 140–179 | 12 | 4 | 3 | 1 | 1 | 1 | 2 | |||
| Cp-SPF-ß01-1 | LC861950 | 539 | 179 | 2 | 1 | 1 | |||||||
| Cp-SPF-ß02-1 | LC861951 | 539 | 179 | 1 | 1 | ||||||||
| Cp-SPF-ß03-1 | LC861952 | 539 | 179 | 1 | 1 | ||||||||
| Cp-SPF-ß04-1 | LC861953 | 539 | 179 | 1 | 1 | ||||||||
| Cp-SPF-ß05-1 | LC861954 | 422 | 140 | 1 | 1 | ||||||||
| Cp-SPF-ß06-1 | LC861955 | 539 | 179 | 1 | 1 | ||||||||
| Cp-SPF-ß07-1 | LC861956 | 506 | 168 | 1 | 1 | ||||||||
| Cp-SPF-ß08-1 | LC861957 | 506 | 168 | 1 | 1 | ||||||||
| Cp-SPF-ß09-1 | LC861958 | 506 | 168 | 1 | 1 | ||||||||
| Cp-SPF-ß10-1 | LC861959 | 506 | 168 | 1 | 1 | ||||||||
| Cp-SPF-ß11-1 | LC861960 | 506 | 168 | 1 | 1 | ||||||||

References
- Arnold, S.J.; Reagan, N.L.; Verrell, P.A. Reproductive isolation and speciation in plethodontid salamanders. Herpetologica 1993, 49, 216–228. [Google Scholar]
- Kikuyama, S.; Toyoda, F.; Ohmiya, Y.; Matsuda, K.; Tanaka, S.; Hayashi, H. Sodefrin: A female-attracting peptide pheromone in newt cloacal glands. Science 1995, 267, 1643–1645. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Kawai, Y.; Hayashi, T.; Ohe, Y.; Hayashi, H.; Toyoda, F.; Kawahara, G.; Iwata, T.; Kikuyama, S. Silefrin, a sodefrin-like pheromone in the abdominal gland of the sword-tailed newt, Cynops ensicauda. FEBS Lett. 2000, 472, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Nakada, T.; Toyoda, F.; Iwata, T.; Yamamoto, K.; Conlon, J.M.; Kato, T.; Kikuyama, S. Isolation, characterization and bioactivity of a region-specific pheromone, [Val8]sodefrin from the newt Cynops pyrrhogaster. Peptides 2007, 28, 774–780. [Google Scholar] [CrossRef]
- Nakada, T.; Toyoda, F.; Matsuda, K.; Nakakura, T.; Hasunuma, I.; Yamamoto, K.; Onoue, S.; Yokosuka, M.; Kikuyama, S. Imorin: A sexual attractiveness pheromone in female red-bellied newts (Cynops pyrrhogaster). Sci. Rep. 2017, 7, 41334. [Google Scholar] [CrossRef]
- Rollmann, S.M.; Houck, L.D.; Feldhoff, R.C. Proteinaceous pheromone affecting female receptivity in a terrestrial salamander. Science 1999, 285, 1907–1909. [Google Scholar] [CrossRef]
- Wabnitz, P.A.; Bowie, J.H.; Tyler, M.J.; Wallace, J.C.; Smith, B.P. Aquatic sex pheromone from a male tree frog. Nature 1999, 401, 444–445. [Google Scholar] [CrossRef]
- Toyoda, F.; Kikuyama, S. Hormonal influence on the olfactory response to a female-attracting pheromone, sodefrin, in the newt, Cynops pyrrhogaster. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2000, 126, 239–245. [Google Scholar] [CrossRef]
- Iwata, T.; Nakada, T.; Toyoda, F.; Yada, T.; Shioda, S.; Kikuyama, S. Responsiveness of vomeronasal cells to a newt peptide pheromone, sodefrin as monitored by changes of intracellular calcium concentrations. Peptides 2013, 45, 15–21. [Google Scholar] [CrossRef]
- Nakada, T.; Hagino-Yamagishi, K.; Nakanishi, K.; Yokosuka, M.; Saito, T.R.; Toyoda, F.; Hasunuma, I.; Nakakura, T.; Kikuyama, S. Expression of G proteins in the olfactory receptor neurons of the newt Cynops pyrrhogaster: Their unique projection into the olfactory bulbs. J. Comp. Neurol. 2014, 522, 3501–3519. [Google Scholar] [CrossRef]
- Iwata, T.; Umezawa, K.; Toyoda, F.; Takahashi, N.; Matsukawa, H.; Yamamoto, K.; Miura, S.; Hayashi, H.; Kikuyama, S. Molecular cloning of newt sex pheromone precursor cDNAs: Evidence for the existence of species-specific forms of pheromones. FEBS Lett. 1999, 457, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Nakada, T.; Ishizuka, Y.; Iwata, T.; Toyoda, F.; Kato, T.; Conlon, J.M.; Kikuyama, S. Evidence for processing enzymes in the abdominal gland of the newt, Cynops pyrrhogaster, that generate sodefrin from its biosynthetic precursor. Zool. Sci. 2007, 24, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.A.; Hollis, D.M.; Watts, R.A.; Houck, L.D.; McCall, M.A.; Gregg, R.G.; Feldhoff, P.W.; Feldhoff, R.C.; Arnold, S.J. Plethodontid modulating factor, a hypervariable salamander courtship pheromone in the three finger protein superfamily. FEBS J. 2007, 274, 2300–2310. [Google Scholar] [CrossRef] [PubMed]
- Houck, L.D.; Watts, R.A.; Mead, L.M.; Palmer, C.A.; Arnold, S.J.; Feldhoff, P.W.; Feldhoff, R.C. A candidate vertebrate pheromone, SPF, increases female receptivity in a salamander. In Chemical Signals in Vertebrates 11; Springer Science+Business Media: New York, NY, USA, 2008; pp. 213–221. [Google Scholar]
- Osikowski, A.; Babik, W.; Grzmil, P.; Szymura, J.M. In the Abdominal Glands of the Smooth Newt (Lissotriton vulgaris) and Montandon’s Newt (L. montandoni) (Salamandridae). Zool. Sci. 2008, 25, 587–592. [Google Scholar] [CrossRef]
- Kiemnec-Tyburczy, K.M.; Watts, R.A.; Gregg, R.G.; von Borstel, D.; Arnold, S.J. Evolutionary shifts in courtship pheromone composition revealed by EST analysis of plethodontid salamander mental glands. Gene 2009, 432, 75–81. [Google Scholar] [CrossRef]
- Wilburn, D.B.; Bowen, K.E.; Doty, K.A.; Arumugam, S.; Lane, A.N.; Feldhoff, P.W.; Feldhoff, R.C. Structural insights into the evolution of a sexy protein: Novel topology and restricted backbone flexibility in a hypervariable pheromone from the red-legged salamander, Plethodon shermani. PLoS ONE 2014, 9, e96975. [Google Scholar] [CrossRef]
- Janssenswillen, S.; Willaert, B.; Treer, D.; Vandebergh, W.; Bossuyt, F.; Van Bocxlaer, I. High pheromone diversity in the male cheek gland of the red-spotted newt Notophthalmus viridescens (Salamandridae). BMC Evol. Biol. 2015, 15, 1–8. [Google Scholar] [CrossRef]
- Hall, K.W.; Eisthen, H.L.; Williams, B.L. Proteinaceous pheromone homologs identified from the cloacal gland transcriptome of a male axolotl, Ambystoma mexicanum. PLoS ONE 2016, 11, e0146851. [Google Scholar] [CrossRef]
- Bossuyt, F.; Schulte, L.M.; Maex, M.; Janssenswillen, S.; Novikova, P.Y.; Biju, S.; Van de Peer, Y.; Matthijs, S.; Roelants, K.; Martel, A. Multiple independent recruitment of sodefrin precursor-like factors in anuran sexually dimorphic glands. Mol. Biol. Evol. 2019, 36, 1921–1930. [Google Scholar] [CrossRef]
- Van Bocxlaer, I.; Treer, D.; Maex, M.; Vandebergh, W.; Janssenswillen, S.; Stegen, G.; Kok, P.; Willaert, B.; Matthijs, S.; Martens, E. Side-by-side secretion of Late Palaeozoic diverged courtship pheromones in an aquatic salamander. Proc. R. Soc. B Biol. Sci. 2015, 282, 20142960. [Google Scholar] [CrossRef]
- Janssenswillen, S.; Vandebergh, W.; Treer, D.; Willaert, B.; Maex, M.; Van Bocxlaer, I.; Bossuyt, F. Origin and diversification of a salamander sex pheromone system. Mol. Biol. Evol. 2015, 32, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Van Bocxlaer, I.; Maex, M.; Treer, D.; Janssenswillen, S.; Janssens, R.; Vandebergh, W.; Proost, P.; Bossuyt, F. Beyond sodefrin: Evidence for a multi-component pheromone system in the model newt Cynops pyrrhogaster (Salamandridae). Sci. Rep. 2016, 6, 21880. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.; Conlon, J.M.; Nakada, T.; Toyoda, F.; Yamamoto, K.; Kikuyama, S. Processing of multiple forms of preprosodefrin in the abdominal gland of the red-bellied newt Cynops pyrrhogaster: Regional and individual differences in preprosodefrin gene expression. Peptides 2004, 25, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Inger, R.F. Preliminary survey of the amphibians of the Riukiu islands. Fieldiana Zool. 1947, 32, 297–352. [Google Scholar]
- Tominaga, A.; Ota, H.; Matsui, M. Phylogeny and phylogeography of the sword-tailed newt, Cynops ensicauda (Amphibia: Caudata), as revealed by nucleotide sequences of mitochondrial DNA. Mol. Phylogenet. Evol. 2010, 54, 910–921. [Google Scholar] [CrossRef]
- Kikuyama, S.; Toyoda, F.; Yamamoto, K.; Iwata, T.; Nakada, T.; Hasunuma, I. Sodefrin and related pheromones. In Handbook of Biologically Active Peptides; Kastin, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 384–390. [Google Scholar]
- Toyoda, F.; Tanaka, S.; Matsuda, K.; Kikuyama, S. Hormonal control of response to and secretion of sex attractants in Japanese newts. Physiol. Behav. 1994, 55, 569–576. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Tominaga, A.; Matsui, M.; Yoshikawa, N.; Nishikawa, K.; Hayashi, T.; Misawa, Y.; Tanabe, S.; Ota, H. Phylogeny and historical demography of Cynops pyrrhogaster (Amphibia: Urodela): Taxonomic relationships and distributional changes associated with climatic oscillations. Mol. Phylogenet. Evol. 2013, 66, 654–667. [Google Scholar] [CrossRef]
- Thom, M.D.; Stockley, P.; Jury, F.; Ollier, W.E.; Beynon, R.J.; Hurst, J.L. The direct assessment of genetic heterozygosity through scent in the mouse. Curr. Biol. 2008, 18, 619–623. [Google Scholar] [CrossRef]
- Abe, T.; Touhara, K. Structure and function of a peptide pheromone family that stimulate the vomeronasal sensory system in mice. Biochem. Soc. Trans. 2014, 42, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Gong, Y.; Wang, B.; Yu, H.; Huang, S.; Liao, X.; Jiang, J.; Ran, J.; Xie, F. Love Hug-Functional Validation of Nuptial Pad-Secreted Pheromone in Anurans. Animals 2024, 14, 1550. [Google Scholar] [CrossRef] [PubMed]
- Watts, R.A.; Palmer, C.A.; Feldhoff, R.C.; Feldhoff, P.W.; Houck, L.D.; Jones, A.G.; Pfrender, M.E.; Rollmann, S.M.; Arnold, S.J. Stabilizing selection on behavior and morphology masks positive selection on the signal in a salamander pheromone signaling complex. Mol. Biol. Evol. 2004, 21, 1032–1041. [Google Scholar] [CrossRef] [PubMed][Green Version]


| Sodefrin or Sodefrin Variant Sequences to Be Contained | Amino Acid Sequences of Sodefrin or Sodefrin Variants | Number of Amino Acid Residues | Number of Isoforms | Total Number of Clones | Number of Clones per Individual ** | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | ||||||
| Cynops ensicauda ensicauda | Presence | SIPSKDALLK (sodefrin) | 189 | 5 | 11 | 6 | 3 | 1 | 1 | ||||
| SIPSKDAQLK ([Gln8]sodefrin) | 102–192 | 8 | 30 | 3 | 4 | 4 | 6 | 5 | 4 | 4 | |||
| Absence * | 163–179 | 10 | 23 | 2 | 2 | 3 | 3 | 2 | 3 | 4 | 4 | ||
| Cynops ensicauda popei | Presence | SIPSKDAQLK ([Gln8]sodefrin) | 192–205 | 2 | 6 | 3 | 1 | 1 | 1 | ||||
| SILSKDAQLK (silefrin) | 173–192 | 5 | 8 | 4 | 2 | 1 | 1 | ||||||
| Absence | 129–179 | 10 | 50 | 4 | 6 | 7 | 7 | 5 | 7 | 7 | 7 | ||
| Cynops pyrrhogaster | Presence | SIPSKDALLK (sodefrin) | 150–189 | 17 | 28 | 4 | 5 | 1 | 3 | 5 | 3 | 4 | 3 |
| SIPSKDALLN ([Asn10]sodefrin) | 173–189 | 11 | 24 | 3 | 4 | 4 | 3 | 4 | 3 | 3 | |||
| Absence | 140–179 | 11 | 12 | 4 | 3 | 1 | 1 | 1 | 2 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakada, T.; Toyoda, F.; Tominaga, A.; Mochida, K.; Yokosuka, M.; Kikuyama, S. Genetic Diversity of Sodefrin-Variant Pheromones and Pheromone Responsiveness in Subspecies of the Japanese Sword-Tail Newt Cynops ensicauda. Animals 2025, 15, 947. https://doi.org/10.3390/ani15070947
Nakada T, Toyoda F, Tominaga A, Mochida K, Yokosuka M, Kikuyama S. Genetic Diversity of Sodefrin-Variant Pheromones and Pheromone Responsiveness in Subspecies of the Japanese Sword-Tail Newt Cynops ensicauda. Animals. 2025; 15(7):947. https://doi.org/10.3390/ani15070947
Chicago/Turabian StyleNakada, Tomoaki, Fumiyo Toyoda, Atsushi Tominaga, Koji Mochida, Makoto Yokosuka, and Sakae Kikuyama. 2025. "Genetic Diversity of Sodefrin-Variant Pheromones and Pheromone Responsiveness in Subspecies of the Japanese Sword-Tail Newt Cynops ensicauda" Animals 15, no. 7: 947. https://doi.org/10.3390/ani15070947
APA StyleNakada, T., Toyoda, F., Tominaga, A., Mochida, K., Yokosuka, M., & Kikuyama, S. (2025). Genetic Diversity of Sodefrin-Variant Pheromones and Pheromone Responsiveness in Subspecies of the Japanese Sword-Tail Newt Cynops ensicauda. Animals, 15(7), 947. https://doi.org/10.3390/ani15070947





