Research Status and Prospect of Amphibian Symbiotic Microbiota
Simple Summary
Abstract
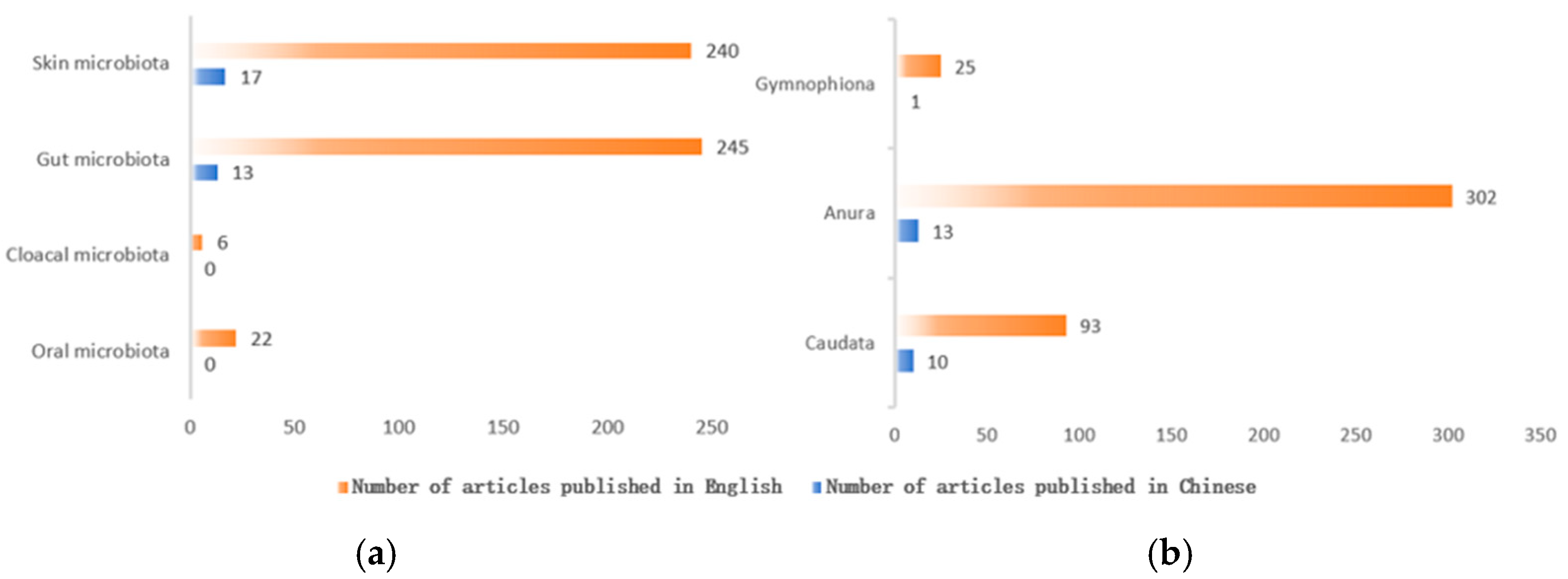
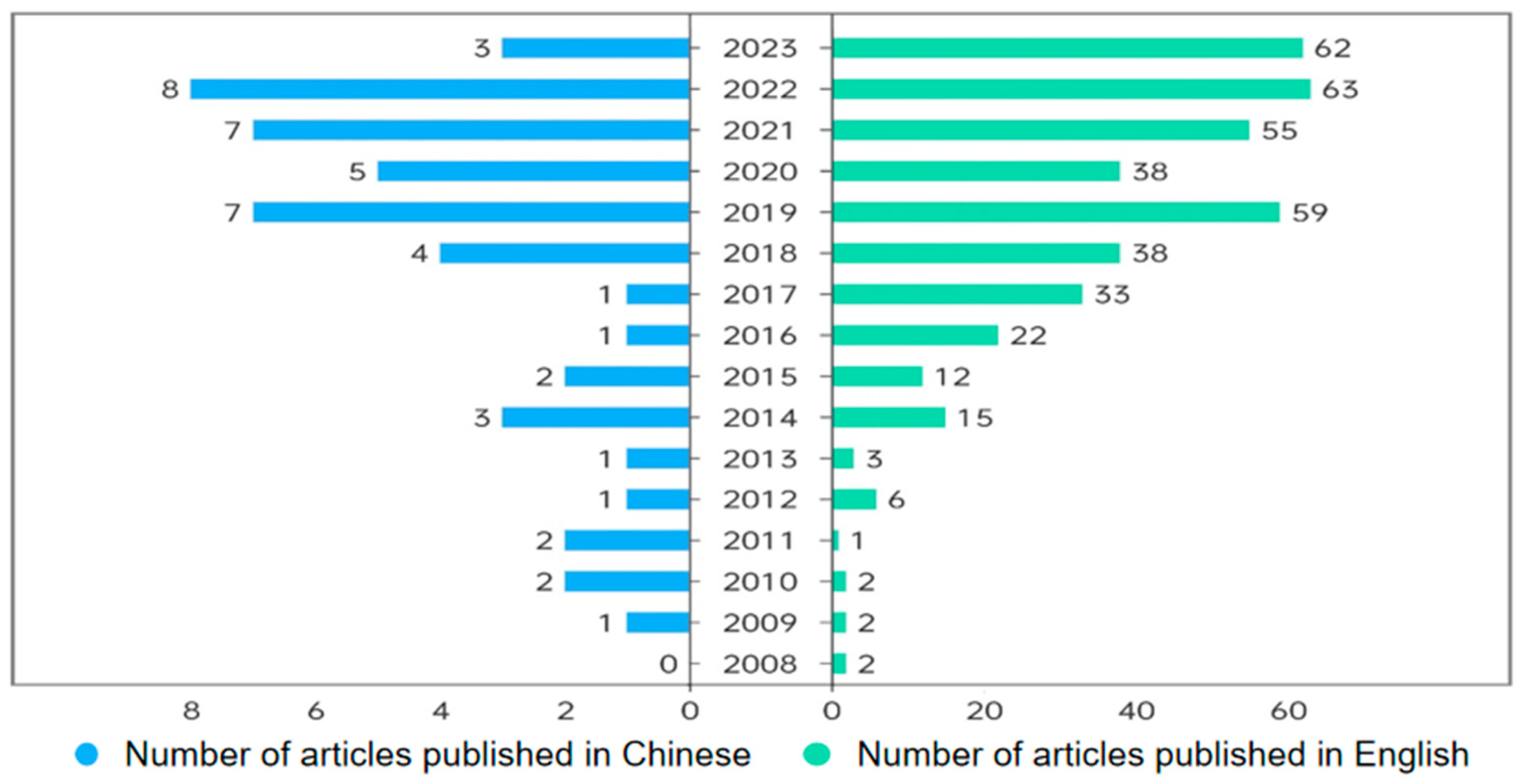
1. Introduction
2. The Importance of Amphibian Symbiotic Microbiota
2.1. The Importance of Amphibian Gut Microbiota
2.2. The Importance of Amphibian Skin Microbiota
3. Research Progress of Microbiological Classification Technology
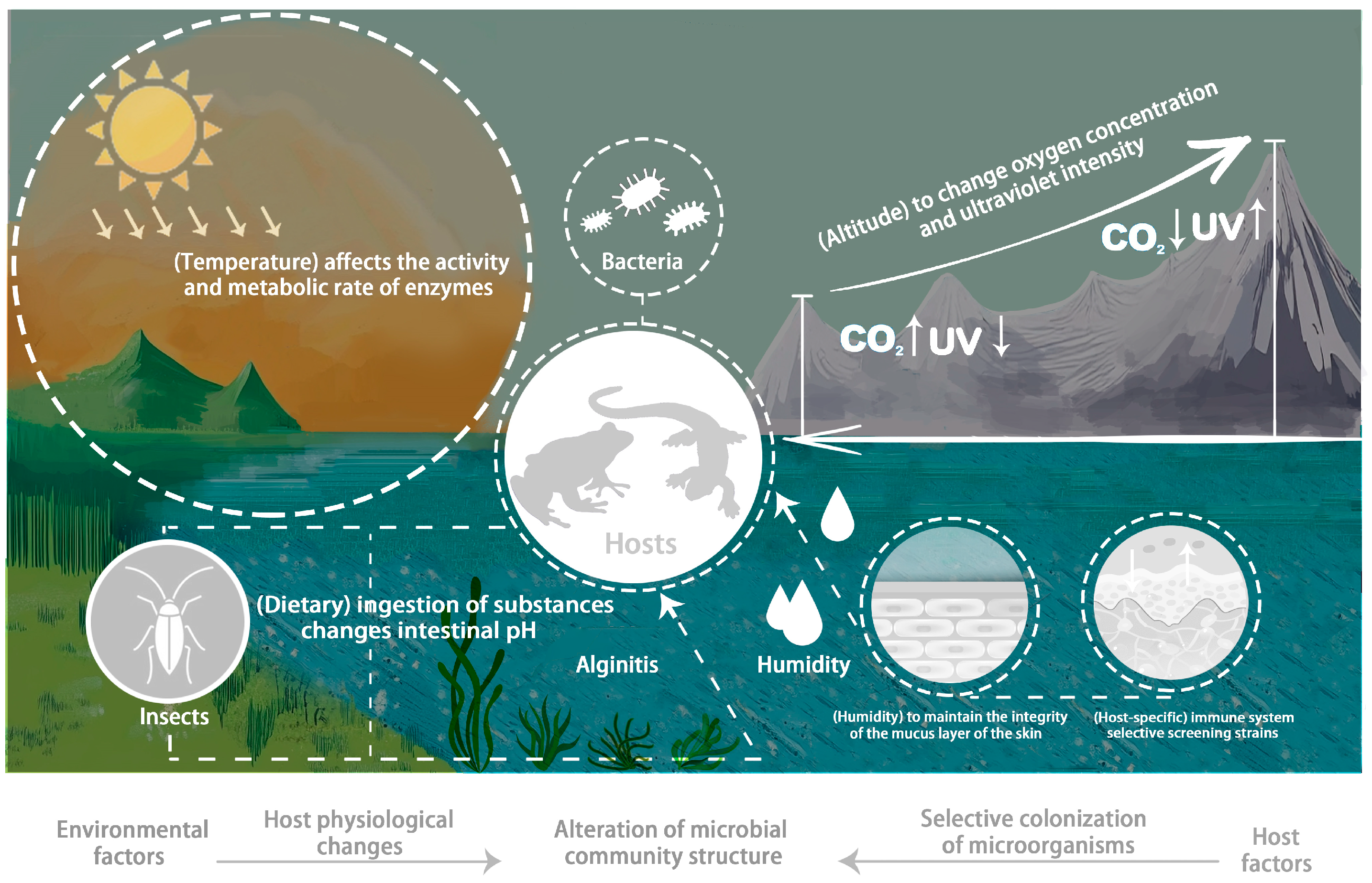
4. Factors Affecting Amphibian Symbiotic Microbiota
4.1. Environmental Factors
4.1.1. The Impact of Temperature on Amphibian Symbiotic Microbiota
The Impact of Temperature on Amphibian Gut Microbiota
The Impact of Temperature on Amphibian Skin Microbiota
4.1.2. The Impact of Altitude on Amphibian Symbiotic Microbiota
The Impact of Altitude on Amphibian Gut Microbiota
The Impact of Altitude on Amphibian Skin Microbiota
4.1.3. The Impact of Host Diet Composition on Amphibian Symbiotic Microbiota
The Impact of Host Diet Composition on Amphibian Gut Microbiota
The Impact of Host Diet Composition on Amphibian Skin Microbiota
4.1.4. The Impact of Humidity on Amphibian Symbiotic Microbiota
4.2. Host Factors
4.2.1. The Impact of Host Specificity on Amphibian Symbiotic Microbiota
The Impact of Host Specificity on Amphibian Gut Microbiota
The Impact of Host Specificity on Amphibian Skin Microbiota
4.2.2. The Impact of Host Development on Amphibian Symbiotic Microbiota
The Impact of Host Development on Amphibian Gut Microbiota
The Impact of Host Development on Amphibian Skin Microbiota
4.3. The Impact of Other Factors on Amphibian Symbiotic Microbiota
5. Outlook for Future Research
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tochimoto, T.; Shimizu, K. Ecological studies on the Japanese giant salamander, Andrias japonicus, and marking of animals for recognition. Hoshizaki Gurin Zaidan Kenkyu Hokoku 2004, 7, 169–178. [Google Scholar]
- Liedtke, H.C.; Wiens, J.J.; Gomez-Mestre, I. The evolution of reproductive modes and life cycles in amphibians. Nat. Commun. 2022, 13, 7039. [Google Scholar] [PubMed]
- Fei, L.; Hu, S.Q.; Ye, C.Y.; Huang, Y.Z. Fauna Sinica•Amphibian; Science Press: Beijing, China, 2006; Volume 1. [Google Scholar]
- Luedtke, J.A.; Chanson, J.; Neam, K.; Hobin, L.; Maciel, A.O.; Catenazzi, A.; Borzee, A.; Hamidy, A.; Aowphol, A.; Jean, A.; et al. Ongoing declines for the world’s amphibians in the face of emerging threats. Nature 2023, 622, 308–314. [Google Scholar]
- Wake, D.B.; Vredenburg, V.T. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc. Natl. Acad. Sci. USA 2008, 105, 11466–11473. [Google Scholar] [CrossRef]
- Xu, W.; Wu, Y.-H.; Zhou, W.-W.; Chen, H.-M.; Zhang, B.-L.; Chen, J.-M.; Xu, W.; Rao, D.-Q.; Zhao, H.; Yan, F.; et al. Hidden hotspots of amphibian biodiversity in China. Proc. Natl. Acad. Sci. USA 2024, 121, e2320674121. [Google Scholar] [CrossRef] [PubMed]
- Browne, R.K.; Kaurova, S.A.; Vasudevan, K.; McGinnity, D.; Venu, G.; Gonzalez, M.; Uteshev, V.K.; Marcec-Greaves, R. Reproduction technologies for the sustainable management of Caudata (salamander) and Gymnophiona (caecilian) biodiversity. Reprod. Fertil. Dev. 2022, 34, 479–497. [Google Scholar] [PubMed]
- Alexiev, A.; Chen, M.Y.; McKenzie, V.J. Identifying fungal-host associations in an amphibian host system. PLoS ONE 2021, 16, e0256328. [Google Scholar]
- Roager, L.; Kempen, P.J.; Bentzon-Tilia, M.; Sonnenschein, E.C.; Gram, L. Impact of host species on assembly, composition, and functional profiles of phycosphere microbiomes. Msystems 2024, 9, e00583-24. [Google Scholar]
- Youngblut, N.D.; Reischer, G.H.; Walters, W.; Schuster, N.; Walzer, C.; Stalder, G.; Ley, R.E.; Farnleitner, A.H. Host diet and evolutionary history explain different aspects of gut microbiome diversity among vertebrate clades. Nat. Commun. 2019, 10, 2200. [Google Scholar]
- Wei, F.W.; Wu, Q.; Hu, Y.B.; Huang, G.P.; Nie, Y.G.; Yan, L. Conservation metagenomics: A new branch of conservation biology. Sci. China-Life Sci. 2019, 62, 168–178. [Google Scholar] [CrossRef]
- Wu, Y.; Zhuang, J.; Song, Y.; Gao, X.; Chu, J.; Han, S. Advances in single-cell sequencing technology in microbiome research. Genes Dis. 2024, 11, 101129. [Google Scholar] [PubMed]
- Bletz, M.C.; Perl, R.G.B.; Bobowski, B.T.C.; Japke, L.M.; Tebbe, C.C.; Dohrmann, A.B.; Bhuju, S.; Geffers, R.; Jarek, M.; Vences, M. Amphibian skin microbiota exhibits temporal variation in community structure but stability of predicted Bd-inhibitory function. ISME J. 2017, 11, 1521–1534. [Google Scholar] [PubMed]
- Harris, R.N.; Brucker, R.M.; Walke, J.B.; Becker, M.H.; Schwantes, C.R.; Flaherty, D.C.; Lam, B.A.; Woodhams, D.C.; Briggs, C.J.; Vredenburg, V.T.; et al. Skin microbes on frogs prevent morbidity and mortality caused by a lethal skin fungus. ISME J. 2009, 3, 818–824. [Google Scholar]
- Zhu, W.; Chang, L.; Shi, S.; Lu, N.; Du, S.; Li, J.; Jiang, J.; Wang, B. Gut microbiota reflect adaptation of cave-dwelling tadpoles to resource scarcity. ISME J. 2024, 18, wrad009. [Google Scholar]
- Brunetti, A.E.; Lyra, M.L.; Melo, W.G.P.; Andrade, L.E.; Palacios-Rodríguez, P.; Prado, B.M.; Haddad, C.F.B.; Pupo, M.T.; Lopes, N.P. Symbiotic skin bacteria as a source for sex-specific scents in frogs. Proc. Natl. Acad. Sci. USA 2019, 116, 2124–2129. [Google Scholar]
- Zhu, W.; Zhao, C.; Feng, J.; Chang, J.; Zhu, W.; Chang, L.; Liu, J.; Xie, F.; Li, C.; Jiang, J.; et al. Effects of Habitat River Microbiome on the Symbiotic Microbiota and Multi-Organ Gene Expression of Captive-Bred Chinese Giant Salamander. Front. Microbiol. 2022, 13, 884880. [Google Scholar]
- Zhu, B.; Shao, C.; Xu, W.J.; Dai, J.H.; Fu, G.H.; Hu, Y. Effects of Thyroid Powder on Tadpole (Lithobates catesbeiana) Metamorphosis and Growth: The Role of Lipid Metabolism and Gut Microbiota. Animals 2024, 14, 208. [Google Scholar] [CrossRef]
- Tong, Q.; Hu, Z.F.; Du, X.P.; Bie, J.; Wang, H.B. Effects of Seasonal Hibernation on the Similarities Between the Skin Microbiota and Gut Microbiota of an Amphibian (Rana dybowskii). Microb. Ecol. 2020, 79, 898–909. [Google Scholar]
- Dallas, J.W.; Meshaka, W.E., Jr.; Zeglin, L.; Warne, R.W. Taxonomy, not locality, influences the cloacal microbiota of two nearctic colubrids: A preliminary analysis. Mol. Biol. Rep. 2021, 48, 6435–6442. [Google Scholar]
- McFall-Ngai, M.; Hadfield, M.G.; Bosch, T.C.G.; Carey, H.V.; Domazet-Loso, T.; Douglas, A.E.; Dubilier, N.; Eberl, G.; Fukami, T.; Gilbert, S.F.; et al. Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl. Acad. Sci. USA 2013, 110, 3229–3236. [Google Scholar] [PubMed]
- Vos, W.M.D.; Tilg, H.; Hul, M.V.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Lize, A.; McKay, R.; Lewis, Z. Gut microbiota and kin recognition. Trends Ecol. Evol. 2013, 28, 325–326. [Google Scholar] [CrossRef]
- Zhu, W.; Chang, L.; Zhang, M.; Chen, Q.; Sui, L.; Shen, C.; Jiang, J. Microbial diversity in mountain-dwelling amphibians: The combined effects of host and climatic factors. iScience 2024, 27, 109907. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Hassan, S.A.; Ismail, I.H.; Chong, S.Y.; Ali, R.A.R.; Nordin, S.A.; Lee, W.S.; Majid, N.A. Gut microbiota in early life and its influence on health and disease: A position paper by the Malaysian Working Group on Gastrointestinal Health. J. Paediatr. Child Health 2017, 53, 1152–1158. [Google Scholar] [CrossRef]
- Anand, S.; Mande, S.S. Host-microbiome interactions: Gut-Liver axis and its connection with other organs. Npj Biofilms Microbiomes 2022, 8, 89. [Google Scholar] [CrossRef]
- Austin, R.M. Cutaneous microbial flora and antibiosis in Plethodon ventralis -: Inferences for parental care in the Plethodontidae. In Proceedings of the 4th Conference on the Biology of Plethodontid Salamanders, Highlands, NC, USA, 12–14 June 1998; pp. 451–462. [Google Scholar]
- Jimenez, R.R.; Sommer, S. The amphibian microbiome: Natural range of variation, pathogenic dysbiosis, and role in conservation. Biodivers. Conserv. 2017, 26, 763–786. [Google Scholar] [CrossRef]
- Varki, A. Biological Roles of Oligosaccharides—All of the Theories Are Correct. Glycobiology 1993, 3, 97–130. [Google Scholar] [CrossRef]
- Woodhams, D.C.; Bletz, M.; Kueneman, J.; McKenzie, V. Managing Amphibian Disease with Skin Microbiota. Trends Microbiol. 2016, 24, 161–164. [Google Scholar] [CrossRef]
- Ross, A.A.; Hoffmann, A.R.; Neufeld, J.D. The skin microbiome of vertebrates. Microbiome 2019, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Woodhams, D.C.; Geiger, C.C.; Reinert, L.K.; Rollins-Smith, L.A.; Lam, B.; Harris, R.N.; Briggs, C.J.; Vredenburg, V.T.; Voyles, J. Treatment of amphibians infected with chytrid fungus: Learning from failed trials with itraconazole, antimicrobial peptides, bacteria, and heat therapy. Dis. Aquat. Org. 2012, 98, 11–25. [Google Scholar] [CrossRef]
- Bartos, O.; Chmel, M.; Swierczkova, I. The overlooked evolutionary dynamics of 16S rRNA revises its role as the “gold standard” for bacterial species identification. Sci. Rep. 2024, 14, 9067. [Google Scholar]
- Li, M.-N.; Han, Q.; Wang, N.; Wang, T.; You, X.-M.; Zhang, S.; Zhang, C.-C.; Shi, Y.-Q.; Qiao, P.-Z.; Man, C.-L.; et al. 16S rRNA gene sequencing for bacterial identification and infectious disease diagnosis. Biochem. Biophys. Res. Commun. 2024, 739, 150974. [Google Scholar] [CrossRef]
- Wu, Z.L. Analysis of symbiotic microbe community on the Ichthyophis bannanicus. Master’s Thesis, Guilin University of Technology, Guilin, China, 2022. (In Chinese). [Google Scholar]
- Hernandez-Gomez, O.; Hua, J. From the organismal to biosphere levels: Environmental impacts on the amphibian microbiota. Fems Microbiol. Rev. 2023, 47, fuad002. [Google Scholar] [CrossRef]
- Fontaine, S.S.; Novarro, A.J.; Kohl, K.D. Environmental temperature alters the digestive performance and gut microbiota of a terrestrial amphibian. J. Exp. Biol. 2018, 221, jeb187559. [Google Scholar] [CrossRef] [PubMed]
- Küng, D.; Bigler, L.; Davis, L.R.; Gratwicke, B.; Griffith, E.; Woodhams, D.C. Stability of Microbiota Facilitated by Host Immune Regulation: Informing Probiotic Strategies to Manage Amphibian Disease. PLoS ONE 2014, 9, e87101. [Google Scholar] [CrossRef]
- Rebollar, E.A.; Hughey, M.C.; Medina, D.; Harris, R.N.; Ibáñez, R.; Belden, L.K. Skin bacterial diversity of Panamanian frogs is associated with host susceptibility and presence of Batrachochytrium dendrobatidis. ISME J. 2016, 10, 1682–1695. [Google Scholar] [CrossRef]
- Walke, J.B.; Becker, M.H.; Loftus, S.C.; House, L.L.; Cormier, G.; Jensen, R.V.; Belden, L.K. Amphibian skin may select for rare environmental microbes. ISME J. 2014, 8, 2207–2217. [Google Scholar] [CrossRef] [PubMed]
- Loudon, A.H.; Woodhams, D.C.; Parfrey, L.W.; Archer, H.; Knight, R.; McKenzie, V.; Harris, R.N. Microbial community dynamics and effect of environmental microbial reservoirs on red-backed salamanders (Plethodon cinereus). ISME J. 2014, 8, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Varela, B.J.; Lesbarrères, D.; Ibáñez, R.; Green, D.M. Environmental and Host Effects on Skin Bacterial Community Composition in Panamanian Frogs. Front. Microbiol. 2018, 9, 298. [Google Scholar] [CrossRef]
- Chang, C.W.; Huang, B.H.; Lin, S.M.; Huang, C.L.; Liao, P.C. Changes of diet and dominant intestinal microbes in farmland frogs. Bmc Microbiol. 2016, 16, 33. [Google Scholar] [CrossRef]
- Huang, S.W.; Zhang, H.Y. The Impact of Environmental Heterogeneity and Life Stage on the Hindgut Microbiota of Holotrichia parallela Larvae (Coleoptera: Scarabaeidae). PLoS ONE 2013, 8, e57169. [Google Scholar]
- Ren, C.L. Effects of Temperature on Intestinal Microbiota, Lipid Metabolism, and Skeletal Development in Chinese Brown Frogs. Master’s Thesis, Shaanxi Normal University, Xi’an, China, 2022. [Google Scholar]
- Emerson, K.J.; Fontaine, S.S.; Kohl, K.D.; Woodley, S.K. Temperature and the microbial environment alter brain morphology in a larval amphibian. J. Exp. Biol. 2023, 226, jeb245333. [Google Scholar]
- Wiggins, P.J.; Smith, J.M.; Harris, R.N.; Minbiole, K.P.C. Gut of Red-backed Salamanders (Plethodon cinereus) May Serve as a Reservoir for an Antifungal Cutaneous Bacterium. J. Herpetol. 2011, 45, 329–332. [Google Scholar] [CrossRef]
- Antwis, R.E.; Haworth, R.L.; Engelmoer, D.J.P.; Ogilvy, V.; Fidgett, A.L.; Preziosi, R.F. Ex situ Diet Influences the Bacterial Community Associated with the Skin of Red-Eyed Tree Frogs (Agalychnis callidryas). PLoS ONE 2014, 9, e85563. [Google Scholar] [CrossRef]
- Lauer, A.; Simon, M.A.; Banning, J.L.; André, E.; Duncan, K.; Harris, R.N. Common cutaneous bacteria from the eastern red-backed salamander can inhibit pathogenic fungi. Copeia 2007, 2007, 630–640. [Google Scholar]
- Colombo, B.M.; Scalvenzi, T.; Benlamara, S.; Pollet, N. Microbiota and mucosal immunity in amphibians. Front. Immunol. 2015, 6, 111. [Google Scholar]
- Sanders, J.G.; Powell, S.; Kronauer, D.J.C.; Vasconcelos, H.L.; Frederickson, M.E.; Pierce, N.E. Stability and phylogenetic correlation in gut microbiota: Lessons from ants and apes. Mol. Ecol. 2014, 23, 1268–1283. [Google Scholar]
- Lima, L.F.O.; Weissman, M.; Reed, M.; Papudeshi, B.; Alker, A.T.; Morris, M.M.; Edwards, R.A.; de Putron, S.J.; Vaidya, N.K.; Dinsdale, E.A. Modeling of the Coral Microbiome: The Influence of Temperature and Microbial Network. Mbio 2020, 11, e02691-19. [Google Scholar] [PubMed]
- Zhao, J.; Xie, X.; Jiang, Y.; Li, J.; Fu, Q.; Qiu, Y.; Fu, X.; Yao, Z.; Dai, Z.; Qiu, Y.; et al. Effects of simulated warming on soil microbial community diversity and composition across diverse ecosystems. Sci. Total Environ. 2024, 911, 168793. [Google Scholar]
- Zhao, P.; Huang, Y.; Liu, B.; Chen, J.; Lei, Z.; Zhang, Y.; Cheng, B.; Zhou, T.; Peng, S. Effects of daytime and nighttime warming on soil microbial diversity. Geoderma 2024, 447, 116909. [Google Scholar]
- Li, J.; Rui, J.; Li, Y.; Tang, N.; Zhan, S.; Jiang, J.; Li, X. Ambient temperature alters body size and gut microbiota of Xenopus tropicalis. Sci. China-Life Sci. 2020, 63, 915–925. [Google Scholar] [PubMed]
- Huey, R.B. Physiological Consequences of Habitat Selection. Am. Nat. 1991, 137, S91–S115. [Google Scholar] [CrossRef]
- Rowley, J.J.L.; Alford, R.A. Hot bodies protect amphibians against chytrid infection in nature. Sci. Rep. 2013, 3, 1515. [Google Scholar] [CrossRef]
- Meyer, E.A.; Cramp, R.L.; Bernal, M.H.; Franklin, C.E. Changes in cutaneous microbial abundance with sloughing: Possible implications for infection and disease in amphibians. Dis. Aquat. Org. 2012, 101, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Ohmer, M.E.B.; Cramp, R.L.; White, C.R.; Franklin, C.E. Skin sloughing rate increases with chytrid fungus infection load in a susceptible amphibian. Funct. Ecol. 2015, 29, 674–682. [Google Scholar] [CrossRef]
- Woodhams, D.C.; Brandt, H.; Baumgartner, S.; Kielgast, J.; Küpfer, E.; Tobler, U.; Davis, L.R.; Schmidt, B.R.; Bel, C.; Hodel, S.; et al. Interacting Symbionts and Immunity in the Amphibian Skin Mucosome Predict Disease Risk and Probiotic Effectiveness. PLoS ONE 2014, 9, e96375. [Google Scholar] [CrossRef]
- Daskin, J.H.; Bell, S.C.; Schwarzkopf, L.; Alford, R.A. Cool Temperatures Reduce Antifungal Activity of Symbiotic Bacteria of Threatened Amphibians—Implications for Disease Management and Patterns of Decline. PLoS ONE 2014, 9, e100378. [Google Scholar] [CrossRef] [PubMed]
- Muletz-Wolz, C.R.; Fleischer, R.C.; Lips, K.R. Fungal disease and temperature alter skin microbiome structure in an experimental salamander system. Mol. Ecol. 2019, 28, 2917–2931. [Google Scholar] [CrossRef]
- Troitsky, T.S.; Laine, V.N.; Lilley, T.M. When the host’s away, the pathogen will play: The protective role of the skin microbiome during hibernation. Anim. Microbiome 2023, 5, 66. [Google Scholar]
- Bresciano, J.C.; Salvador, C.A.; Paz-y-Miño, C.; Parody-Merino, A.M.; Bosch, J.; Woodhams, D.C. Variation in the Presence of Anti-Batrachochytrium dendrobatidis Bacteria of Amphibians Across Life Stages and Elevations in Ecuador. Ecohealth 2015, 12, 310–319. [Google Scholar] [CrossRef]
- Wang, J.J.; Soininen, J.; Zhang, Y.; Wang, B.X.; Yang, X.D.; Shen, J. Contrasting patterns in elevational diversity between microorganisms and macroorganisms. J. Biogeogr. 2011, 38, 595–603. [Google Scholar] [CrossRef]
- Xu, L.L. The Impact of Altitude on Amphibian Symbiotic Microbiota (Skin and Gut Microbiomes). Master’s Thesis, Nanjing Normal University, Nanjing, China, 2019. (In Chinese). [Google Scholar]
- Zhao, J.S.; Yao, Y.F.; Li, D.Y.; Xu, H.M.; Wu, J.Y.; Wen, A.X.; Xie, M.; Ni, Q.Y.; Zhang, M.W.; Peng, G.N.; et al. Characterization of the Gut Microbiota in Six Geographical Populations of Chinese Rhesus Macaques (Macaca mulatta), Implying an Adaptation to High-Altitude Environment. Microb. Ecol. 2018, 76, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.G.; Xu, D.M.; Wang, L.; Hao, J.J.; Wang, J.F.; Zhou, X.; Wang, W.W.; Qiu, Q.; Huang, X.D.; Zhou, J.W.; et al. Convergent Evolution of Rumen Microbiomes in High-Altitude Mammals. Curr. Biol. 2016, 26, 1873–1879. [Google Scholar] [CrossRef]
- Satanower, S. Microbiome Research: An Overview. Aldrichimica Acta 2022, 55, 80–81. [Google Scholar]
- Guo, J.; Zhao, R.; Li, K.; Tan, Y.; Wang, L.; Ling, H.; Zhang, H.; Dharmarajan, G.; Bi, Y.; Yang, R. Altitude adaptation: The unseen work of gut microbiota. hLife 2025, 3, 5–20. [Google Scholar] [CrossRef]
- Muletz Wolz, C.R.; Yarwood, S.A.; Campbell Grant, E.H.; Fleischer, R.C.; Lips, K.R.; Hoye, B. Effects of host species and environment on the skin microbiome of Plethodontid salamanders. J. Anim. Ecol. 2017, 87, 341–353. [Google Scholar] [CrossRef]
- Zeng, B.; Zhao, J.C.; Guo, W.; Zhang, S.Y.; Hua, Y.T.; Tang, J.S.; Kong, F.L.; Yang, X.W.; Fu, L.Z.; Liao, K.; et al. High-Altitude Living Shapes the Skin Microbiome in Humans and Pigs. Front. Microbiol. 2017, 8, 1929. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Z.; Wang, S.; Li, J.; Zhang, L.; Liao, Z. A novel framework unveiling the importance of heterogeneous selection and drift on the community structure of symbiotic microbial indicator taxa across altitudinal gradients in amphibians. Microbiol. Spectr. 2025, 13, e04192-23. [Google Scholar] [CrossRef]
- Secor, S.M.; Carey, H.V. Integrative Physiology of Fasting. Compr. Physiol. 2016, 6, 773–825. [Google Scholar]
- Carey, H.V.; Assadi-Porter, F.M. The Hibernator Microbiome: Host-Bacterial Interactions in an Extreme Nutritional Symbiosis. In Annual Review of Nutrition; Stover, P.J., Balling, R., Eds.; Annual Review of Nutrition: San Mateo, CA, USA, 2017; Volume 37, pp. 477–500. [Google Scholar]
- Dai, Y.F. Research Progress on the Functional Regulation of Intestinal Microbial in Chinese Giant Salamander. Adv. Microbiol. 2024, 13, 87–91. [Google Scholar] [CrossRef]
- Lemieux-Labonté, V.; Vigliotti, C.; Tadic, Z.; Wehrle, B.; Lopez, P.; Bapteste, E.; Lapointe, F.J.; German, D.P.; Herrel, A. Proximate Drivers of Population-Level Lizard Gut Microbial Diversity: Impacts of Diet, Insularity, and Local Environment. Microorganisms 2022, 10, 1550. [Google Scholar] [CrossRef] [PubMed]
- Kohl, K.D.; Yahn, J. Effects of environmental temperature on the gut microbial communities of tadpoles. Environ. Microbiol. 2016, 18, 1561–1565. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Y.; Wu, S.; Zhang, W.; Cheng, X.; Li, Z.; Han, F.; Shi, J.; Shi, Y.; He, Z.; et al. Effects of Dietary Changes on the Gut Microbiota of Cynops orientalis. Asian Herpetol. Res. 2024, 15, 63–72. [Google Scholar]
- Tong, Q.; Liu, X.-N.; Hu, Z.-F.; Ding, J.-F.; Bie, J.; Wang, H.-B.; Zhang, J.-T. Effects of Captivity and Season on the Gut Microbiota of the Brown Frog (Rana dybowskii). Front. Microbiol. 2019, 10, 1912. [Google Scholar]
- Knutie, S.A.; Shea, L.A.; Kupselaitis, M.; Wilkinson, C.L.; Kohl, K.D.; Rohr, J.R. Early-Life Diet Affects Host Microbiota and Later-Life Defenses Against Parasites in Frogs. Integr. Comp. Biol. 2017, 57, 732–742. [Google Scholar]
- Demircan, T.; Ovezmyradov, G.; Yildirim, B.; Keskin, I.; Ilhan, A.E.; Fescioglu, E.C.; Ozturk, G.; Yildirim, S. Experimentally induced metamorphosis in highly regenerative axolotl (ambystoma mexicanum) under constant diet restructures microbiota. Sci. Rep. 2018, 8, 10974. [Google Scholar]
- Yang, B.; Cui, Z.; Ning, M.; Chen, Y.; Wu, Z.; Huang, H. Variation in the intestinal microbiota at different developmental stages of Hynobius maoershanensis. Ecol. Evol. 2022, 12, e8712. [Google Scholar]
- Sabino-Pinto, J.; Bletz, M.C.; Islam, M.M.; Shimizu, N.; Bhuju, S.; Geffers, R.; Jarek, M.; Kurabayashi, A.; Vences, M. Composition of the Cutaneous Bacterial Community in Japanese Amphibians: Effects of Captivity, Host Species, and Body Region. Microb. Ecol. 2016, 72, 460–469. [Google Scholar]
- Hernández-Gómez, O.; Briggler, J.T.; Williams, R.N. Captivity-Induced Changes in the Skin Microbial Communities of Hellbenders (Cryptobranchus alleganiensis). Microb. Ecol. 2018, 77, 782–793. [Google Scholar] [CrossRef]
- Wilson, B.A.; Passos, L.F.; Garcia, G.; Young, R.J. Comparing the bacterial communities of wild and captive golden mantella frogs: Implications for amphibian conservation. PLoS ONE 2018, 13, e0205652. [Google Scholar]
- Kueneman, J.G.; Parfrey, L.W.; Woodhams, D.C.; Archer, H.M.; Knight, R.; McKenzie, V.J. The amphibian skin-associated microbiome across species, space and life history stages. Mol. Ecol. 2014, 23, 1238–1250. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, V.J.; Bowers, R.M.; Fierer, N.; Knight, R.; Lauber, C.L. Co-habiting amphibian species harbor unique skin bacterial communities in wild populations. ISME J. 2012, 6, 588–596. [Google Scholar] [CrossRef]
- Vences, M.; Dohrmann, A.B.; Künzel, S.; Granzow, S.; Baines, J.F.; Tebbe, C.C. Composition and variation of the skin microbiota in sympatric species of European newts (Salamandridae). Amphib. -Reptil. 2015, 36, 5–12. [Google Scholar] [CrossRef]
- Bates, K.A.; Friesen, J.; Loyau, A.; Butler, H.; Vredenburg, V.T.; Laufer, J.; Chatzinotas, A.; Schmeller, D.S. Environmental and Anthropogenic Factors Shape the Skin Bacterial Communities of a Semi-Arid Amphibian Species. Microb. Ecol. 2022, 86, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Lucia, Z.; Giulio, G.; Matteo, G.; Stefano, C.; Irene, L.P.; Paolo, P.; Giorgio, B.; Hauffe, H.C. More Than Meets the Eye: Unraveling the Interactions Between Skin Microbiota and Habitat in an Opportunistic Amphibian. Microb. Ecol. 2024, 87, 176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.P. Rana dybowskii Surface Bacteria Identification and the Habitat Microbe Analysis. Master’s Thesis, Northeast Forestry University, Haerbing, China, 2013. (In Chinese). [Google Scholar]
- Bletz, M.C.; Goedbloed, D.J.; Sanchez, E.; Reinhardt, T.; Tebbe, C.C.; Bhuju, S.; Geffers, R.; Jarek, M.; Vences, M.; Steinfartz, S. Amphibian gut microbiota shifts differentially in community structure but converges on habitat-specific predicted functions. Nat. Commun. 2016, 7, 13699. [Google Scholar] [CrossRef]
- Xu, L.L.; Chen, H.; Zhang, M.; Zhu, W.; Chang, Q.; Lu, G.; Chen, Y.; Jiang, J.; Zhu, L. Changes in the community structure of the symbiotic microbes of wild amphibians from the eastern edge of the Tibetan Plateau. Microbiologyopen 2020, 9, e1004. [Google Scholar] [CrossRef]
- Park, J.-K.; Do, Y. The difference and variation of gut bacterial community and host physiology can support adaptation during and after overwintering in frog population. Integr. Zool. 2024, 19, 631–645. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, Y.; Hu, H.; Wang, G.-H. Nasonia-microbiome associations: A model for evolutionary hologenomics research. Trends Parasitol. 2023, 39, 101–112. [Google Scholar] [CrossRef]
- Korpita, T.M.M.; Muths, E.L.L.; Watry, M.K.; McKenzie, V.J.J. Captivity, Reintroductions, and the Rewilding of Amphibian-associated Bacterial Communities. Microb. Ecol. 2023, 86, 2271–2281. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Chen, H.; Xiao, H.; Zhang, H.M.; Li, L.Z.; Guo, C.; Chen, J.; Wei, Q. Amphibian diversity and habitat selection in karst desertification areas of northwest Guizhou. Biodivers. Sci. 2020, 28, 485–495. [Google Scholar] [CrossRef]
- Cani, P.D.; Delzenne, N.M.; Amar, J.; Burcelin, R. Role of gut microflora in the development of obesity and insulin resistance following high-fat diet feeding. Pathol. Biol. 2008, 56, 305–309. [Google Scholar]
- Chen, Z.; Chen, J.-Q.; Liu, Y.; Zhang, J.; Chen, X.-H.; Qu, Y.-F. Comparative study on gut microbiota in three Anura frogs from a mountain stream. Ecol. Evol. 2022, 12, e8854. [Google Scholar] [CrossRef]
- Kouete, M.T.; Bletz, M.C.; LaBumbard, B.C.; Woodhams, D.C.; Blackburn, D.C. Parental care contributes to vertical transmission of microbes in a skin-feeding and direct-developing caecilian. Anim. Microbiome 2023, 5, 28. [Google Scholar] [CrossRef]
- Hasan, N.; Yang, H.Y. Factors affecting the composition of the gut microbiota, and its modulation. Peerj 2019, 7, e7502. [Google Scholar] [CrossRef] [PubMed]
- Bird, A.K.; Prado-Irwin, S.R.; Vredenburg, V.T.; Zink, A.G. Skin Microbiomes of California Terrestrial Salamanders Are Influenced by Habitat More Than Host Phylogeny. Front. Microbiol. 2018, 9, 442. [Google Scholar] [CrossRef]
- Bletz, M.C.; Perl, R.G.B.; Vences, M. Skin microbiota differs drastically between co-occurring frogs and newts. R. Soc. Open Sci. 2017, 4, 170107. [Google Scholar]
- Chen, Y.E.; Fischbach, M.A.; Belkaid, Y. Skin microbiota–host interactions. Nature 2018, 555, 427–436. [Google Scholar]
- Weng, F.C.-H.; Yang, Y.-J.; Wang, D. Functional analysis for gut microbes of the brown tree frog (Polypedates megacephalus) in artificial hibernation. BMC Genom. 2016, 17, 31–42. [Google Scholar]
- Kohl, K.D.; Cary, T.L.; Karasov, W.H.; Dearing, M.D. Restructuring of the amphibian gut microbiota through metamorphosis. Environ. Microbiol. Rep. 2013, 5, 899–903. [Google Scholar] [CrossRef] [PubMed]
- Weng, F.C.-H.; Shaw, G.T.-W.; Weng, C.-Y.; Yang, Y.-J.; Wang, D. Inferring Microbial Interactions in the Gut of the Hong Kong Whipping Frog (Polypedates megacephalus) and a Validation Using Probiotics. Front. Microbiol. 2017, 8, 525. [Google Scholar] [CrossRef] [PubMed]
- Faszewski, E.E.; Kaltenbach, J.C. Histology and lectin-binding patterns in the skin of the terrestrial horned frog ceratophrys ornata. Cell Tissue Res. 1995, 281, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.H.; Heintzelman, M.B. Morphology of ventral epidermis of rana-catesbeiana during metamorphosis. Anat. Rec. 1987, 217, 305–317. [Google Scholar] [CrossRef]
- Bosch, J.; Martínez-Solano, I.; García-París, M. Evidence of a chytrid fungus infection involved in the decline of the common midwife toad (Alytes obstetricans) in protected areas of central Spain. Biol. Conserv. 2001, 97, 331–337. [Google Scholar] [CrossRef]
- Shade, A.; Peter, H.; Allison, S.D.; Baho, D.L.; Berga, M.; Buergmann, H.; Huber, D.H.; Langenheder, S.; Lennon, J.T.; Martiny, J.B.H.; et al. Fundamentals of microbial community resistance and resilience. Front. Microbiol. 2012, 3, 417. [Google Scholar] [CrossRef]
- Van Veelen, H.P.J.; Salles, J.F.; Matson, K.D.; van der Velde, M.; Tieleman, B.I. Microbial environment shapes immune function and cloacal microbiota dynamics in zebra finches Taeniopygia guttata. Anim. Microbiome 2020, 2, 21. [Google Scholar] [CrossRef] [PubMed]
- Kruger, A. Frog Skin Microbiota Vary with Host Species and Environment but Not Chytrid Infection. Front. Microbiol. 2020, 11, 1330. [Google Scholar] [CrossRef]
- Bataille, A.; Lee-Cruz, L.; Tripathi, B.; Kim, H.; Waldman, B. Microbiome Variation Across Amphibian Skin Regions: Implications for Chytridiomycosis Mitigation Efforts. Microb. Ecol. 2016, 71, 221–232. [Google Scholar]
- Jani, A.J.; Bushell, J.; Arisdakessian, C.G.; Belcaid, M.; Boiano, D.M.; Brown, C.; Knapp, R.A. The amphibian microbiome exhibits poor resilience following pathogen-induced disturbance. ISME J. 2021, 15, 1628–1640. [Google Scholar] [CrossRef]
- Zhu, D.-q.; Dong, W.-j.; Long, X.-z.; Yang, X.-m.; Han, X.-y.; Kou, Y.-h.; Tong, Q. Skin ulcers and microbiota in Rana dybowskii: Uncovering the role of the gut-skin axis in amphibian health. Aquaculture 2024, 585, 740724. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wang, Y.; He, Z.; Wu, S.; Wang, S.; Zhao, N.; Zhu, W.; Jiang, J.; Wang, S. Research Status and Prospect of Amphibian Symbiotic Microbiota. Animals 2025, 15, 934. https://doi.org/10.3390/ani15070934
Wang Z, Wang Y, He Z, Wu S, Wang S, Zhao N, Zhu W, Jiang J, Wang S. Research Status and Prospect of Amphibian Symbiotic Microbiota. Animals. 2025; 15(7):934. https://doi.org/10.3390/ani15070934
Chicago/Turabian StyleWang, Ziyi, Yuting Wang, Zhirong He, Siyu Wu, Suyue Wang, Na Zhao, Wei Zhu, Jianping Jiang, and Supen Wang. 2025. "Research Status and Prospect of Amphibian Symbiotic Microbiota" Animals 15, no. 7: 934. https://doi.org/10.3390/ani15070934
APA StyleWang, Z., Wang, Y., He, Z., Wu, S., Wang, S., Zhao, N., Zhu, W., Jiang, J., & Wang, S. (2025). Research Status and Prospect of Amphibian Symbiotic Microbiota. Animals, 15(7), 934. https://doi.org/10.3390/ani15070934







