Effects of Grape Pomace Complete Pellet Feed on Growth Performance, Fatty Acid Composition, and Rumen Fungal Composition in Beef Cattle
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Determination of the Nutritional Level of the Ration
2.2. Animal Management and Experiment Design
2.3. Determination of Growth Performance
2.4. Blood Biochemical Analysis
2.5. Fatty Acid Content in Muscle
2.6. Determination of Rumen Fermentation Parameters
2.7. Rumen Microbial Community Analysis
2.8. Statistical Analysis of Data
3. Results
3.1. Growth Performance
3.2. Blood Biochemical Analysis
3.3. Fatty Acid Composition of Beef Cattle Muscle
3.4. Rumen Fermentation Parameters in Beef Cattle
3.5. Effects of the Rumen Microbial Community
3.6. Economic Benefits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, Q.; Liu, H.; Wang, Z.; Lan, X.; An, J.; Shen, W.; Wan, F. Recent Advances in Feed and Nutrition of Beef Cattle in China—A Review. Anim. Biosci. 2023, 36, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Yeganehpour, E.; Taghizadeh, A.; Paya, H.; Hossein-Khani, A.; Palangi, V.; Shirmohammadi, S.; Abachi, S. Utilization of Fruit and Vegetable Wastes as an Alternative Renewable Energy Source in Ruminants’ Diet. Biomass Convers. Biorefin. 2023, 13, 7909–7917. [Google Scholar] [CrossRef]
- Patel, K.P.; Katole, S.B. Un-Conventional Feeds—Need for a Conventional Approach. Indian Farmer 2023, 10, 67–71. [Google Scholar]
- Constantin, O.E.; Stoica, F.; Ratu, R.N.; Stanciuc, N.; Bahrim, G.E.; Rapeanu, G. Bioactive Components, Applications, Extractions, and Health Benefits of Winery By-Products from a Circular Bioeconomy Perspective: A Review. Antioxidants 2024, 13, 100. [Google Scholar] [CrossRef]
- Costa, M.M.; Alfaia, C.M.; Lopes, P.A.; Pestana, J.M.; Prates, J.A.M. Grape By-Products as Feedstuff for Pig and Poultry Production. Animals 2022, 12, 2239. [Google Scholar] [CrossRef]
- Khiaosa-ard, R.; Mahmood, M.; Mickdam, E.; Pacífico, C.; Meixner, J.; Traintinger, L.-S. Winery By-Products as a Feed Source with Functional Properties: Dose–Response Effect of Grape Pomace, Grape Seed Meal, and Grape Seed Extract on Rumen Microbial Community and Their Fermentation Activity in RUSITEC. J. Anim. Sci. Biotechnol. 2023, 14, 92. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards Integral Utilization of Grape Pomace from Winemaking Process: A Review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- Juráček, M.; Vašeková, P.; Massányi, P.; Kováčik, A.; Bíro, D.; Šimko, M.; Gálik, B.; Rolinec, M.; Hanušovský, O.; Kolláthová, R.; et al. The Effect of Dried Grape Pomace Feeding on Nutrients Digestibility and Serum Biochemical Profile of Wethers. Agriculture 2021, 11, 1194. [Google Scholar] [CrossRef]
- Bešlo, D.; Došlić, G.; Agić, D.; Rastija, V.; Šperanda, M.; Gantner, V.; Lučić, B. Polyphenols in Ruminant Nutrition and Their Effects on Reproduction. Antioxidants 2022, 11, 970. [Google Scholar] [CrossRef]
- Ramdani, D.; Yuniarti, E.; Jayanegara, A.; Chaudhry, A.S. Roles of Essential Oils, Polyphenols, and Saponins of Medicinal Plants as Natural Additives and Anthelmintics in Ruminant Diets: A Systematic Review. Animals 2023, 13, 767. [Google Scholar] [CrossRef]
- Molosse, V.L.; Deolindo, G.L.; Lago, R.V.P.; Cecere, B.G.O.; Zotti, C.A.; Vedovato, M.; Copetti, P.M.; Fracasso, M.; Morsch, V.M.; Xavier, A.C.H.; et al. The Effects of the Inclusion of Ensiled and Dehydrated Grape Pomace in Beef Cattle Diet: Growth Performance, Health, and Economic Viability. Anim. Feed Sci. Technol. 2023, 302, 115671. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Munekata, P.E.S.; Zhang, W.; Garcia-Oliveira, P.; Carpena, M.; Prieto, M.A.; Bohrer, B.; Lorenzo, J.M. Protein Oxidation in Muscle Foods: A Comprehensive Review. Antioxidants 2022, 11, 60. [Google Scholar] [CrossRef]
- Hadidi, M.; Orellana-Palacios, J.C.; Aghababaei, F.; Gonzalez-Serrano, D.J.; Moreno, A.; Lorenzo, J.M. Plant By-Product Antioxidants: Control of Protein-Lipid Oxidation in Meat and Meat Products. LWT 2022, 169, 114003. [Google Scholar] [CrossRef]
- Beigh, Y.A.; Ganai, A.M.; Ahmad, H.A. Prospects of Complete Feed System in Ruminant Feeding: A Review. Vet. World 2017, 10, 424–437. [Google Scholar] [CrossRef]
- Trinci, A.P.J.; Davies, D.R.; Gull, K.; Lawrence, M.I.; Bonde Nielsen, B.; Rickers, A.; Theodorou, M.K. Anaerobic Fungi in Herbivorous Animals. Mycol. Res. 1994, 98, 129–152. [Google Scholar] [CrossRef]
- Wu, X.; Elekwachi, C.O.; Bai, S.; Luo, Y.; Zhang, K.; Forster, R.J. Characterizing the Alteration in Rumen Microbiome and Carbohydrate-Active Enzymes Profile with Forage of Muskoxen Rumen through Comparative Metatranscriptomics. Microorganisms 2022, 10, 71. [Google Scholar] [CrossRef]
- Cheng, X.; Du, X.; Liang, Y.; Degen, A.A.; Wu, X.; Ji, K.; Gao, Q.; Xin, G.; Cong, H.; Yang, G. Effect of Grape Pomace Supplement on Growth Performance, Gastrointestinal Microbiota, and Methane Production in Tan Lambs. Front. Microbiol. 2023, 14, 1264840. [Google Scholar] [CrossRef]
- Pauletto, M.; Elgendy, R.; Ianni, A.; Marone, E.; Giantin, M.; Grotta, L.; Ramazzotti, S.; Bennato, F.; Dacasto, M.; Martino, G. Nutrigenomic Effects of Long-Term Grape Pomace Supplementation in Dairy Cows. Animals 2020, 10, 714. [Google Scholar] [CrossRef]
- Liao, C.; Sullivan, P.J.; Barrett, C.B.; Kassam, K.-A.S. Socioenvironmental Threats to Pastoral Livelihoods: Risk Perceptions in the Altay and Tianshan Mountains of Xinjiang, China. Risk Anal. 2014, 34, 640–655. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990; Volume 1. [Google Scholar]
- Vansoest, P.; Robertson, J.; Lewis, B. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Amarowicz, R.; Troszyńska, A.; Baryłko-Pikielna, N.; Shahidi, F. Polyphenolics Extracts from Legume Seeds: Correlations Between Total Antioxidant Activity, Total Phenolics Content, Tannins Content and Astringency. J. Food Lipids 2004, 11, 278–286. [Google Scholar] [CrossRef]
- NY/T815-2004; Ministry of Agriculture and Rural Affairs of the People’s Republic of China. Feeding Standard of Beef Cattle. China Agriculture Press: Beijing, China, 2004. (In Chinese)
- Xavier, C.; Driesen, C.; Siegenthaler, R.; Dohme-Meier, F.; Le Cozler, Y.; Lerch, S. Estimation of Empty Body and Carcass Chemical Composition of Lactating and Growing Cattle: Comparison of Imaging, Adipose Cellularity, and Rib Dissection Methods. Transl. Anim. Sci. 2022, 6, txac066. [Google Scholar] [CrossRef] [PubMed]
- Dannenberger, D.; Nuernberg, K.; Herdmann, A.; Nuernberg, G.; Hagemann, E.; Kienast, W. Dietary PUFA Intervention Affects Fatty Acid- and Micronutrient Profiles of Beef and Related Beef Products. Foods 2013, 2, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Brito, A.F.; Broderick, G.A. Effect of Varying Dietary Ratios of Alfalfa Silage to Corn Silage on Production and Nitrogen Utilization in Lactating Dairy Cows. J. Dairy Sci. 2006, 89, 3924–3938. [Google Scholar] [CrossRef]
- Zinn, R.; Owens, F. A Rapid Procedure for Purine Measurement and Its Use for Estimating Net Ruminal Protein-Synthesis. Can. J. Anim. Sci. 1986, 66, 157–166. [Google Scholar] [CrossRef]
- Dimou, C.; Koutelidakis, A. Value Added Alternatives of Winemaking Process Residues: A Health Based Oriented Perspective. BAOJ Biotechnol. 2016, 2, 016. [Google Scholar]
- Tayengwa, T.; Chikwanha, O.C.; Raffrenato, E.; Dugan, M.E.R.; Mutsvangwa, T.; Mapiye, C. Comparative Effects of Feeding Citrus Pulp and Grape Pomace on Nutrient Digestibility and Utilization in Steers. Animal 2021, 15, 100020. [Google Scholar] [CrossRef]
- Caetano, M.; Wilkes, M.J.; Pitchford, W.S.; Lee, S.J.; Hynd, P.I. Effect of Ensiled Crimped Grape Marc on Energy Intake, Performance and Gas Emissions of Beef Cattle. Anim. Feed Sci. Technol. 2019, 247, 166–172. [Google Scholar] [CrossRef]
- Escalera-Valente, F.; Alonso, M.E.; Lomillos-Pérez, J.M.; Gaudioso-Lacasa, V.R.; Alonso, A.J.; González-Montaña, J.R. Blood Biochemical Variables Found in Lidia Cattle after Intense Exercise. Animals 2021, 11, 2866. [Google Scholar] [CrossRef]
- Carrillo-Muro, O.; Rodríguez-Cordero, D.; Hernández-Briano, P.; Correa-Aguado, P.I.; Medina-Flores, C.A.; Huerta-López, L.A.; Rodríguez-Valdez, F.J.; Rivera-Villegas, A.; Plascencia, A. Enzymic Activity, Metabolites, and Hematological Responses in High-Risk Newly Received Calves for “Clinical Health” Reference Intervals. Animals 2024, 14, 2342. [Google Scholar] [CrossRef]
- Chedea, V.S.; Pelmus, R.S.; Lazar, C.; Pistol, G.C.; Calin, L.G.; Toma, S.M.; Dragomir, C.; Taranu, I. Effects of a diet containing dried grape pomace on blood metabolites and milk composition of dairy cows. J. Sci. Food Agric. 2017, 97, 2516–2523. [Google Scholar] [CrossRef] [PubMed]
- Onasanya, G.O.; Oke, F.O.; Sanni, T.M.; Muhammad, A.I. Parameters Influencing Haematological, Serum and Bio-Chemical References in Livestock Animals under Different Management Systems. Open J. Vet. Med. 2015, 05, 181. [Google Scholar] [CrossRef]
- Martins Flores, D.R.; Franco da Fonseca, P.A.; Schmitt, J.; Tonetto, C.J.; Rosado Junior, A.G.; Hammerschmitt, R.K.; Facco, D.B.; Brunetto, G.; Nornberg, J.L. Lambs Fed with Increasing Levels of Grape Pomace Silage: Effects on Productive Performance, Carcass Characteristics, and Blood Parameters. Livest. Sci. 2020, 240, 104169. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Dai, C.; Li, J.; Huang, P.; Li, Y.; Ding, X.; Huang, J.; Hussain, T.; Yang, H. Effects of Dietary Energy on Growth Performance, Carcass Characteristics, Serum Biochemical Index, and Meat Quality of Female Hu Lambs. Anim. Nutr. 2020, 6, 499–506. [Google Scholar] [CrossRef]
- Alba, D.F.; Campigotto, G.; Cazarotto, C.J.; Dos Santos, D.S.; Gebert, R.R.; Reis, J.H.; Souza, C.F.; Baldissera, M.D.; Gindri, A.L.; Kempka, A.P.; et al. Use of Grape Residue Flour in Lactating Dairy Sheep in Heat Stress: Effects on Health, Milk Production and Quality. J. Therm. Biol. 2019, 82, 197–205. [Google Scholar] [CrossRef]
- He, P.; Lei, Y.; Zhang, K.; Zhang, R.; Bai, Y.; Li, Z.; Jia, L.; Shi, J.; Cheng, Q.; Ma, Y.; et al. Dietary Oregano Essential Oil Supplementation Alters Meat Quality, Oxidative Stability, and Fatty Acid Profiles of Beef Cattle. Meat Sci. 2023, 205, 109317. [Google Scholar] [CrossRef]
- De Nazaré Santos Torres, R.; Bertoco, J.P.A.; Arruda, M.C.G.; De Melo Coelho, L.; Paschoaloto, J.R.; Ezequiel, J.M.B.; Almeida, M.T.C. The Effect of Dietary Inclusion of Crude Glycerin on Performance, Ruminal Fermentation, Meat Quality and Fatty Acid Profile of Beef Cattle: Meta-Analysis. Res. Vet. Sci. 2021, 140, 171–184. [Google Scholar] [CrossRef]
- Bennato, F.; Martino, C.; Ianni, A.; Giannone, C.; Martino, G. Dietary Grape Pomace Supplementation in Lambs Affects the Meat Fatty Acid Composition, Volatile Profiles and Oxidative Stability. Foods 2023, 12, 1257. [Google Scholar] [CrossRef]
- Ponnampalam, E.N.; Sinclair, A.J.; Holman, B.W.B. The Sources, Synthesis and Biological Actions of Omega-3 and Omega-6 Fatty Acids in Red Meat: An Overview. Foods 2021, 10, 1358. [Google Scholar] [CrossRef]
- Nogoy, K.M.C.; Sun, B.; Shin, S.; Lee, Y.; Li, X.Z.; Choi, S.H.; Park, S. Fatty Acid Composition of Grain- and Grass-Fed Beef and Their Nutritional Value and Health Implication. Food Sci. Anim. Resour. 2022, 42, 18–33. [Google Scholar] [CrossRef]
- Arend, F.A.; Murdoch, G.K.; Doumit, M.E.; Chibisa, G.E. Inclusion of Grape Pomace in Finishing Cattle Diets: Carcass Traits, Meat Quality and Fatty Acid Composition. Animals 2022, 12, 2597. [Google Scholar] [CrossRef] [PubMed]
- Vahmani, P.; Ponnampalam, E.N.; Kraft, J.; Mapiye, C.; Bermingham, E.N.; Watkins, P.J.; Proctor, S.D.; Dugan, M.E.R. Bioactivity and Health Effects of Ruminant Meat Lipids. Invited Review. Meat Sci. 2020, 165, 108114. [Google Scholar] [CrossRef] [PubMed]
- Sakowski, T.; Grodkowski, G.; Gołebiewski, M.; Slósarz, J.; Kostusiak, P.; Solarczyk, P.; Puppel, K. Genetic and Environmental Determinants of Beef Quality—A Review. Front. Vet. Sci. 2022, 9, 819605. [Google Scholar] [CrossRef]
- Molosse, V.L.; Deolindo, G.L.; Lago, R.V.P.; Klein, B.; Zotti, C.A.; Vedovato, M.; da Silveira, M.V.; Copetti, P.M.; Schetinger, M.R.C.; Favero, J.F.; et al. The Use of Secondary Grape Biomass in Beef Cattle Nutrition on Carcass Characteristics, Quality and Shelf Life of Meat. Food Nutr. Sci. 2024, 15, 447–469. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, H.; Lu, Z.; Nian, F.; Zheng, C.; Li, F.; Tang, D. Improving Shelf Life and Content of Unsaturated Fatty Acids in Meat of Lambs Fed a Diet Supplemented with Grape Dregs. Foods 2023, 12, 4204. [Google Scholar] [CrossRef]
- Tayengwa, T.; Chikwanha, O.C.; Neethling, J.; Dugan, M.E.R.; Mutsvangwa, T.; Mapiye, C. Polyunsaturated Fatty Acid, Volatile and Sensory Profiles of Beef from Steers Fed Citrus Pulp or Grape Pomace. Food Res. Int. 2021, 139, 109923. [Google Scholar] [CrossRef]
- Makmur, M.; Zain, M.; Sholikin, M.M.; Suharlina; Jayanegara, A. Modulatory Effects of Dietary Tannins on Polyunsaturated Fatty Acid Biohydrogenation in the Rumen: A Meta-Analysis. Heliyon 2022, 8, e09828. [Google Scholar] [CrossRef]
- Del Bianco, S.; Natalello, A.; Luciano, G.; Valenti, B.; Campidonico, L.; Gkarane, V.; Monahan, F.; Biondi, L.; Favotto, S.; Sepulcri, A.; et al. Influence of Dietary Inclusion of Tannin Extracts from Mimosa, Chestnut and Tara on Volatile Compounds and Flavour in Lamb Meat. Meat Sci. 2021, 172, 108336. [Google Scholar] [CrossRef]
- Xiong, Y.; Guo, C.; Wang, L.; Chen, F.; Dong, X.; Li, X.; Ni, K.; Yang, F. Effects of Paper Mulberry Silage on the Growth Performance, Rumen Microbiota and Muscle Fatty Acid Composition in Hu Lambs. Fermentation 2021, 7, 286. [Google Scholar] [CrossRef]
- Hennessy, A.A.; Kenny, D.A.; Byrne, C.J.; Childs, S.; Ross, R.P.; Devery, R.; Stanton, C. Fatty Acid Concentration of Plasma, Muscle, Adipose and Liver from Beef Heifers Fed an Encapsulated n-3 Polyunsaturated Fatty Acid Supplement. Animal 2021, 15, 100039. [Google Scholar] [CrossRef]
- Pogorzelski, G.; Pogorzelska-Nowicka, E.; Pogorzelski, P.; Półtorak, A.; Hocquette, J.-F.; Wierzbicka, A. Towards an Integration of Pre- and Post-Slaughter Factors Affecting the Eating Quality of Beef. Livest. Sci. 2022, 255, 104795. [Google Scholar] [CrossRef]
- Davis, H.; Magistrali, A.; Butler, G.; Stergiadis, S. Nutritional Benefits from Fatty Acids in Organic and Grass-Fed Beef. Foods 2022, 11, 646. [Google Scholar] [CrossRef] [PubMed]
- Nuernberg, K.; Dannenberger, D.; Nuernberg, G.; Ender, K.; Voigt, J.; Scollan, N.D.; Wood, J.D.; Nute, G.R.; Richardson, R.I. Effect of a Grass-Based and a Concentrate Feeding System on Meat Quality Characteristics and Fatty Acid Composition of Longissimus Muscle in Different Cattle Breeds. Livest. Prod. Sci. 2005, 94, 137–147. [Google Scholar] [CrossRef]
- Wang, Q.; Zeng, Y.; Zeng, X.; Wang, X.; Wang, Y.; Dai, C.; Li, J.; Huang, P.; Huang, J.; Hussain, T.; et al. Effects of Dietary Energy Levels on Rumen Fermentation, Gastrointestinal Tract Histology, and Bacterial Community Diversity in Fattening Male Hu Lambs. Front. Microbiol. 2021, 12, 695445. [Google Scholar] [CrossRef]
- Li, J.; Yan, H.; Chen, J.; Duan, C.; Guo, Y.; Liu, Y.; Zhang, Y.; Ji, S. Correlation of Ruminal Fermentation Parameters and Rumen Bacterial Community by Comparing Those of the Goat, Sheep, and Cow In Vitro. Fermentation 2022, 8, 427. [Google Scholar] [CrossRef]
- Zhan, J.; Liu, M.; Wu, C.; Su, X.; Zhan, K.; Zhao, G. qi Effects of Alfalfa Flavonoids Extract on the Microbial Flora of Dairy Cow Rumen. Asian-Australas. J. Anim. Sci. 2017, 30, 1261–1269. [Google Scholar] [CrossRef]
- Li, F.; Cao, Y.; Liu, N.; Yang, X.; Yao, J.; Yan, D. Subacute Ruminal Acidosis Challenge Changed in Situ Degradability of Feedstuffs in Dairy Goats. J. Dairy Sci. 2014, 97, 5101–5109. [Google Scholar] [CrossRef]
- Ramos, S.C.; Jeong, C.D.; Mamuad, L.L.; Kim, S.H.; Kang, S.H.; Kim, E.T.; Cho, Y.I.; Lee, S.S.; Lee, S.S. Diet Transition from High-Forage to High-Concentrate Alters Rumen Bacterial Community Composition, Epithelial Transcriptomes and Ruminal Fermentation Parameters in Dairy Cows. Animals 2021, 11, 838. [Google Scholar] [CrossRef]
- Sato, S. Pathophysiological Evaluation of Subacute Ruminal Acidosis (SARA) by Continuous Ruminal pH Monitoring. Anim. Sci. J. 2016, 87, 168–177. [Google Scholar]
- Ren, C.; Zhang, X.; Wei, H.; Wang, S.; Wang, W.; He, L.; Lu, Y.; Zhang, K.; Zhang, Z.; Wang, G.; et al. Effect of Replacing Alfalfa Hay with Common Vetch Hay in Sheep Diets on Growth Performance, Rumen Fermentation and Rumen Microbiota. Animals 2024, 14, 2182. [Google Scholar] [CrossRef]
- Dewhurst, R.J.; Newbold, J.R. Effect of Ammonia Concentration on Rumen Microbial Protein Production in Vitro. Br. J. Nutr. 2022, 127, 847–849. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, D.; Chen, W.; Li, Y.; Wu, H.; Meng, Q.; Zhou, Z. Estimating Ruminal Crude Protein Degradation from Beef Cattle Feedstuff. Sci. Rep. 2019, 9, 11368. [Google Scholar] [CrossRef] [PubMed]
- (PDF) Effect of Carbohydrate Source on Ammonia Utilization in Lactating Dairy Cow. Available online: https://www.researchgate.net/publication/8085997_Effect_of_carbohydrate_source_on_ammonia_utilization_in_lactating_dairy_cow (accessed on 12 March 2025).
- Vinyard, J.R.; Myers, C.A.; Murdoch, G.K.; Rezamand, P.; Chibisa, G.E. Optimum Grape Pomace Proportion in Feedlot Cattle Diets: Ruminal Fermentation, Total Tract Nutrient Digestibility, Nitrogen Utilization, and Blood Metabolites. J. Anim. Sci. 2021, 99, skab044. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, G.; Li, Y.; Zhang, Y. Effects of High Forage/Concentrate Diet on Volatile Fatty Acid Production and the Microorganisms Involved in VFA Production in Cow Rumen. Animals 2020, 10, 223. [Google Scholar] [CrossRef]
- Guerra-Rivas, C.; Gallardo, B.; Mantecon, A.R.; del Alamo-Sanza, M.; Manso, T. Evaluation of Grape Pomace from Red Wine By-Product as Feed for Sheep. J. Sci. Food Agric. 2017, 97, 1885–1893. [Google Scholar] [CrossRef]
- Norris, A.B.; Crossland, W.L.; Tedeschi, L.O.; Foster, J.L.; Muir, J.P.; Pinchak, W.E.; Fonseca, M.A. Inclusion of Quebracho Tannin Extract in a High-Roughage Cattle Diet Alters Digestibility, Nitrogen Balance, and Energy Partitioning. J. Anim. Sci. 2020, 98, skaa047. [Google Scholar] [CrossRef]
- Kara, K.; Öztaş, M.A. The Effect of Dietary Fermented Grape Pomace Supplementation on In Vitro Total Gas and Methane Production, Digestibility, and Rumen Fermentation. Fermentation 2023, 9, 741. [Google Scholar] [CrossRef]
- Newbold, C.J.; Ramos-Morales, E. Review: Ruminal Microbiome and Microbial Metabolome: Effects of Diet and Ruminant Host. Animal 2020, 14, S78–S86. [Google Scholar] [CrossRef]
- Krol, B.; Slupczynska, M.; Wilk, M.; Asghar, M.U.; Cwynar, P. Anaerobic Rumen Fungi and Fungal Direct-Fed Microbials in Ruminant Feeding. J. Anim. Feed Sci. 2023, 32, 3–16. [Google Scholar] [CrossRef]
- Péter, G. Rumen Microbiology: From Evolution to Revolution; Springer: New Delhi, India, 2015. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, F.; Chen, Y.; Wu, H.; Meng, Q.; Guan, L.L. Metatranscriptomic Profiling Reveals the Effect of Breed on Active Rumen Eukaryotic Composition in Beef Cattle With Varied Feed Efficiency. Front. Microbiol. 2020, 11, 367. [Google Scholar] [CrossRef]
- da Silva, E.B.R.; da Silva, J.A.R.; da Silva, W.C.; Belo, T.S.; Sousa, C.E.L.; Santos, M.R.P.D.; Neves, K.A.L.; Rodrigues, T.C.G.D.C.; Camargo-Junior, R.N.C.; Lourenco-Junior, J.D.B. A Review of the Rumen Microbiota and the Different Molecular Techniques Used to Identify Microorganisms Found in the Rumen Fluid of Ruminants. Animals 2024, 14, 1448. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, R.A.; Dagar, S.S.; Griffith, G.W.; Pratt, C.J.; Youssef, N.H.; Elshahed, M.S. Taxonomy of the Anaerobic Gut Fungi (Neocallimastigomycota): A Review of Classification Criteria and Description of Current Taxa. Int. J. Syst. Evol. Microbiol. 2022, 72, 005322. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Q.; Xu, T.; Hu, L.; Zhao, N.; Liu, H.; Xu, S. Effects of Winter Barn Feeding and Grazing on Growth Performance, Meat Quality and Rumen Fungal Community of Tibetan Sheep. Ital. J. Anim. Sci. 2023, 22, 959–971. [Google Scholar] [CrossRef]
- Carberry, C.A.; Waters, S.M.; Kenny, D.A.; Creevey, C.J. Rumen Methanogenic Genotypes Differ in Abundance According to Host Residual Feed Intake Phenotype and Diet Type. Appl. Environ. Microbiol. 2014, 80, 2039. [Google Scholar] [CrossRef]
- Saminathan, M.; Ramiah, S.K.; Gan, H.M.; Abdullah, N.; Wong, C.M.V.L.; Ho, Y.W.; Idrus, Z. In Vitro Study on the Effects of Condensed Tannins of Different Molecular Weights on Bovine Rumen Fungal Population and Diversity. Ital. J. Anim. Sci. 2019, 18, 1451–1462. [Google Scholar] [CrossRef]
- Khiaosa-ard, R.; Pacifico, C.; Mahmood, M.; Mickdam, E.; Meixner, J.; Traintinger, L.-S.; Zebeli, Q. Changes in the Solid-Associated Bacterial and Fungal Communities Following Ruminal in Vitro Fermentation of Winery by-Products: Aspects of the Bioactive Compounds and Feed Safety. Anaerobe 2024, 89, 102893. [Google Scholar] [CrossRef]
- Nidhina, N.; Bhavya, M.L.; Bhaskar, N.; Muthukumar, S.P.; Murthy, P.S. Aflatoxin Production by Aspergillus Flavus in Rumen Liquor and Its Implications. Food Control 2017, 71, 26–31. [Google Scholar] [CrossRef]
- Vilela, A. Non-Saccharomyces Yeasts and Organic Wines Fermentation: Implications on Human Health. Fermentation 2020, 6, 54. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, N.; Zhang, J.; Gu, Q.; Dong, C.; Lin, B.; Zou, C. Microbiome and Fermentation Parameters in the Rumen of Dairy Buffalo in Response to Ingestion Associated with a Diet Supplemented with Cysteamine and Hemp Seed Oil. J. Anim. Physiol. Anim. Nutr. 2022, 106, 471–484. [Google Scholar] [CrossRef]
- Belanche, A.; Doreau, M.; Edwards, J.E.; Moorby, J.M.; Pinloche, E.; Newbold, C.J. Shifts in the Rumen Microbiota Due to the Type of Carbohydrate and Level of Protein Ingested by Dairy Cattle Are Associated with Changes in Rumen Fermentation. J. Nutr. 2012, 142, 1684–1692. [Google Scholar] [CrossRef]
- Shen, J.; Zheng, W.; Xu, Y.; Yu, Z. The Inhibition of High Ammonia to in Vitro Rumen Fermentation Is pH Dependent. Front. Vet. Sci. 2023, 10, 1163021. [Google Scholar] [CrossRef]
- Guo, W.; Zhou, M.; Ma, T.; Bi, S.; Wang, W.; Zhang, Y.; Huang, X.; Guan, L.L.; Long, R. Survey of Rumen Microbiota of Domestic Grazing Yak during Different Growth Stages Revealed Novel Maturation Patterns of Four Key Microbial Groups and Their Dynamic Interactions. Anim. Microbiome 2020, 2, 23. [Google Scholar] [CrossRef]
- Srinivasan, R.; Prabhu, G.; Prasad, M.; Mishra, M.; Chaudhary, M.; Srivastava, R. Penicillium. In Beneficial Microbes in Agro-Ecology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 651–667. ISBN 978-0-12-823414-3. [Google Scholar]
- Lin, M.; Feng, L.; Cheng, Z.; Wang, K. Effect of Ethanol or Lactic Acid on Volatile Fatty Acid Profile and Microbial Community in Short-Term Sequentially Transfers by Ruminal Fermented with Wheat Straw in Vitro. Process Biochem. 2021, 102, 369–375. [Google Scholar] [CrossRef]
- Sosa, A.; Saro, C.; Mateos, I.; Díaz, A.; Galindo, J.; Carro, M.D.; Ranilla, M.J. Efecto de Aspergillus oryzae en la fermentación ruminal de una dieta de heno de alfalfa: Concentrado con la utilización de la técnica de simulación de rumen (Rusitec). Cuba. J. Agric. Sci. 2020, 54, 183–192. [Google Scholar]
- Kong, F.; Lu, N.; Liu, Y.; Zhang, S.; Jiang, H.; Wang, H.; Wang, W.; Li, S. Aspergillus Oryzae and Aspergillus Niger Co-Cultivation Extract Affects In Vitro Degradation, Fermentation Characteristics, and Bacterial Composition in a Diet-Specific Manner. Animals 2021, 11, 1248. [Google Scholar] [CrossRef] [PubMed]
- Donyadoust, M.; KHalilvandi Behrouzyar, H.; Pirmohammadi, R.; Donyadoust, M. Evaluation the Effects of Biological Processing of Wheat Straw by Aspergillus Oryzae on Rumen Fermentation Parameters and Fiber Degradability in Ruminants. J. Rumin. Res. 2023, 10, 1–20. [Google Scholar] [CrossRef]
- Wang, C.; You, Y.; Huang, W.; Zhan, J. The High-Value and Sustainable Utilization of Grape Pomace: A Review. Food Chem. X 2024, 24, 101845. [Google Scholar] [CrossRef]
- Bakasa, C. Modelling the Environmental, Social, and Economic Implications of Using Fruit Pomace as an Alternative Livestock Feed Resource: A System Dynamic Modelling Approach. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2022. [Google Scholar]
- Kokkinomagoulos, E.; Kandylis, P. Grape Pomace, an Undervalued by-Product: Industrial Reutilization within a Circular Economy Vision. Rev. Environ. Sci. Biotechnol. 2023, 22, 739–773. [Google Scholar] [CrossRef]
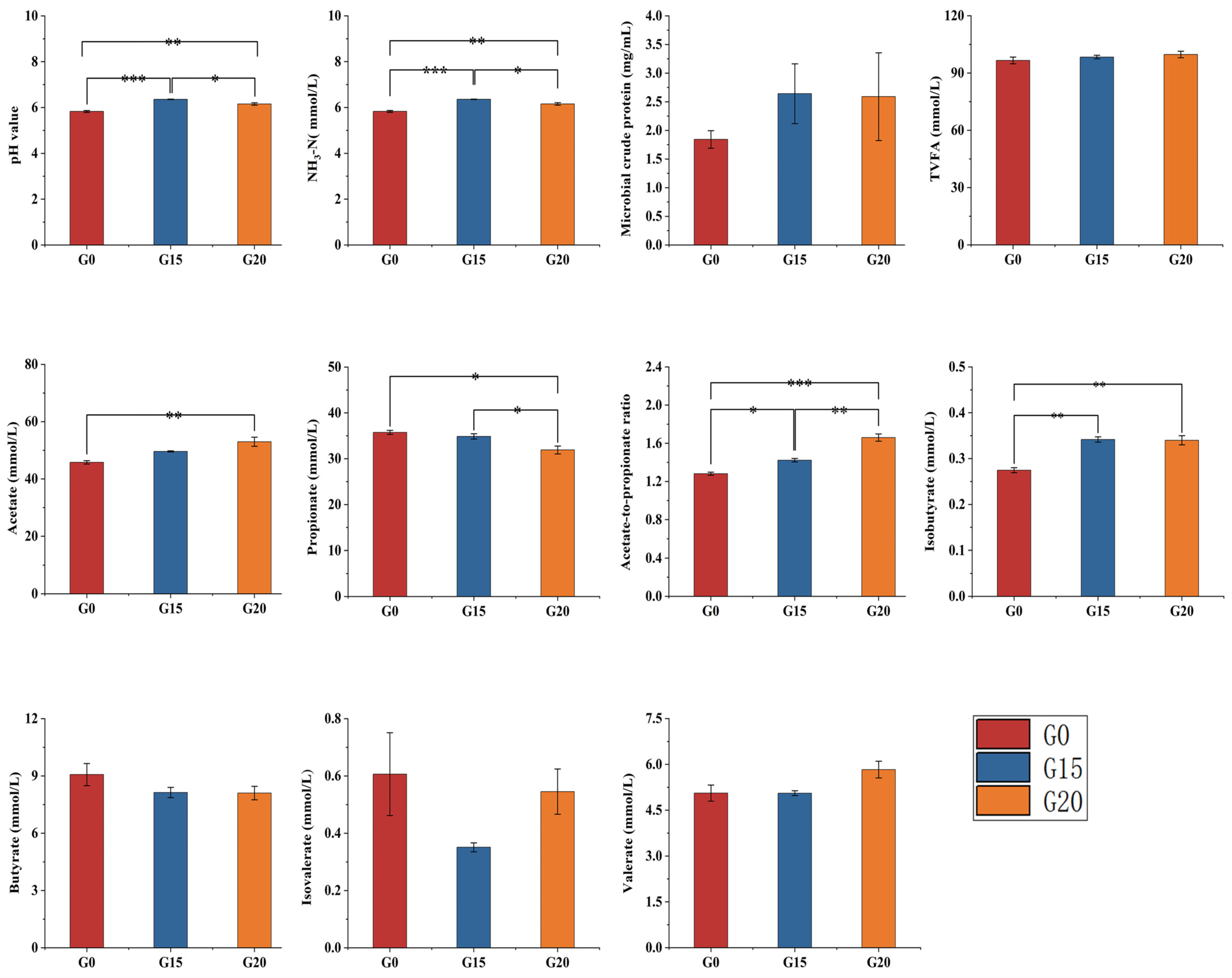
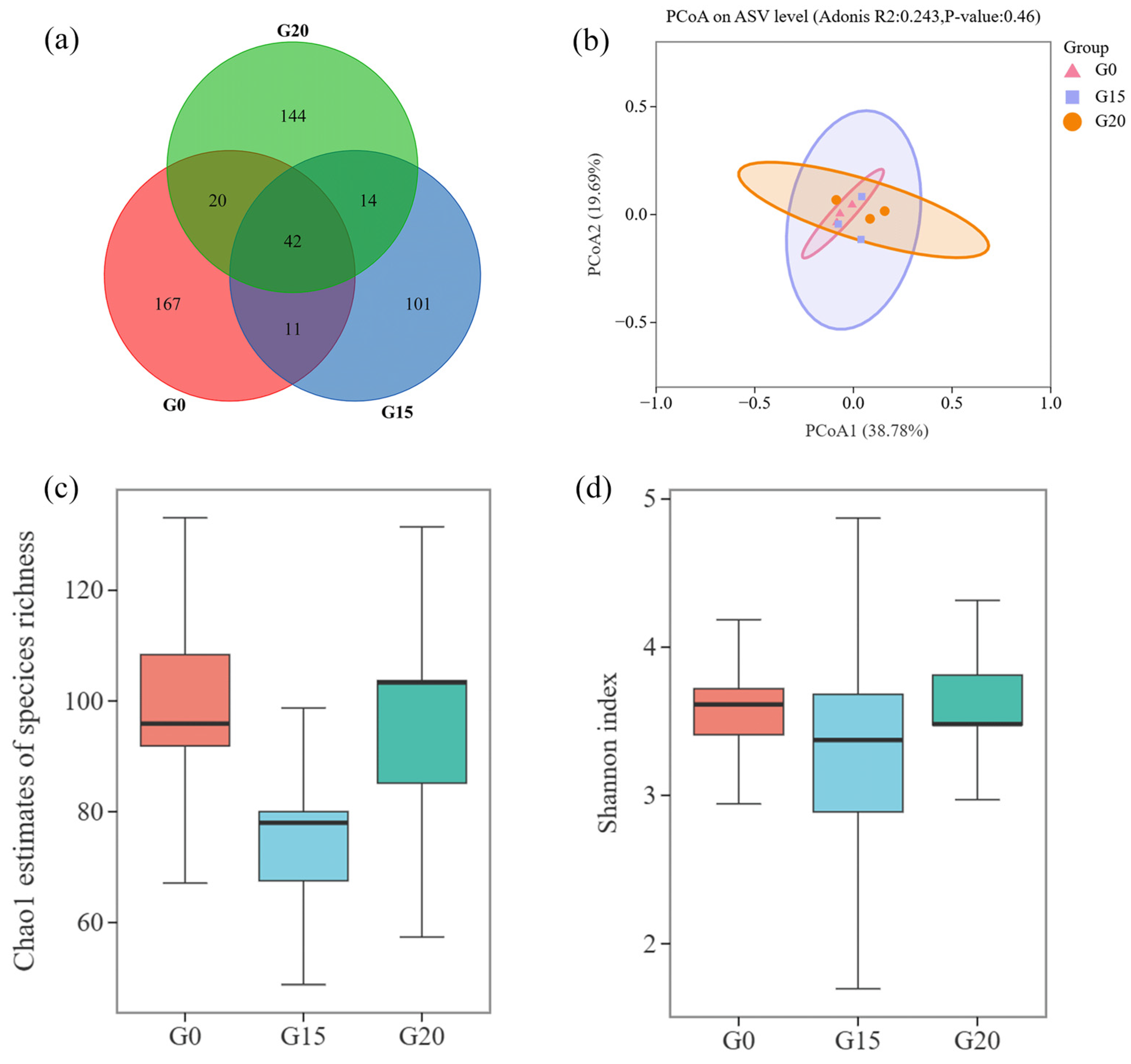
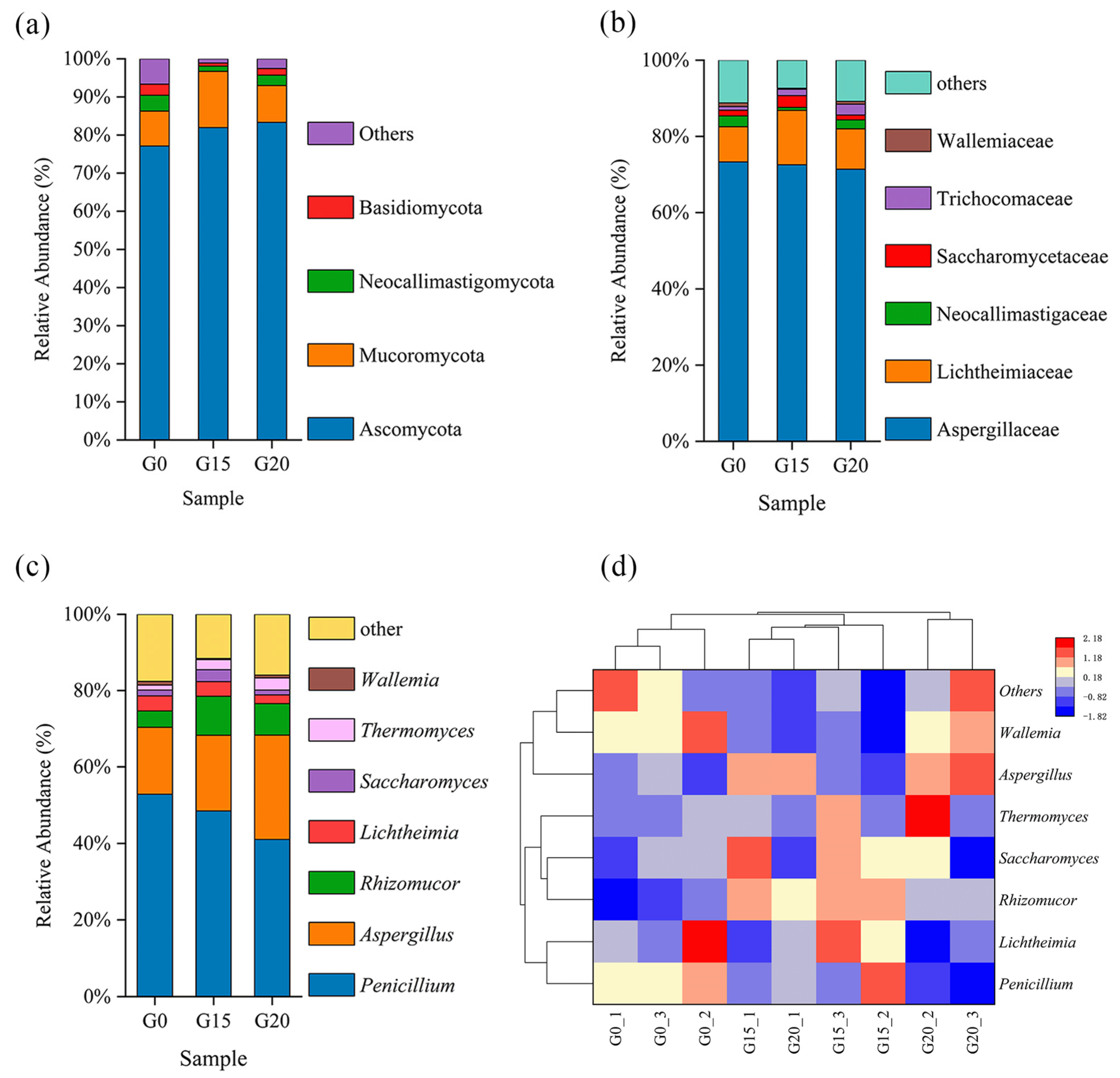

| Items | Content |
|---|---|
| Dry matter/(DM/%) | 93.50 |
| Crude protein/(DM/%) | 13.11 |
| Crude fat/(DM/%) | 12.70 |
| Neutral detergent fiber/(DM/%) | 38.10 |
| Acid detergent fiber/(DM/%) | 30.82 |
| Ash/(DM/%) | 8.50 |
| Condensed tannin/(g/kg) | 2.51 |
| Items | Groups | ||
|---|---|---|---|
| G0 | G15 | G20 | |
| Corn | 42.12 | 39.04 | 37.17 |
| Wheat bran | 6.79 | 10.34 | 12.96 |
| Soybean meal | 7.19 | 4.87 | 3.88 |
| Salt | 1.00 | 1.00 | 1.00 |
| Premix 1 | 1.00 | 1.00 | 1.00 |
| Corn bran | 1.96 | 1.05 | 0.65 |
| Alfalfa hay | 12.32 | 3.66 | 0.00 |
| Corn straw | 11.52 | 7.34 | 6.12 |
| Grape pomace | 0.00 | 15.00 | 20.00 |
| Wheat Straw | 12.17 | 7.64 | 8.00 |
| Corn cob | 3.93 | 9.05 | 9.22 |
| Total | 100.00 | 100.00 | 100.00 |
| Chemical composition (g/kg DM) | |||
| Combined net energy (NEmf) 2, MJ/kg | 6.03 | 6.02 | 6.03 |
| Crude protein (CP), % of DM | 11.13 | 11.15 | 11.16 |
| Crude fat (CF), % of DM | 2.6 | 3.31 | 3.58 |
| Neutral detergent fiber (NDF), % of DM | 36.09 | 35.61 | 35.34 |
| Acid detergent fiber (ADF), % of DM | 20.59 | 19.95 | 19.91 |
| Crude ash (Ash), % of DM | 4.65 | 4.89 | 5.11 |
| Items | Groups | p-Value | ||
|---|---|---|---|---|
| G0 | G15 | G20 | ||
| Initial body weight, kg | 292.83 ± 8.82 | 284.17 ± 7.54 | 285.17 ± 3.44 | 0.651 |
| Final body weight, kg | 342.02 ± 3.61 | 339.53 ± 4.65 | 359.11 ± 8.44 | 0.112 |
| Average daily feed intake, kg/day | 9.06 ± 0.11 b | 9.40 ± 0.20 ab | 9.81 ± 0.13 a | 0.036 |
| Average daily gain, kg/day | 0.82 ± 0.11 b | 0.93 ± 0.07 b | 1.23 ± 0.09 a | 0.036 |
| Feed conversion, kg/kg | 11.50 ± 1.72 | 10.27 ± 0.79 | 8.06 ± 0.66 | 0.099 |
| Items | Groups | p-Value | ||
|---|---|---|---|---|
| G0 | G15 | G20 | ||
| Urea nitrogen (mmol/L) | 1.01 ± 0.11 | 0.96 ± 0.17 | 1.75 ± 0.36 | 0.101 |
| Total Protein (g/L) | 61.70 ± 1.18 b | 65.60 ± 1.05 ab | 70.93 ± 3.14 a | 0.049 |
| Albumin (g/L) | 34.63 ± 1.17 b | 36.27 ± 0.33 ab | 38.83 ± 0.83 a | 0.034 |
| Globulin (g/L) | 27.07±1.96 | 29.33 ± 0.84 | 32.1 ± 3.76 | 0.416 |
| A:G | 1.30 ± 0.14 | 1.24 ± 0.03 | 1.24 ± 0.15 | 0.919 |
| A:T | 0.56 ± 0.03 | 0.55 ± 0.01 | 0.55 ± 0.03 | 0.899 |
| G:T | 0.44 ± 0.03 | 0.45 ± 0.01 | 0.45 ± 0.03 | 0.899 |
| Triglyceride (mmol/L) | 0.32 ± 0.06 | 0.37 ± 0.02 | 0.32 ± 0.04 | 0.599 |
| Total cholesterol (mmol/L) | 4.54 ± 0.23 | 5.23 ± 0.42 | 5.41 ± 0.21 | 0.183 |
| Items (μg/g) | Groups | p-Value | ||
|---|---|---|---|---|
| G0 | G15 | G20 | ||
| C8-0 | 1.04 ± 0.15 b | 1.54 ± 0.10 a | 1.28 ± 0.05 ab | 0.043 |
| C9-0 | 0.49 ± 0.03 | 0.66 ± 0.07 | 0.58 ± 0.01 | 0.074 |
| C10-0 | 2.96 ± 0.4 | 4.25 ± 0.61 | 4.38 ± 0.27 | 0.124 |
| C11-0 | 0.17 ± 0.01 | 0.20 ± 0.02 | 0.20 ± 0.01 | 0.221 |
| C12-0 | 3.05 ± 0.6 | 4.59 ± 0.83 | 5.23 ± 1.29 | 0.322 |
| C14-0 | 44.76 ± 7.64 | 65.49 ± 9.00 | 71.08 ± 9.35 | 0.158 |
| C15-0 | 13.40 ± 3.62 | 17.19 ± 1.45 | 18.21 ± 3.77 | 0.553 |
| C16-0 | 520.19 ± 13.58 b | 595.36 ± 11.37 a | 604.92 ± 9.67 a | p < 0.01 |
| C17-0 | 22.79 ± 2.53 | 27.98 ± 2.33 | 29.80 ± 2.00 | 0.162 |
| C18-0 | 503.91 ± 5.32 c | 566.97 ± 5.52 b | 608.87 ± 8.53 a | p < 0.01 |
| C20-0 | 4.63 ± 0.46 b | 5.99 ± 0.15 a | 6.18 ± 0.19 a | 0.021 |
| SFA | 1117.39 ± 33.11 b | 1290.23 ± 24.02 a | 1350.72 ± 24.26 a | p < 0.01 |
| C14-1 | 7.15 ± 0.78 b | 11.84 ± 1.45 ab | 16.10 ± 2.46 a | 0.028 |
| C16-1 | 46.14 ± 2.94 | 59.63 ± 12.09 | 68.43 ± 5.36 | 0.208 |
| C18-1n9c | 341.73 ± 8.94 b | 366.19 ± 7.24 b | 448.52 ± 7.85 a | p < 0.01 |
| C20-1(cis-11) | 5.52 ± 1.51 | 4.98 ± 0.83 | 4.63 ± 0.41 | 0.832 |
| MUFA | 409.88 ± 8.12 b | 442.64 ± 21.41 b | 537.69 ± 14.65 a | p < 0.01 |
| C22-2 | 5.78 ± 2.32 | 3.20 ± 0.12 | 1.91 ± 0.02 | 0.198 |
| C18-2n6c | 528.39 ± 5.69 c | 655.71 ± 5.06 b | 760.95 ± 3.55 a | p < 0.01 |
| C18-3n3 | 17.69 ± 1.1 | 22.18 ± 2.59 | 22.22 ± 2.77 | 0.339 |
| C18-3n6 | 2.86 ± 0.29 b | 3.75 ± 0.19 a | 3.68 ± 0.05 a | 0.036 |
| C20-2 | 11.28 ± 1.76 | 11.8 ± 0.53 | 12.89 ± 0.69 | 0.618 |
| PUFA | 566.00 ± 6.77 c | 696.64 ± 7.91 b | 801.65 ± 2.94 a | p < 0.01 |
| Items | Groups | ||
|---|---|---|---|
| G0 | G15 | G20 | |
| Feed price, CNY/kg | 2.60 | 2.09 | 2.04 |
| Total intake, kg/head | 492.70 | 532.17 | 562.51 |
| Total feed cost, kg/head | 1281.02 | 1112.24 | 1147.52 |
| Weight gain income, kg/head | 1106.62 | 1245.85 | 1663.73 |
| Beef cattle prices, CNY/kg | 22.50 | 22.50 | 22.50 |
| Gross profit, CNY/kg | −174.4 | 133.61 | 516.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, M.; Li, Y.; Qi, J.; Wu, W.; Sun, X.; Gao, C.; Zhang, X.; Mamtimin, T.; Wan, J. Effects of Grape Pomace Complete Pellet Feed on Growth Performance, Fatty Acid Composition, and Rumen Fungal Composition in Beef Cattle. Animals 2025, 15, 930. https://doi.org/10.3390/ani15070930
Teng M, Li Y, Qi J, Wu W, Sun X, Gao C, Zhang X, Mamtimin T, Wan J. Effects of Grape Pomace Complete Pellet Feed on Growth Performance, Fatty Acid Composition, and Rumen Fungal Composition in Beef Cattle. Animals. 2025; 15(7):930. https://doi.org/10.3390/ani15070930
Chicago/Turabian StyleTeng, Meimei, Yuanqiu Li, Jiangjiao Qi, Wenda Wu, Xinchang Sun, Chengze Gao, Xia Zhang, Tursunay Mamtimin, and Jiangchun Wan. 2025. "Effects of Grape Pomace Complete Pellet Feed on Growth Performance, Fatty Acid Composition, and Rumen Fungal Composition in Beef Cattle" Animals 15, no. 7: 930. https://doi.org/10.3390/ani15070930
APA StyleTeng, M., Li, Y., Qi, J., Wu, W., Sun, X., Gao, C., Zhang, X., Mamtimin, T., & Wan, J. (2025). Effects of Grape Pomace Complete Pellet Feed on Growth Performance, Fatty Acid Composition, and Rumen Fungal Composition in Beef Cattle. Animals, 15(7), 930. https://doi.org/10.3390/ani15070930






