Impact of Iron Supplementation on Growth Performance, Iron Homeostasis and Redox Balance of Suckling Piglets
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets, and Experimental Design
2.2. Blood Sample Collection
2.3. Serum Iron Concentration
2.4. Serum Antioxidant Capacity Detection
2.5. Statistical Analysis
3. Results
3.1. Growth Performance and Diarrhea Incidence
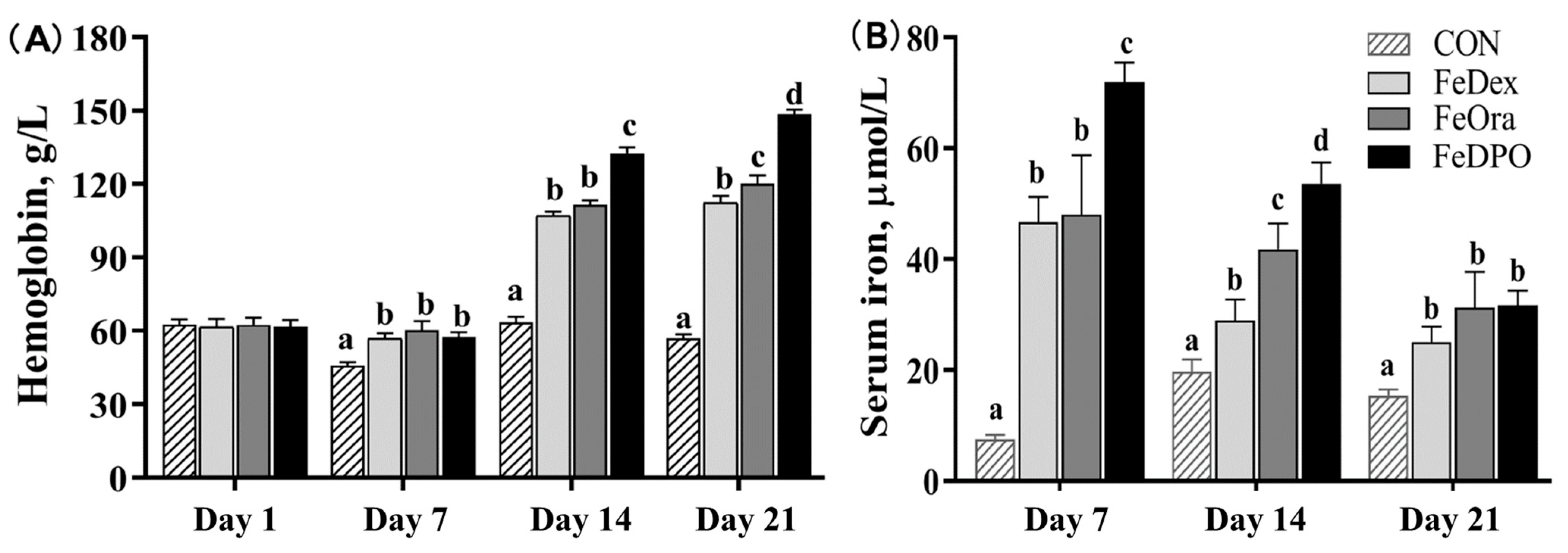
3.2. Blood Biochemical Parameters
3.3. Serum Antioxidant Capacity Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Streyl, K.; Carlstron, J.; Dantos, E.; Mendoza, R.; Islas, J.A.T.; Bhushan, C. Field Evaluation of the Effectiveness of an oral toltrazuril and iron combination (Baycox® Iron) in maintaining weaning weight by preventing coccidiosis and anaemia in neonatal piglets. Parasitol. Res. 2015, 114, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, E.D. Iron and infection. Microbiol. Rev. 1978, 42, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Venn, J.A.J.; Mccance, R.A.; Widdowson, E.M. Iron metabolism in piglet anaemia. J. Comp. Pathol. Ther. 1947, 57, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Committee on Nutrient Requirements of Swine, National Research Council. Nutrient Requirements of Swine, 11th ed.; National Academy Press: Washington, DC, USA, 2012. [Google Scholar]
- Kim, J.C.; Wilcock, P.; Bedford, M.R. Iron status of piglets and impact of phytase superdosing on iron physiology: A review. Anim. Feed Sci. Technol. 2017, 235, 8–14. [Google Scholar] [CrossRef]
- Bauckman, K.A.; Mysorekar, I.U. Ferritinophagy drives uropathogenic Escherichia coli persistence in bladder epithelial cells. Autophagy 2016, 12, 850–863. [Google Scholar] [CrossRef]
- Hallquist, N.A.; Mcneil, I.K.; Lockwood, J.F.; Sherman, A.R. Maternal-iron deficiency effects on peritoneal macrophage and peritoneal natural-killer-cell cytotoxicity in rat pups. Am. J. Clin. Nutr. 1992, 55, 741–746. [Google Scholar] [CrossRef]
- Michels, K.R.; Lambrecht, N.J.; Carson, W.F.; Schaller, M.A.; Lukacs, N.W.; Bermick, J.R. The role of iron in the susceptibility of neonatal mice to Escherichia coli K1 sepsis. J. Infect. Dis. 2019, 220, 1219–1229. [Google Scholar] [CrossRef]
- Sawhney, S.; Bhattacharya, D.; Ponraj, P.; Sunder, J.; Sujatha, T.; Mondal, S.; Chakurkar, E.B.; De, A.K. Control of iron deficiency anaemia in piglets through 2-7-10-15 module of oral iron supplementation. Explor. Anim. Med. Res. 2022, 12, 187–194. [Google Scholar] [CrossRef]
- Pu, Y.T.; Li, S.H.; Xiong, H.T.; Zhang, X.F.; Wang, Y.Z.; Du, H.H. Iron promotes intestinal development in neonatal piglets. Nutrients 2018, 10, 726–737. [Google Scholar] [CrossRef]
- Johnson, E.E.; Wessling-Resnick, M. Iron metabolism and the innate immune response to infection. Microbes Infect. 2012, 14, 207–216. [Google Scholar] [CrossRef]
- Egeli, A.K.; Framstad, T. Evaluation of the efficacy of perorally administered glutamic acid-chelated iron and iron-dextran injected subcutaneously in Duroc and Norwegian Landrace piglets. J. Vet. Med. Ser. A 2010, 45, 53–61. [Google Scholar] [CrossRef]
- Staroń, R.; Van Swelm, R.P.; Lipiński, P.; Gajowiak, A.; Lenartowicz, M.; Bednarz, A.; Gajewska, M.; Pieszka, M.; Laarakkers, C.M.M.; Swinkels, D.W.; et al. Urinary hepcidin levels in iron-deficient and iron-supplemented piglets correlate with hepcidin hepatic mRNA and serum levels and with body iron status. PLoS ONE 2015, 10, e0136695. [Google Scholar] [CrossRef]
- Hu, P.; Zhao, D.Y.; Zhao, F.Z.; Wang, J.; Zhu, W.Y. The effects of the combination of oral lactoferrin and iron injection on iron homestasis, antioxidative abilities and cytokines activities of suckling piglets. Animals 2019, 9, 438–451. [Google Scholar] [CrossRef]
- Dong, Z.L.; Wan, D.; Li, G.Y.; Zhang, Y.M.; Yang, H.S.; Wu, X.; Yin, Y.L. Comparison of oral and parenteral iron administration on iron homeostasis, oxidative and immune status in anemic neonatal pigs. Biol. Trace Elem. Res. 2019, 5, 117–124. [Google Scholar] [CrossRef]
- Glawischnig, E.; Baumgartner, W.; Gewessler, F. Über die Wirkung einer einmaligen oralen eisen-dextran-gabe zur anämieprophylaxe beim saugferkel. Dtsch. Tieraerztliche Wochenschr. 1987, 94, 237–324. [Google Scholar]
- Witschi, F.; Heinritzi, K. Research into the applicability of a per os iron preparation as a prophylaxis against piglet anaemia (iron-deficiency anaemia in sucking pigs). Tierärztl. Prax. 2001, 29, 36–44. [Google Scholar]
- Szabo, P.; Bilkei, G. Iron deficiency in outdoor pig production. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2002, 49, 390–391. [Google Scholar] [CrossRef]
- Peters, J.C.; Mahan, D.C.; Wiseman, T.G.; Fastinger, N.D. Effect of dietary organic and inorganic micromineral source and level on sow body, liver, colostrum, mature milk, and progeny mineral compositions over six parities. J. Anim. Sci. 2010, 88, 626–837. [Google Scholar] [CrossRef]
- Stojanac, N.; Stevancevic, O.; Cincović, M.; Belić1, B.; Plavsa, N.; Urosevic, M. Effects of iron administration method on anemia prevention and production performance of piglets. Acta Sci. Vet. 2016, 44, 1361–1369. Available online: https://www.ufrgs.br/actavet/44/PUB%201361.pdf (accessed on 30 May 2016).
- Chevalier, T.B.; Monegue, J.; Lindemann, M.D. Effects of iron dosage administered to newborn piglets on hematological measures, preweaning and postweaning growth performance, and postweaning tissue mineral content. J. Swine Health Prod. 2021, 29, 189–199. [Google Scholar] [CrossRef]
- Thanner, S.; Gutzwiller, A. Effects of a reduced dose of injected iron on health, iron status and growth of suckling piglets with access to iron enriched soil. Schweiz. Arch. Tierheilkd. 2018, 160, 123–127. [Google Scholar] [CrossRef]
- Zimmerman, D.R. Iron in swine nutrition. In National Feed Ingredients Association Literature Review on Iron in Animal and Poultry Nutrition; National Feed Ingredients Association: West Des Moines, IA, USA, 1980. [Google Scholar]
- Murphy, K.A.; Friendship, R.M.; Dewey, C.E. Effects of weaning age and dosage of supplemented iron on the hemoglobin concentrations and growth rate of piglets. J. Swine Health Prod. 1997, 5, 135–138. Available online: http://www.aasp.org/shap/issues/v5n4/index.html (accessed on 15 August 1997).
- Bryszewska, M.A.; Laghi, L.; Zannoni, A.; Gianotti, A.; Barone, F.; Saa, D.L.T.; Bacci, M.L.; Ventrella, D.; Forni, M. Bioavailability of microencapsulated iron from fortified bread assessed using piglet model. Nutrients 2017, 9, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Maes, D.; Steyaert, M.; Vanderhaeghe, C.; López Rodríguez, A.; de Jong, E.; Del Pozo Sacristán, R.; Vangroenweghe, F.; Dewulf, J. Comparison of oral versus parenteral iron supplementation on the health and productivity of piglets. Vet. Rec. 2011, 168, 168–188. [Google Scholar] [CrossRef]
- Egeli, A.K.; Framstad, T. Effect of an oral starter dose of iron on haematology and weight gain in piglets having voluntary access to glutamic acid-chelated iron solution. Acta Vet. Scand. 1998, 39, 359–365. [Google Scholar] [CrossRef]
- Iben, B. Importance of oral iron supplementation in piglets in the first hours of life. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 1998, 26, 36–40. [Google Scholar] [PubMed]
- Fernández-Menéndez, S.; Fernández-Sánchez, M.L.; González-Iglesias, H.; Fernández-Colomer, B.; López-Sastre, J.; Sanz-Medel, A. Iron bioavailability from supplemented formula milk: Effect of lactoferrin addition. Eur. J. Nutr. 2016, 56, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Pomorska-Mól, M.; Markowska-Daniel, I. Influence of iron and immunomodulators on growth performance and immunohematological status of piglets. Cent. Eur. J. Immunol. 2010, 35, 63–68. [Google Scholar]
- Churio, O.; Durán, E.; Guzmán-Pino, S.A.; Valenzuela, C. Use of encapsulation technology to improve the efficiency of an iron oral supplement to prevent anemia in suckling pigs. Animals 2018, 20, 1. [Google Scholar] [CrossRef]
- Purnamasidhi, C.A.W.; Suega, K.; Bakta, I.M. Role of Red Cell Distribution Width (RDW) in the diagnosis of iron deficiency anemia. Indones. J. Biomed. Sci. 2019, 13, 12–15. [Google Scholar] [CrossRef]
- Chandra, H.; Chandra, S.; Rawat, A.; Verma, S.K. Role of mean platelet volume as discriminating guide for bone marrow disease in patients with thrombocytopenia. Int. J. Lab. Hematol. 2010, 32, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xie, Y.F. Correlation analysis of iron deficiency anemia with PLT, MPV and PDW. J. Yanan Univ. 2013, 11, 55–56. [Google Scholar]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. Available online: https://www.nature.com/articles/35041687 (accessed on 9 November 2000). [PubMed]

| CON | FeDex | FeOra | FeDPO | p | |
|---|---|---|---|---|---|
| Bodyweight, kg | |||||
| Day 1 | 1.53 ± 0.07 | 1.50 ± 0.07 | 1.58 ± 0.06 | 1.49 ± 0.06 | 0.75 |
| Day 7 | 2.82 ± 0.13 | 2.83 ± 0.14 | 2.60 ± 0.11 | 2.63 ± 0.11 | 0.37 |
| Day 14 | 4.00 ± 0.19 | 4.17 ± 0.26 | 4.29 ± 0.26 | 4.59 ± 0.22 | 0.33 |
| Day 21 | 5.25 ± 0.22 a | 5.71 ± 0.31 ab | 5.79 ± 0.33 ab | 6.30 ± 0.29 b | 0.04 |
| Diarrhea index | |||||
| Day 1–7 | 0.02 ± 0.02 | 0.00 ± 0.00 | 0.03 ± 0.03 | 0.00 ± 0.00 | 0.59 |
| Day 8–14 | 0.22 ± 0.10 | 0.11 ± 0.01 | 0.17 ± 0.13 | 0.07 ± 0.04 | 0.67 |
| Day 15–21 | 0.18 ± 0.05 | 0.15 ± 0.02 | 0.15 ± 0.05 | 0.18 ± 0.07 | 0.89 |
| Day 1–21 | 0.14 ± 0.07 | 0.09 ± 0 01 | 0.11 ± 0.06 | 0.08 ± 0.03 | 0.82 |
| CON | FeDex | FeOra | FeDPO | p | |
|---|---|---|---|---|---|
| PND 14 | |||||
| WBC, 109/L | 9.62 ± 0.99 | 11.55 ± 1.16 | 9.90 ± 0.88 | 10.52 ± 0.65 | 0.48 |
| RBC, 1012/L | 4.26 ± 0.14 a | 5.24 ± 0.18 b | 5.68 ± 0.25 b | 5.60 ± 0.10 b | <0.01 |
| HCT, % | 21.90 ± 0.82 a | 36.42 ± 1.38 b | 37.87 ± 1.04 b | 42.68 ± 0.56 c | <0.01 |
| MCV, fL | 51.53 ± 0.73 a | 69.59 ± 1.35 b | 67.58 ± 2.30 b | 76.38 ± 1.14 c | <0.01 |
| MCH, pg | 15.29 ± 0.24 a | 22.16 ± 0.4 b | 20.03 ± 0.5 b | 24.46 ± 0.36 c | <0.01 |
| MCHC, g/L | 298.1 ± 4.05 a | 319.2 ± 0.96 b | 312.64 ± 3.76 b | 320.73 ± 1.42 b | <0.01 |
| RDW, % | 38.11 ± 0.34 b | 18.31 ± 0.42 a | 19.19 ± 0.57 a | 19.16 ± 0.41 a | <0.01 |
| PLT, 109/L | 781.63 ± 26.79 b | 614 ± 25.56 a | 645.25 ± 50.05 a | 669.56 ± 31.71 a | 0.01 |
| MPV, fL | 7.98 ± 0.21 a | 9.49 ± 0.17 b | 8.86 ± 0.18 b | 8.97 ± 0.08 b | <0.01 |
| PDW | 15.65 ± 0.09 a | 17.35 ± 0.11 b | 17.5 ± 0.19 b | 17.69 ± 0.07 b | <0.01 |
| PCT, % | 0.58 ± 0.02 b | 0.59 ± 0.02 b | 0.49 ± 0.02 a | 0.58 ± 0.02 b | 0.01 |
| PND 21 | |||||
| WBC, 109/L | 9.76 ± 0.82 a | 12.79 ± 1.96 ab | 18.51 ± 1.67 b | 16.6 ± 1.57 b | <0.01 |
| RBC, 1012/L | 4.03 ± 0.19 a | 5.57 ± 0.10 b | 6.13 ± 0.13 c | 6.20 ± 0.13 c | <0.01 |
| HCT, % | 18.09 ± 0.64 a | 34.28 ± 1.04 b | 36.49 ± 1.13 b | 43.56 ± 0.56 c | <0.01 |
| MCV, fL | 45.45 ± 1.27 a | 61.51 ± 1.46 b | 59.73 ± 1.78 b | 70.54 ± 1.33 c | <0.01 |
| MCH, pg | 14.42 ± 0.77 a | 19.39 ± 0.37 b | 19.18 ± 0.64 b | 23.08 ± 0.36 c | <0.01 |
| MCHC, g/L | 299.14 ± 3.01 a | 322.8 ± 2.29 b | 321.58 ± 3.19 b | 328.00 ± 2.75 b | <0.01 |
| RDW, % | 36.47 ± 0.60 c | 20.23 ± 0.85 b | 19.67 ± 0.90 b | 17.24 ± 0.42 a | <0.01 |
| PLT, 109/L | 1232.4 ± 295.44 c | 715.88 ± 33.26 a | 857.83 ± 54.42 ab | 803.4 ± 78.98 ab | <0.01 |
| MPV, fL | 6.87 ± 0.10 a | 8.55 ± 0.26 b | 8.20 ± 0.15 b | 8.75 ± 0.12 b | <0.01 |
| PDW | 16.06 ± 0.33 a | 17.19 ± 0.19 b | 16.95 ± 0.17 b | 17.72 ± 0.10 b | <0.01 |
| PCT, % | 0.46 ± 0.04 a | 0.60 ± 0.03 b | 0.60 ± 0.03 b | 0.51 ± 0.08 ab | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Q.; Wu, Q.; Zhou, Q.; Tang, J.; Zhuo, Y.; Fang, Z.; Lin, Y.; Xu, S.; Feng, B.; Hua, L.; et al. Impact of Iron Supplementation on Growth Performance, Iron Homeostasis and Redox Balance of Suckling Piglets. Animals 2025, 15, 924. https://doi.org/10.3390/ani15070924
Meng Q, Wu Q, Zhou Q, Tang J, Zhuo Y, Fang Z, Lin Y, Xu S, Feng B, Hua L, et al. Impact of Iron Supplementation on Growth Performance, Iron Homeostasis and Redox Balance of Suckling Piglets. Animals. 2025; 15(7):924. https://doi.org/10.3390/ani15070924
Chicago/Turabian StyleMeng, Qingwei, Qing Wu, Qiang Zhou, Jiayong Tang, Yong Zhuo, Zhengfeng Fang, Yan Lin, Shengyu Xu, Bin Feng, Lun Hua, and et al. 2025. "Impact of Iron Supplementation on Growth Performance, Iron Homeostasis and Redox Balance of Suckling Piglets" Animals 15, no. 7: 924. https://doi.org/10.3390/ani15070924
APA StyleMeng, Q., Wu, Q., Zhou, Q., Tang, J., Zhuo, Y., Fang, Z., Lin, Y., Xu, S., Feng, B., Hua, L., Jiang, X., Wu, D., & Che, L. (2025). Impact of Iron Supplementation on Growth Performance, Iron Homeostasis and Redox Balance of Suckling Piglets. Animals, 15(7), 924. https://doi.org/10.3390/ani15070924











