Comparative Analysis of Short-Chain Fatty Acids and the Immune Barrier in Cecum of Dahe Pigs and Dahe Black Pigs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Sample Collection
2.2. Test Diet
2.3. Detection of SCFAs in Cecal Contents
2.4. Detection of the Expression Levels of Immune Genes Related to SCFAs
2.5. Functional Annotation of SCFAs Immune Genes
2.6. Statistic Analysis
3. Results
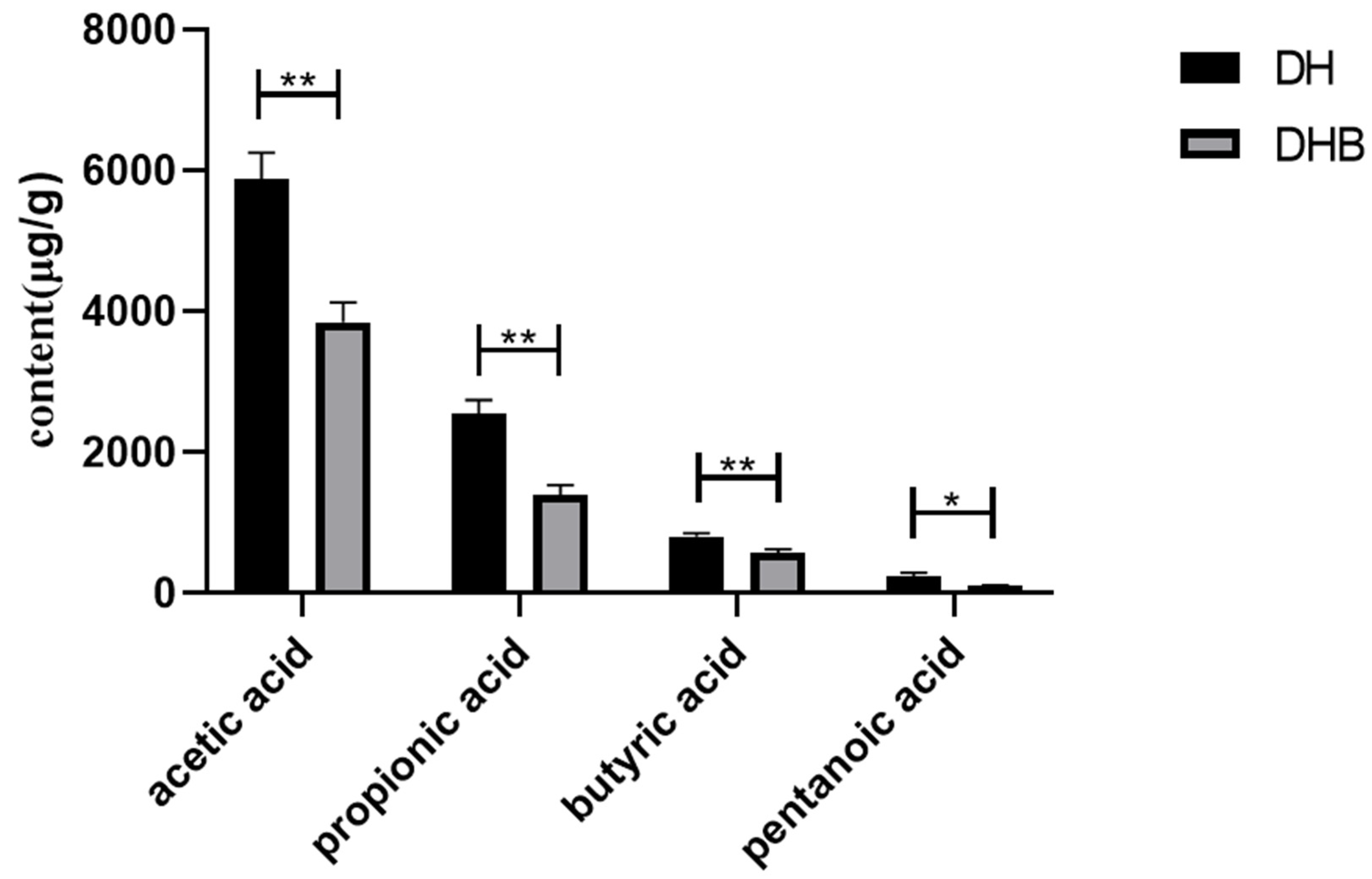
3.1. Content of Short Chain Fatty Acids in Cecum Tissue
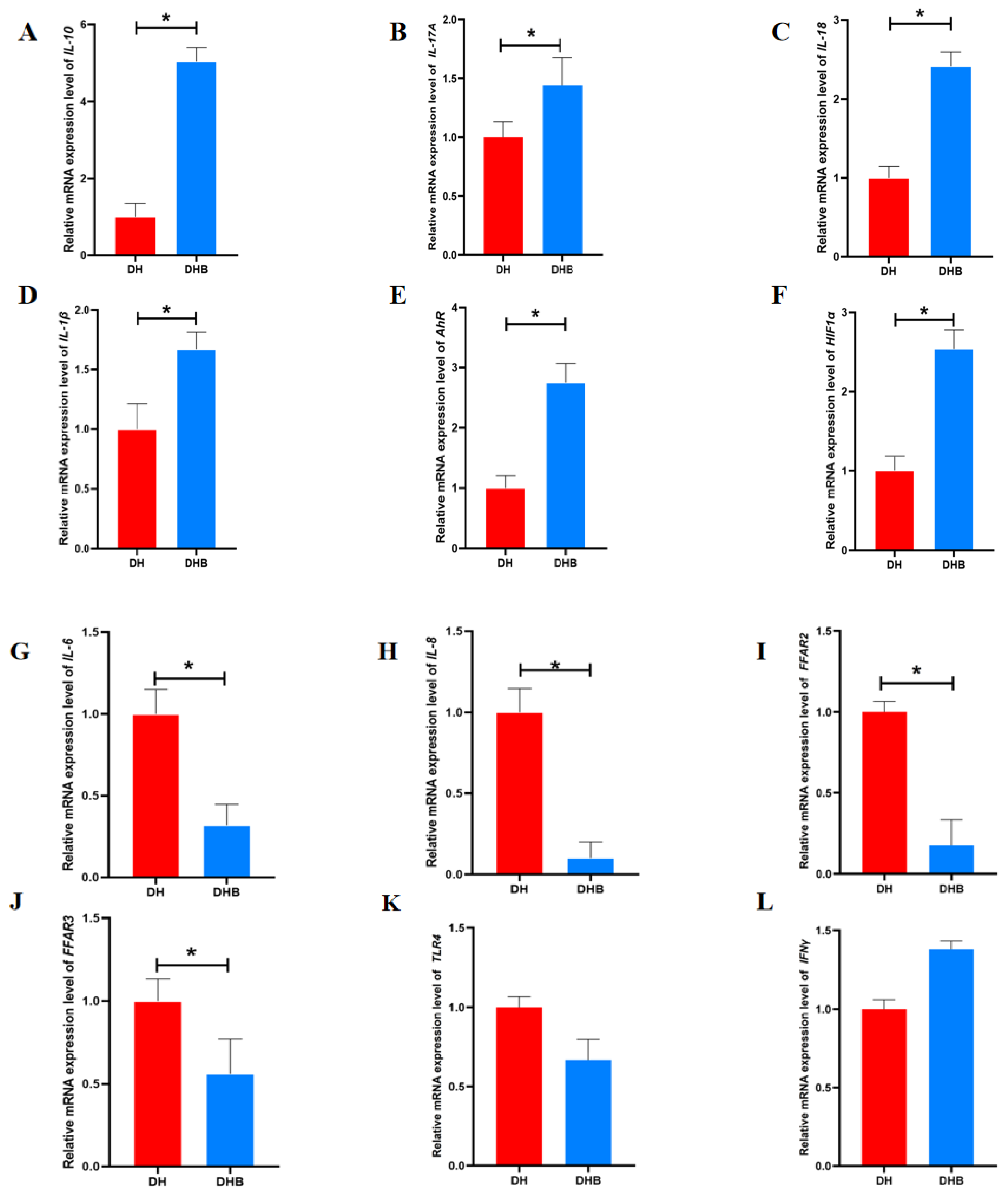
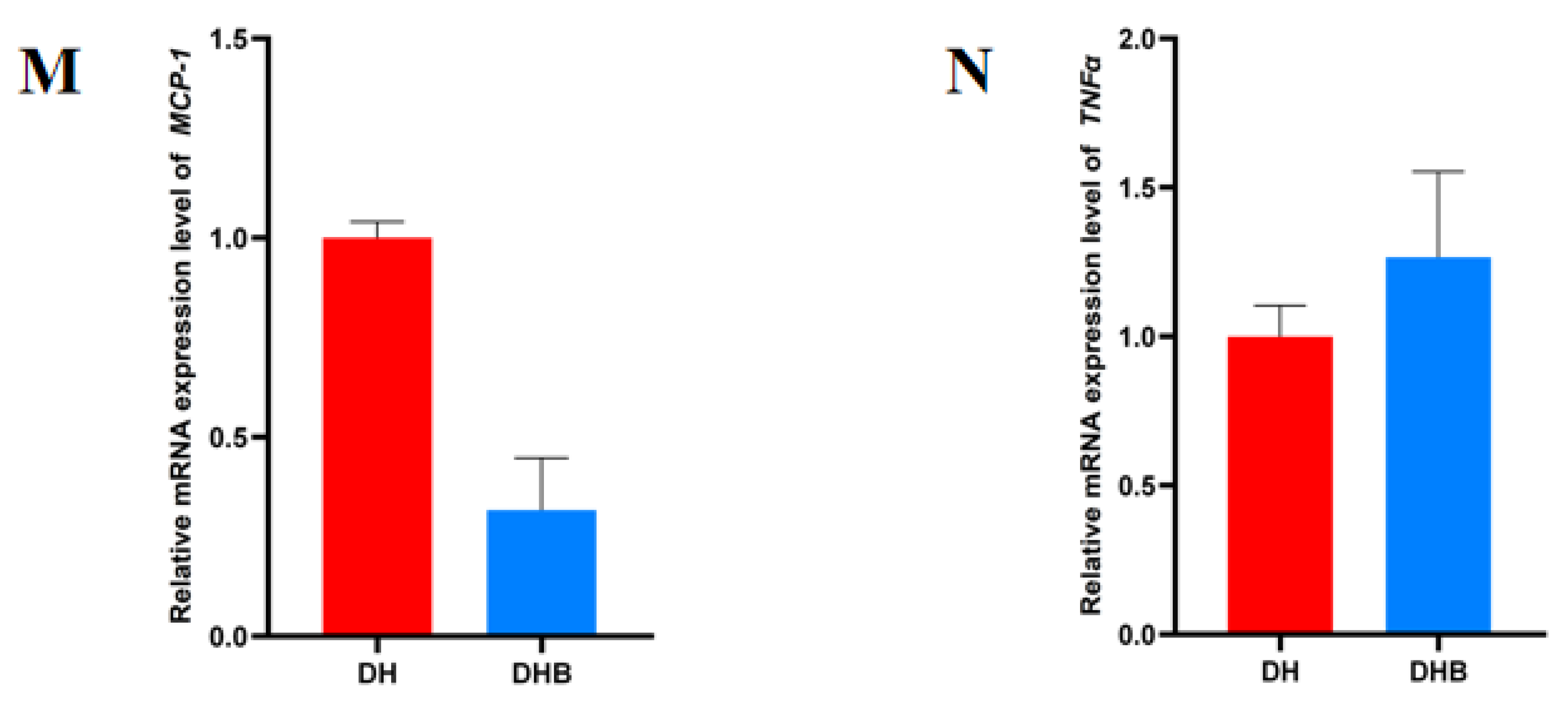
3.2. The mRNA Expression Levels of Immune Genes Associated with SCFAs in Cecum Tissue
3.3. Go Functional Annotation of Short-Chain Fatty Acid Immune Genes in Cecum Tissue
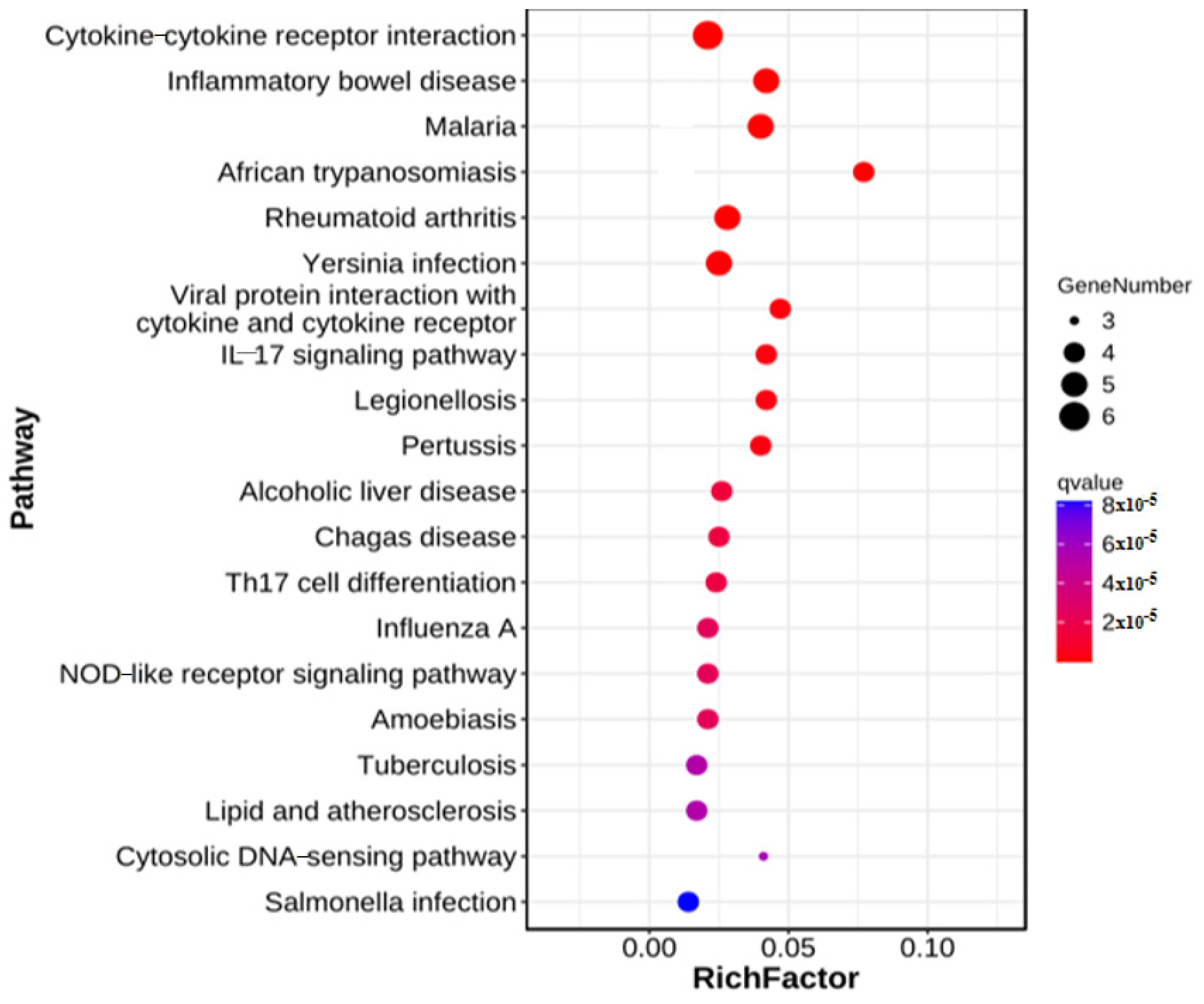
3.4. KEGG Functional Annotation of Short-Chain Fatty Acid Immune Genes in Cecum Tissue
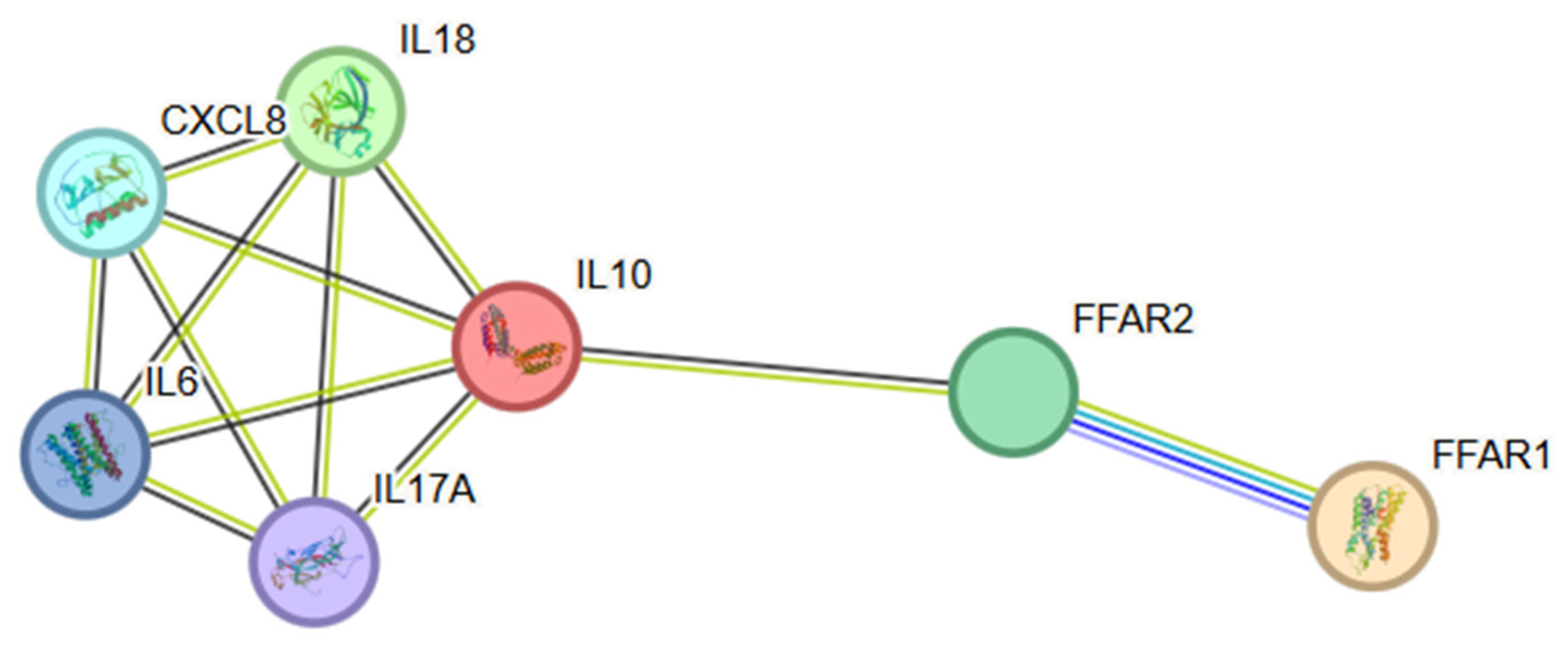
3.5. PPI Network Construction and Hub Gene Identification
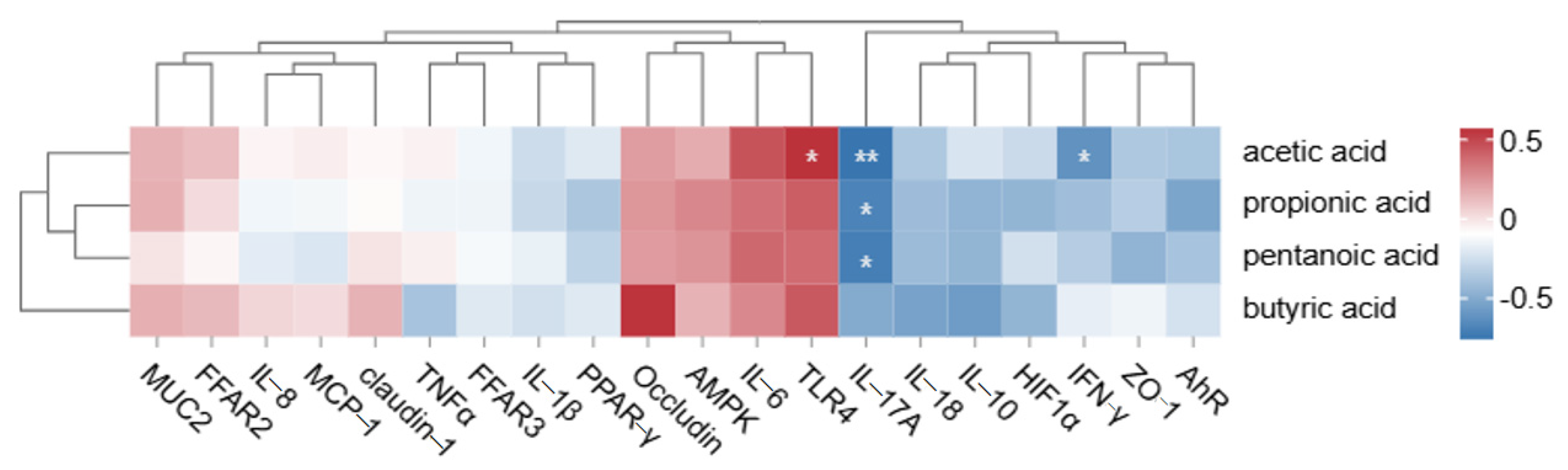
3.6. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Composition and Nutrient Levels of Basal Diets (Air-Dry Basis)
| Item | BW (kg) | |
| 30~60 | 60~120 | |
| Raw material (%) | ||
| Corn | 64.21 | 61.50 |
| Soybean meal | 16.11 | 15.00 |
| Rice bran | 10.00 | 10.00 |
| Wheat bran | 7.10 | 10.00 |
| Stone powder | 1.08 | 2.00 |
| Premix | 1.00 | 1.00 |
| Calcium hydrogen phosphate | 0.32 | 0.00 |
| Salt | 0.18 | 0.50 |
| Total | 100 | 100 |
| Nutrient level | ||
| pigs digestive energy (MJ/kg) | 13.81 | 13.51 |
| Crude protein * | 15.17 | 14.85 |
| Lysine * | 0.63 | 0.55 |
| Calcium * | 0.55 | 0.81 |
| Total Phosphorus * | 0.53 | 0.49 |
| (1) Meat fat type growth and finishing pigs (30~60 kg) premix provides the following: vitamin A, 5000 IU; vitamin D3, 180 IU; vitamin E, 15 mg; vitamin K3, 0.50 mg; thiamine vitamin B1, 1.50 mg; riboflavin vitamin B2, 2.50 mg; niacin VPP, 12.00 mg; pantothenic acid, B5 9.00 mg; pyridoxine B6, 1.00 mg; biotin VH, 0.08 mg; folic acid, 0.30 mg; vitamin B12, 10.00 mg; choline, 350 mg; iron, 148.34 mg; copper, 199.50 mg; manganese, 113.50 mg; zinc, 65.00 mg; iodine, 0.15 mg; selenium, 0.25 mg. Meat fat type growth and finishing pigs (60~100 kg) provides the following: vitamin A, 5000 IU; vitamin D3, 160 IU; vitamin E, 15 mg; vitamin K3, 0.50 mg; thiamin B1, 1.50 mg; riboflavin B2, 2.50 mg; niacin Vpp, 9.00 mg; pantothenic acid B5, 8.00 mg; pyridoxine B6, 1 mg; biotin VH, 0.08 mg per kg of diet; folic acid, 0.30 mg; vitamin B12, 6.00 mg; choline, 0.40 g; iron, 258.56 mg; copper, 199.50 mg; manganese, 113.50 mg; zinc, 55.00 mg; iodine, 0.15 mg; selenium, 0.25 mg. (2) * is the measured value. | ||
Appendix B. The Primer Sequence and Annealing Temperature of the Target Gene and Internal Reference Gene
| Genes | Primer Sequences (5′-3′) | Annealing Temperature |
| 18S rRNA | F: GTCCACTGGTGTCTTCACGA R: GCTGACGATCTTGAGGGAGT | 57.5 °C |
| FFAR2 | F: CGTGTTCATCGTTCAGTA R: GAAGTTCTCATAGCAGGTA | 51.5 °C |
| FFAR3 | F: TGGAGACCTTACGTGTTG R: CGAGGATGAGAAGTAGTAGAT | 57 °C |
| IL-1β | F: CCCAAAAGTTACCCGAAGAGG R: TCTGCTTGAGAGGTGCTGATG | 57 °C |
| IL-6 | F: GGACGCCTGAAGAAGAT R: TGAACCCAATTGGAAGC | 51.5 °C |
| CXCL8 | F: ACTGGCTGTTGCCTTCTT R: CAGTTCTCTTCAAAAATATCTG | 57 °C |
| IL-10 | F: GCTGAAGACCCTCAGGCTGA R: TTGCTCTTGCTTCTCCACT | 59 °C |
| IL-17A | F: CAGATTACTCCAAACGCTTCACC R: GCAGCCCACTGTCACCATCACTT | 57 °C |
| IL-18 | F: GCAGTAACCATCTCTGTGCAGTGTA R: TCATCAATATTATCAGGAGGACTCATTT | 59 °C |
| TNFα | F: ACCACGCTCTTCTGCCTACTGC R: TCCCTCGGCTTTGACATTGGCTAC | 61 °C |
| TLR4 | F: ACCAGACTTTCTTGCAGTGGGTCA R: AATGACGGCCTCGCTTATCTGACA | 51.5 °C |
| IFN-γ | F: GGCCATTCAAAGGAGCATGG R: AGTTCACTGATGGCTTTGCG | 51.5 °C |
| MCP-1 | F: CAGCCCTCCTGTGCCT R: TTCAAGGCTTCGGAGTTT | 51.5 °C |
| AhR | F: ACTACCACCCATCTTCACCCG R: CAACACACATCAATGCTTCCC | 57 °C |
| HIF1α | F: CATCAGTTGCCACTTCCCCAT R: CAAAACCATCCAAGGCTTTCA | 57 °C |
References
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, S.X.; Li, M.L.; Yan, D.W.; Ge, C.R. Germplasm Characteristics, Conservation and Diversified Utilization of Yunnan Local pigs Breeds. J. Yunnan Agric. Univ. 2020, 35, 1096–1105. [Google Scholar] [CrossRef]
- Jiao, X.H. Research Status of the Development of Wujin pigs. Swine Ind. Sci. 2023, 40, 118–120. [Google Scholar] [CrossRef]
- Miao, X.H.; Wu, L.F.; Zhu, R.; Shen, Y.C.; Zhang, M.D.; Gao, C.G.; Han, M.; Chen, K.K.; Cao, X.Y.; Wei, B.; et al. Practice and Thinking on the Conservation and Utilization of Genetic Resources of Main Livestock Breeds in Qujing City. Yunnan J. Anim. Sci. Vet. Med. 2021, 4, 41–44. [Google Scholar] [CrossRef]
- Sivaprakasam, S.; Gurav, A.; Paschall, A.V.; Coe, G.L.; Chaudhary, K.; Cai, Y.; Kolhe, R.; Martin, P.; Browning, D.; Huang, L.; et al. An essential role of Ffar2 (Gpr43) in dietary fibre-mediated promotion of healthy composition of gut microbiota and suppression of intestinal carcinogenesis. Oncogenesis 2016, 5, e238. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef] [PubMed]
- Taggart, A.K.; Kero, J.; Gan, X.; Cai, T.Q.; Cheng, K.; Ippolito, M.; Ren, N.; Kaplan, R.; Wu, K.; Wu, T.J.; et al. (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J. Biol. Chem. 2005, 280, 26649–26652. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.M.; de Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Chen, Y.; Ma, Z.; Zhang, X.; Shi, D.; Khan, J.A.; Liu, H. Gut microbiota-derived short chain fatty acids are potential mediators in gut inflammation. Anim. Nutr. 2021, 8, 350–360. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frampton, J.; Murphy, K.G.; Frost, G.; Chambers, E.S. Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat. Metab. 2020, 2, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, X.W.; Shuai, S.R.; Li, M.Z.; Chen, L. Analysis of Muscle Nutrient Components of Dahe pigs and Dahe Black pigs. Chin. J. Anim. Husb. 2008, 7, 6–9. [Google Scholar]
- Li, W.Z.; Zhao, S.M.; Huang, Y.; Yang, M.H.; Pan, H.B.; Zhang, X.; Ge, C.R.; Gao, S.Z. Expression of lipogenic genes during porcine intramuscular preadipocyte differentiation. Res. Vet. Sci. 2012, 93, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Y.; Wang, J.; Huang, Y.; Pan, H.B.; Zhang, X.; Huang, Z.X.; Zhao, S.M.; Gao, S.Z. The effect of dietary protein levels on the expression of genes coding for four selected protein translation initiation factors in muscle tissue of Wujin pigs. J. Anim. Physiol. Anim. Nutr. 2014, 98, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.D.; Li, S.F.; Li, X. The Effect of the Conservation and Breeding Process of Dahe pigs on Carcass Quality and Meat Chemical Composition. Swine Prod. 2014, 1, 47–48. [Google Scholar]
- Shin, Y.; Han, S.; Kwon, J.; Ju, S.; Choi, T.G.; Kang, I.; Kim, S.S. Roles of Short-Chain Fatty Acids in Inflammatory Bowel Disease. Nutrients 2023, 15, 4466. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, N.; Gao, Y.; Zhu, W.; Meng, D.; Walker, W.A. Short chain fatty acids produced by colonizing intestinal commensal bacterial interaction with expressed breast milk are anti-inflammatory in human immature enterocytes. PLoS ONE 2020, 15, e0229283. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450, Erratum in Nature 2014, 506, 254. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, Y.M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Luu, M.; Pautz, S.; Kohl, V.; Singh, R.; Romero, R.; Lucas, S.; Hofmann, J.; Raifer, H.; Vachharajani, N.; Carrascosa, L.C.; et al. The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigsenetic crosstalk in lymphocytes. Nat. Commun. 2019, 10, 760. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kasubuchi, M.; Hasegawa, S.; Hiramatsu, T.; Ichimura, A.; Kimura, I. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients 2015, 7, 2839–2849. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chambers, E.S.; Morrison, D.J.; Frost, G. Control of appetite and energy intake by SCFAs: What are the potential underlying mechanisms? Proc. Nutr. Soc. 2015, 74, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Hao, M.; Zhu, J.; Yi, L.; Cheng, W.; Xie, Y.; Zhao, S. Comparison of differentially expressed genes in longissimus dorsi muscle of Diannan small ears, Wujin and landrace pigs using RNA-seq. Front. Vet. Sci. 2024, 10, 1296208. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miller, T.L.; Wolin, M.J. Pathways of acetate, propionate, and butyrate formation by the human fecal microbial flora. Appl. Env. Microbiol. 1996, 62, 1589–1592. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang, P.V.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meng, H.; Xu, D.; Wang, Q.; Liu, L.; Liu, W.; Wang, J. Maintaining immune homeostasis with Coptis Chinensis water extract to mitigate sepsis severity via modulating gut microbiome and metabolism. J. Pharm. Biomed. Anal. 2023, 236, 115719. [Google Scholar] [CrossRef] [PubMed]
- Madella, A.M.; Van Bergenhenegouwen, J.; Garssen, J.; Masereeuw, R.; Overbeek, S.A. Microbial-Derived Tryptophan Catabolites, Kidney Disease and Gut Inflammation. Toxins 2022, 14, 645. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meynier, M.; Baudu, E.; Rolhion, N.; Defaye, M.; Straube, M.; Daugey, V.; Modoux, M.; Wawrzyniak, I.; Delbac, F.; Villéger, R.; et al. AhR/IL-22 pathway as new target for the treatment of post-infectious irritable bowel syndrome symptoms. Gut. Microbes. 2022, 14, 2022997. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abdulla, O.A.; Neamah, W.; Sultan, M.; Alghetaa, H.K.; Singh, N.; Busbee, P.B.; Nagarkatti, M.; Nagarkatti, P. The Ability of AhR Ligands to Attenuate Delayed Type Hypersensitivity Reaction Is Associated with Alterations in the Gut Microbiota. Front. Immunol. 2021, 12, 684727. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, S.; Wang, J.; Song, X.; Zhang, X.; Ge, C.; Gao, S. Impact of dietary protein on lipid metabolism-related gene expression in porcine adipose tissue. Nutr. Metab. 2010, 7, 6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kalina, U.; Koyama, N.; Hosoda, T.; Nuernberger, H.; Sato, K.; Hoelzer, D.; Herweck, F.; Manigold, T.; Singer, M.V.; Rossol, S.; et al. Enhanced production of IL-18 in butyrate-treated intestinal epithelium by stimulation of the proximal promoter region. Eur. J. Immunol. 2002, 32, 2635–2643. [Google Scholar] [CrossRef] [PubMed]
- Qamar, A.; Waheed, J.; Hamza, A.; Mohyuddin, S.G.; Lu, Z.; Namula, Z.; Chen, Z.; Chen, J. The role of intestinal microbiota in chicken health, intestinal physiology and immunity. JAPS: J. Anim. Plant Sci. 2021, 31, 342–351. [Google Scholar] [CrossRef]
- Wells, J.M.; Brummer, R.J.; Derrien, M.; MacDonald, T.T.; Troost, F.; Cani, P.D.; Theodorou, V.; Dekker, J.; Méheust, A.; de Vos, W.M.; et al. Homeostasis of the gut barrier and potential biomarkers. Am. J. Physiol. Gastrointest Liver. Physiol. 2017, 312, G171–G193. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gou, H.Z.; Zhang, Y.L.; Ren, L.F.; Li, Z.J.; Zhang, L. How do intestinal probiotics restore the intestinal barrier? Front. Microbiol. 2022, 13, 929346. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fukata, M.; Vamadevan, A.S.; Abreu, M.T. Toll-like receptors (TLRs) and Nod-like receptors (NLRs) in inflammatory disorders. Semin. Immunol. 2009, 21, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, N.; Mizote, K.; Honda, H.; Saeki, A.; Watanabe, Y.; Yamaguchi-Miyamoto, T.; Fukui, R.; Tanimura, N.; Motoi, Y.; Akashi-Takamura, S.; et al. Funiculosin variants and phosphorylated derivatives promote innate immune responses via the Toll-like receptor 4/myeloid differentiation factor-2 complex. J. Biol. Chem. 2017, 292, 15378–15394. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ryu, J.K.; Kim, S.J.; Rah, S.H.; Kang, J.I.; Jung, H.E.; Lee, D.; Lee, H.K.; Lee, J.O.; Park, B.S.; Yoon, T.Y.; et al. Reconstruction of LPS Transfer Cascade Reveals Structural Determinants within LBP, CD14, and TLR4-MD2 for Efficient LPS Recognition and Transfer. Immunity 2017, 46, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dong, B.; Feng, Z.; Yu, S.; Bao, Y. A study on immunomodulatory mechanism of Polysaccharopeptide mediated by TLR4 signaling pathway. BMC Immunol. 2015, 16, 34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoon, Y.D.; Han, S.B.; Kang, J.S.; Lee, C.W.; Park, S.K.; Lee, H.S.; Kang, J.S.; Kim, H.M. Toll-like receptor 4-dependent activation of macrophages by polysaccharide isolated from the radix of Platycodon grandiflorum. Int. Immunopharmacol. 2003, 3, 1873–1882. [Google Scholar] [CrossRef] [PubMed]
- Ando, I.; Tsukumo, Y.; Wakabayashi, T.; Akashi, S.; Miyake, K.; Kataoka, T.; Nagai, K. Safflower polysaccharides activate the transcription factor NF-kappa B via Toll-like receptor 4 and induce cytokine production by macrophages. Int. Immunopharmacol. 2002, 2, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Hua, K.F.; Lin, C.C.; Lin, C.H.; Hsu, J.; Wong, C.H. Extract of Reishi polysaccharides induces cytokine expression via TLR4-modulated protein kinase signaling pathways. J. Immunol. 2004, 173, 5989–5999. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Chen, L.; Liu, Y.; Yang, J.; Ma, C.; Yao, Z.; Yang, L.; Wei, L.; Li, M. Enhancement of the innate immune response of bladder epithelial cells by Astragalus polysaccharides through upregulation of TLR4 expression. Biochem. Biophys. Res. Commun. 2010, 397, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Zhang, H.; Li, C.F.; Meng, S.D. Research progress in the regulation of Tregs function by toll-like receptor pathway. J. Bioeng. 2020, 36, 1071–1712. [Google Scholar] [CrossRef]
- Jun, J.C.; Jones, M.B.; Oswald, D.M.; Sim, E.S.; Jonnalagadda, A.R.; Kreisman, L.S.C.; Cobb, B.A. T cell-intrinsic TLR2 stimulation promotes IL-10 expression and suppressive activity by CD45RbHi T cells. PLoS ONE 2017, 12, e0180688. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Netea, M.G.; van de Veerdonk, F.L.; Kullberg, B.J.; Van der Meer, J.W.; Joosten, L.A. The role of NLRs and TLRs in the activation of the inflammasome. Expert Opin. Biol. Ther. 2008, 8, 1867–1872. [Google Scholar] [CrossRef] [PubMed]
- Li, X.K.; Yang, X.; Zhang, X.; Zheng, N. Research progress on protective effects of short-chain fatty acids on intestinal barrier. Chin. J. Anim. Nutr. 2024, 36, 4861–4871. [Google Scholar]
- Sadler, R.; Cramer, J.V.; Heindl, S.; Kostidis, S.; Betz, D.; Zuurbier, K.R.; Northoff, B.H.; Heijink, M.; Goldberg, M.P.; Plautz, E.J.; et al. Short-Chain Fatty Acids Improve Poststroke Recovery via Immunological Mechanisms. J. Neurosci. 2020, 40, 1162–1173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ragsdale, S.W.; Pierce, E. Acetogenesis and the Wood-Ljungdahl pathway of CO(2) fixation. Biochim. Biophys. Acta. 2008, 1784, 1873–1898. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, M.; Wu, W.; Liu, Z.; Cong, Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 2017, 52, 1–8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Duncan, S.H.; McCrae, S.I.; Millar, J.; Jackson, M.S.; Flint, H.J. Restricted distribution of the butyrate kinase pathway among butyrate-producing bacteria from the human colon. J. Bacteriol. 2004, 186, 2099–2106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Merli, G.; Becci, A.; Amato, A.; Beolchini, F. Acetic acid bioproduction: The technological innovation change. Sci. Total. Environ. 2021, 798, 149292. [Google Scholar] [CrossRef] [PubMed]
- Bedford, A.; Gong, J. Implications of butyrate and its derivatives for gut health and animal production. Anim. Nutr. 2018, 4, 151–159. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tap, J.; Furet, J.P.; Bensaada, M.; Philippe, C.; Roth, H.; Rabot, S.; Lakhdari, O.; Lombard, V.; Henrissat, B.; Corthier, G.; et al. Gut microbiota richness promotes its stability upon increased dietary fibre intake in healthy adults. Environ. Microbiol. 2015, 17, 4954–4964. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Nusbaum, O.; Chen, X.; Zhu, Y. Valeric Acid Suppresses Liver Cancer Development by Acting as a Novel HDAC Inhibitor. Mol. Ther. Oncolytics. 2020, 19, 8–18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, Y.; Dong, J.; Xiao, H.; Zhang, S.; Wang, B.; Cui, M.; Fan, S. Gut commensal derived-valeric acid protects against radiation injuries. Gut Microbes 2020, 11, 789–806. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiao, A.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Luo, Y.; Luo, J.; Yan, H.; Wang, Q.; Wang, H.; et al. Sodium acetate, propionate, and butyrate reduce fat accumulation in mice via modulating appetite and relevant genes. Nutrition 2021, 87–88, 111198. [Google Scholar] [CrossRef] [PubMed]
- Haghikia, A.; Zimmermann, F.; Schumann, P.; Jasina, A.; Roessler, J.; Schmidt, D.; Heinze, P.; Kaisler, J.; Nageswaran, V.; Aigner, A.; et al. Propionate attenuates atherosclerosis by immune-dependent regulation of intestinal cholesterol metabolism. Eur. Heart J. 2022, 43, 518–533. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dang, C.S.; Zhao, K.X.; Li, H.W.; Zhu, H.; Wang, X.; Wang, S.J. Research progress on the correlation between intestinal short-chain fatty acids and type 2 diabetes mellitus. Chin. J. Microecol. 2021, 33, 1471–1475. [Google Scholar]
- Liu, J.; Li, H.; Gong, T.; Chen, W.; Mao, S.; Kong, Y.; Yu, J.; Sun, J. Anti-neuroinflammatory Effect of Short-Chain Fatty Acid Acetate against Alzheimer’s Disease via Upregulating GPR41 and Inhibiting ERK/JNK/NF-κB. J. Agric. Food Chem. 2020, 68, 7152–7161. [Google Scholar] [CrossRef] [PubMed]
- Dahlstrand Rudin, A.; Khamzeh, A.; Venkatakrishnan, V.; Basic, A.; Christenson, K.; Bylund, J. Short chain fatty acids released by Fusobacterium nucleatum are neutrophil chemoattractants acting via free fatty acid receptor 2 (FFAR2). Cell. Microbiol. 2021, 23, e13348. [Google Scholar] [CrossRef] [PubMed]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.Y.; Lannoy, V.; Decobecq, M.E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef] [PubMed]
- Karaki, S.; Tazoe, H.; Hayashi, H.; Kashiwabara, H.; Tooyama, K.; Suzuki, Y.; Kuwahara, A. Expression of the short-chain fatty acid receptor, GPR43, in the human colon. J. Mol. Histol. 2008, 39, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Senga, T.; Iwamoto, S.; Yoshida, T.; Yokota, T.; Adachi, K.; Azuma, E.; Hamaguchi, M.; Iwamoto, T. LSSIG is a novel murine leukocyte-specific GPCR that is induced by the activation of STAT3. Blood 2003, 101, 1185–1187. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.U.; In, H.J.; Kwon, M.S.; Park, B.O.; Jo, M.; Kim, M.O.; Cho, S.; Lee, S.; Lee, H.J.; Kwak, Y.S.; et al. β-Arrestin 2 mediates G protein-coupled receptor 43 signals to nuclear factor-κB. Biol. Pharm. Bull. 2013, 36, 1754–1759. [Google Scholar] [CrossRef] [PubMed]
- Inoue, D.; Kimura, I.; Wakabayashi, M.; Tsumoto, H.; Ozawa, K.; Hara, T.; Takei, Y.; Hirasawa, A.; Ishihama, Y.; Tsujimoto, G. Short-chain fatty acid receptor GPR41-mediated activation of sympathetic neurons involves synapsin 2b phosphorylation. FEBS Lett. 2012, 586, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Sam, Q.H.; Ling, H.; Yew, W.S.; Tan, Z.; Ravikumar, S.; Chang, M.W.; Chai, L.Y.A. The Divergent Immunomodulatory Effects of Short Chain Fatty Acids and Medium Chain Fatty Acids. Int. J. Mol. Sci. 2021, 22, 6453. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Altan-Bonnet, G.; Mukherjee, R. Cytokine-mediated communication: A quantitative appraisal of immune complexity. Nat. Rev. Immunol. 2019, 19, 205–217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dang, D.T. Molecular Approaches to Protein Dimerization: Opportunities for Supramolecular Chemistry. Front. Chem. 2022, 10, 829312. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goto, Y.; Kurashima, Y.; Kiyono, H. The gut microbiota and inflammatory bowel disease. Curr. Opin. Rheumatol. 2015, 27, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587, Erratum in Microorganisms 2020, 8, E2046. https://doi.org/10.3390/microorganisms8122046. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kwiatkowski, D.P.; Luoni, G. Host genetic factors in resistance and susceptibility to malaria. Parassitologia 2006, 48, 450–467. [Google Scholar] [PubMed]
- Albuquerque, S.S.; Carret, C.; Grosso, A.R.; Tarun, A.S.; Peng, X.; Kappe, S.H.; Prudêncio, M.; Mota, M.M. Host cell transcriptional profiling during malaria liver stage infection reveals a coordinated and sequential set of biological events. BMC Genomics. 2009, 10, 270. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seabaugh, J.A.; Anderson, D.M. Pathogenicity and virulence of Yersinia. Virulence 2024, 15, 2316439. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chung, L.K.; Bliska, J.B. Yersinia versus host immunity: How a pathogen evades or triggers a protective response. Curr. Opin. Microbiol. 2016, 29, 56–62. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Qian, Y.; Kang, Z.; Liu, C.; Li, X. IL-17 signaling in host defense and inflammatory diseases. Cell. Mol. Immunol. 2010, 7, 328–333. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aliyu, M.; Zohora, F.T.; Anka, A.U.; Ali, K.; Maleknia, S.; Saffarioun, M.; Azizi, G. Interleukin-6 cytokine: An overview of the immune regulation, immune dysregulation, and therapeutic approach. Int. Immunopharmacol. 2022, 111, 109130. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Y.; Wang, X.; Li, M.; Yan, H.; Shi, H.; Shi, D.; Chen, J.; Guo, L.; Feng, L. Elevation of CXCL8 secretion induced by PEDV infection via NF-κB signaling pathway. Front. Cell. Infect. Microbiol. 2024, 14, 1422560. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Milburn, J.V.; Hoog, A.; Villanueva-Hernández, S.; Mair, K.H.; Gerner, W. Identification of IL-10 competent B cells in swine. Dev. Comp. Immunol. 2022, 135, 104488. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Bai, D.; Zheng, X.; Zhu, L.; Ou, K.; Wang, X.; Tong, H.; Zhang, Y.; Wang, J.; Zeng, J.; et al. Comparing HD knockin pigs and mice reveals the pathological role of IL-17. Cell. Rep. 2023, 42, 113443. [Google Scholar] [CrossRef] [PubMed]
- Muneta, Y.; Mori, Y.; Shimoji, Y.; Yokomizo, Y. Porcine interleukin 18: Cloning, characterization of the cDNA and expression with the baculovirus system. Cytokine 2000, 12, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.H.; Meng, X.; Hu, J.W.; Ding, Y.F.; Peng, Y.R. Research progress on the correlation between TLR4-MyD88-NF-kB signaling pathway and the hepatitis-fibrosis-liver cancer axis. Int. J. Pharm. Res. 2017, 44, 396–401. [Google Scholar]
- Young, H.A. Unraveling the pros and cons of interferon-gamma gene regulation. Immunity 2006, 24, 506–507. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kak, G.; Raza, M.; Tiwari, B.K. Interferon-gamma (IFN-γ): Exploring its implications in infectious diseases. Biomol. Concepts. 2018, 9, 64–79. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.T.; Liu, Q.; Ning, M.X.; Qi, Y.X.; Rehman, S.; Chen, D.K. Development and applications of a monoclonal antibody against caprine interferon-gamma. BMC Biotechnol. 2019, 19, 102. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, X.D.; Kang, R.M.; Wei, Y.; Ye, Y.G. Advances in the role of CD4+T cell-associated cytokines in zoonotic parasitic infections. Adv. Vet. Med. 2024, 45, 104–109. [Google Scholar]
- Zhao, Y.F.; Yu, Y.H.; Wang, J.M.; Zhang, J.H.; Sun, Z.L.; Niu, R.Y.; Wang, J.D. Effect of IL-17A gene knockout on fluorine-induced hepatitis response and hepatocyte apoptosis in mice. J. Anim. Husb. Vet. Sci. 2023, 54, 3108–3117. [Google Scholar]
- Berry, S.P.D.; Dossou, C.; Kashif, A.; Sharifinejad, N.; Azizi, G.; Hamedifar, H.; Sabzvari, A.; Zian, Z. The role of IL-17 and anti-IL-17 agents in the immunopathogenesis and management of autoimmune and inflammatory diseases. Int. Immunopharmacol. 2022, 102, 108402. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, J.; Zhang, J.; Sun, Z.; Niu, R.; Manthari, R.K.; Ommati, M.M.; Wang, S.; Wang, J. Fluoride exposure induces mitochondrial damage and mitophagy via activation of the IL-17A pathway in hepatocytes. Sci. Total. Environ. 2022, 804, 150184. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Shi, P.; Nian, D. Research progress on the role of IL-17 in autoimmune diseases of nervous system. Shandong Med. 2021, 61, 107–111. [Google Scholar]
- Yang, M.; Song, Y.; Zhang, H.J.; Ji, J.B.; Yang, J.M. IL-17 regulates the PI3K/AKT/FAS/FASL signaling pathway to inhibit apoptosis of Hep-2 cells. J. Anhui Med. Univ. 2018, 53, 1681–1684. [Google Scholar]
- Li, M.; Han, X.; Sun, L.; Liu, X.; Zhang, W.; Hao, J. Indole-3-acetic acid alleviates DSS-induced colitis by promoting the production of R-equol from Bifidobacterium pseudolongum. Gut. Microbes. 2024, 16, 2329147. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, M.; Ding, Y.; Wei, J.; Dong, Y.; Wang, J.; Dai, X.; Yan, J.; Chu, F.; Zhang, K.; Meng, F.; et al. Gut microbiota metabolite indole-3-acetic acid maintains intestinal epithelial homeostasis through mucin sulfation. Gut. Microbes. 2024, 16, 2377576. [Google Scholar] [CrossRef]
- Liu, S.; Cai, P.; You, W.; Yang, M.; Tu, Y.; Zhou, Y.; Valencak, T.; Xiao, Y.; Wang, Y.; Shan, T. Enhancement of Gut Barrier Integrity by a Bacillus Subtilis Secreted Metabolite Through the GADD45A-Wnt/β-Catenin Pathway. iMeta 2025, 23, e70005. [Google Scholar] [CrossRef]
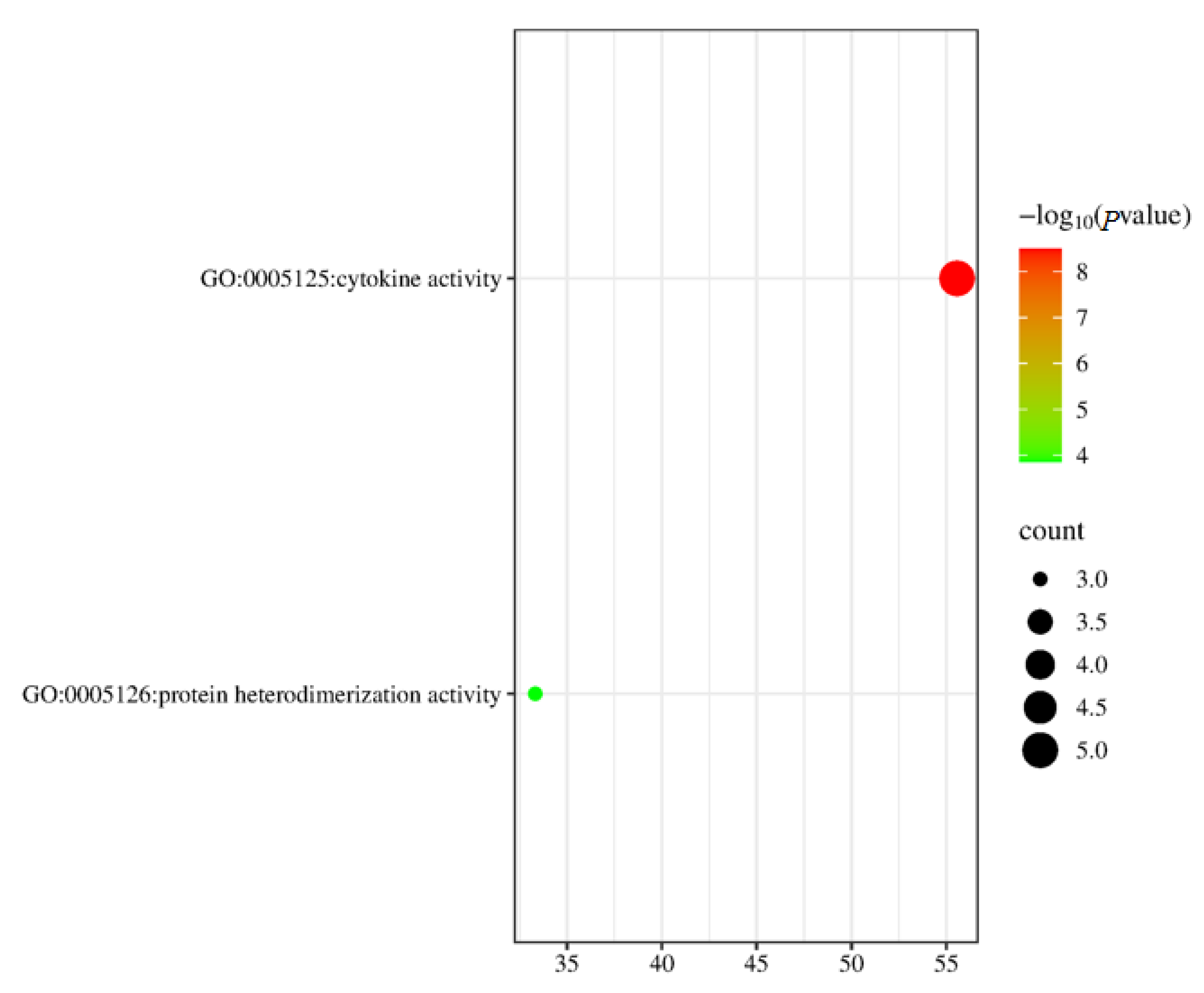
| Groups | Term ID | Description | p Value | Gene Number |
|---|---|---|---|---|
| DH vs. DHB | GO:0005125 | cytokine activity | 3.2359365693 × 10−9 | 5 |
| GO:0046982 | protein heterodimerization activity | 0.000141253754462 | 3 |
| Groups | Pathway ID | Description | p Value | Gene Number |
|---|---|---|---|---|
| DH vs. DHB | ko05321 | Inflammatory bowel disease | 5.60283 × 10−9 | 5 |
| ko05144 | Malaria | 7.48289 × 10−9 | 5 | |
| ko05135 | Yersinia infection | 8.262792 × 10−8 | 5 | |
| ko04657 | IL-17 signaling pathway | 3.107567 × 10−7 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, H.; Xie, Y.; Yi, L.; Cheng, W.; Song, G.; Shi, W.; Zhu, J.; Zhao, S. Comparative Analysis of Short-Chain Fatty Acids and the Immune Barrier in Cecum of Dahe Pigs and Dahe Black Pigs. Animals 2025, 15, 920. https://doi.org/10.3390/ani15070920
Jia H, Xie Y, Yi L, Cheng W, Song G, Shi W, Zhu J, Zhao S. Comparative Analysis of Short-Chain Fatty Acids and the Immune Barrier in Cecum of Dahe Pigs and Dahe Black Pigs. Animals. 2025; 15(7):920. https://doi.org/10.3390/ani15070920
Chicago/Turabian StyleJia, Huijin, Yuxiao Xie, Lanlan Yi, Wenjie Cheng, Guangyao Song, Wenzhe Shi, Junhong Zhu, and Sumei Zhao. 2025. "Comparative Analysis of Short-Chain Fatty Acids and the Immune Barrier in Cecum of Dahe Pigs and Dahe Black Pigs" Animals 15, no. 7: 920. https://doi.org/10.3390/ani15070920
APA StyleJia, H., Xie, Y., Yi, L., Cheng, W., Song, G., Shi, W., Zhu, J., & Zhao, S. (2025). Comparative Analysis of Short-Chain Fatty Acids and the Immune Barrier in Cecum of Dahe Pigs and Dahe Black Pigs. Animals, 15(7), 920. https://doi.org/10.3390/ani15070920





