Comparative Analysis of the Gut Microbiota of Thai Indigenous Chicken Fed House Crickets
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Chicken Samples and Rearing Management
2.3. Feed and Design
2.4. Sample Collection
2.5. DNA Sequencing
2.6. Amplification of the Full-Length 16S rDNA
2.7. Amplicon Sequencing Based on Oxford Nanopore Technology (ONT)
2.8. Data Analysis
2.9. Statistical Analysis
3. Results
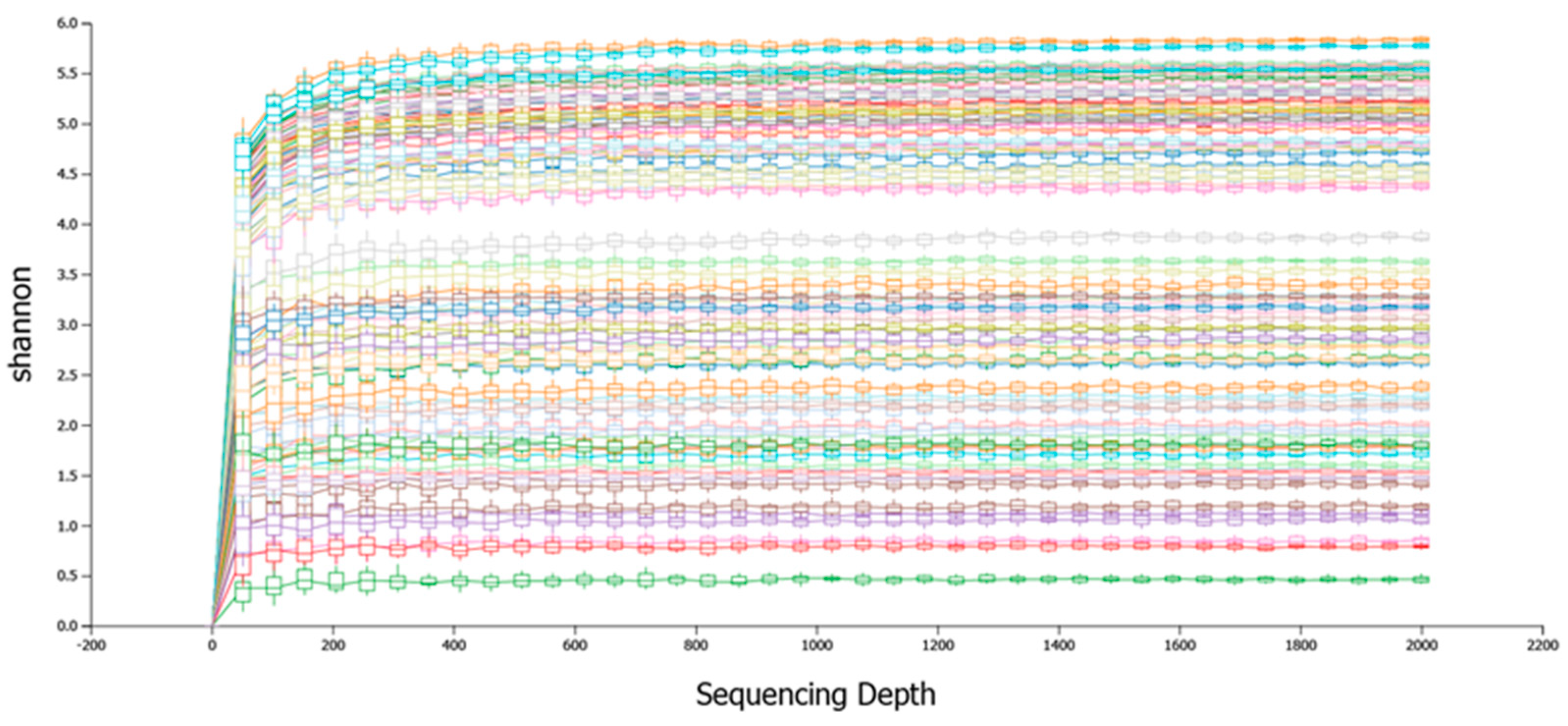
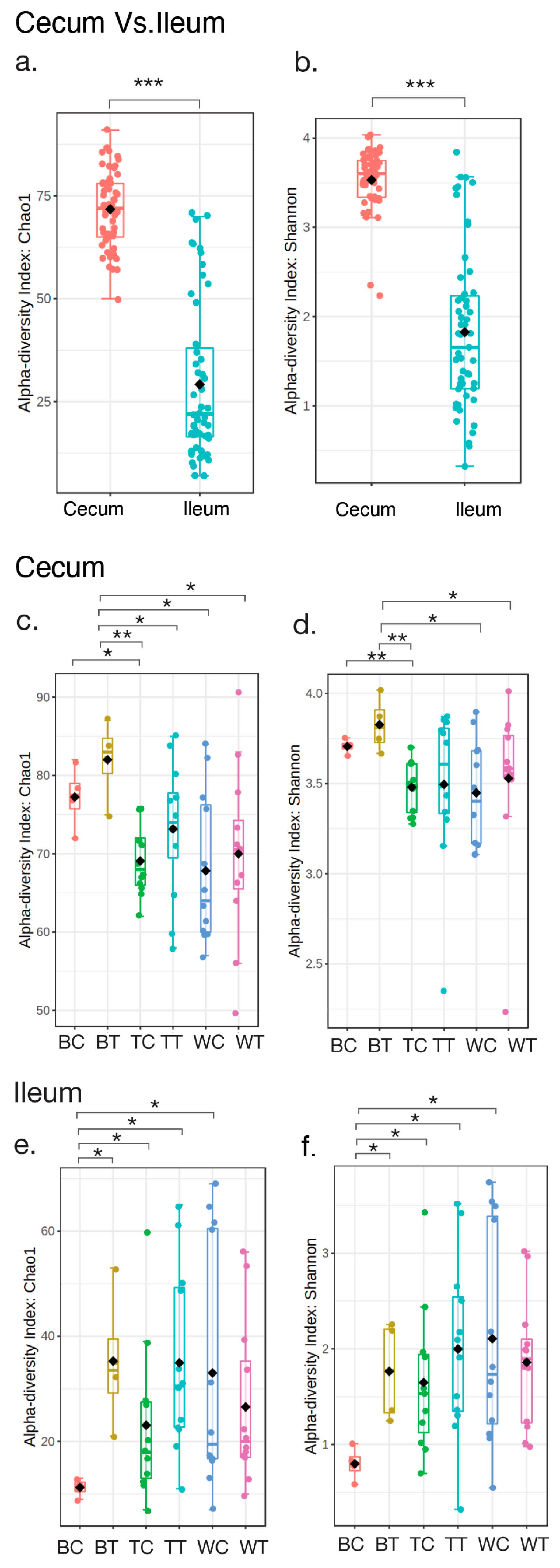
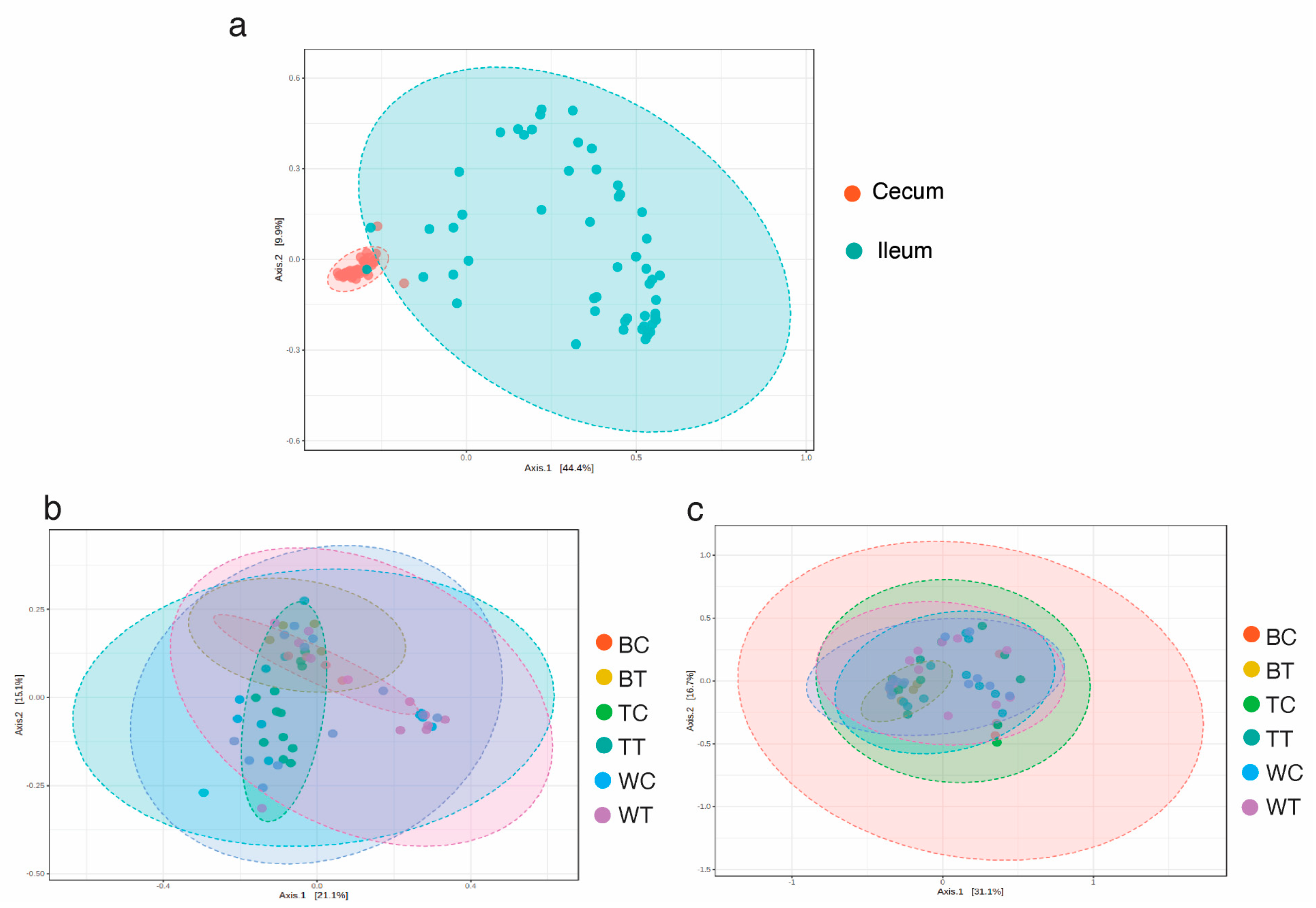
3.1. Sequencing Summary and Diversity Analysis
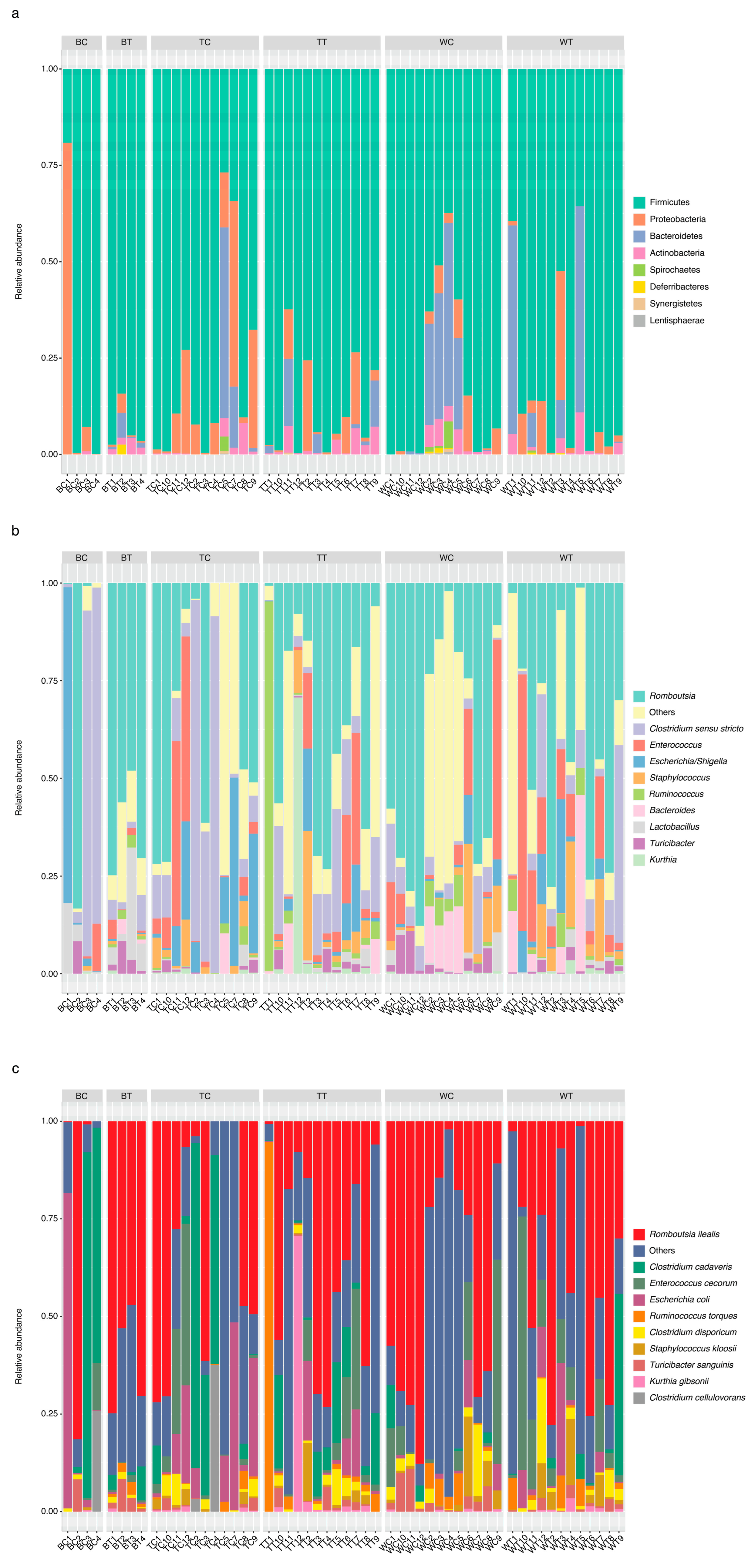
3.2. Relative Abundance of Bacteria in Cecum and Ileum Samples
3.3. Linear Discriminant Analysis of Effect Size (LEfSe)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TC | Betong Chicken (KU line) control |
| TT | Betong Chicken (KU line) test fed on crickets |
| WC | White feather, black bone control |
| WT | White feather, black bone test fed on crickets |
| BC | Black feather, black bone control |
| BT | Black feather, black bone test fed on crickets |
References
- Bahrndorff, S.; Alemu, T.; Alemneh, T.; Lund Nielsen, J. The Microbiome of Animals: Implications for Conservation Biology. Int. J. Genom. 2016, 2016, 5304028. [Google Scholar] [CrossRef]
- Bäumler, A.J.; Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 2016, 535, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Aruwa, C.E.; Pillay, C.; Nyaga, M.M.; Sabiu, S. Poultry gut health—Microbiome functions, environmental impacts, microbiome engineering and advancements in characterization technologies. J. Anim. Sci. Biotechnol. 2021, 12, 119. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human gut microbiota in health and disease: Unveiling the relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.; Hughes, R.J.; Moore, R.J. Microbiota of the chicken gastrointestinal tract: Influence on health, productivity and disease. Appl. Microbiol. Biotechnol. 2014, 98, 4301–4310. [Google Scholar] [CrossRef]
- Maki, J.J.; Klima, C.L.; Sylte, M.J.; Looft, T. The Microbial Pecking Order: Utilization of Intestinal Microbiota for Poultry Health. Microorganisms 2019, 7, 376. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Kumar, S.; Oakley, B.; Kim, W.K. Chicken Gut Microbiota: Importance and Detection Technology. Front. Vet. Sci. 2018, 5, 254. [Google Scholar] [CrossRef] [PubMed]
- Shehata, A.A.; Yalçın, S.; Latorre, J.D.; Basiouni, S.; Attia, Y.A.; El-Wahab, A.A.; Visscher, C.; El-Seedi, H.R.; Huber, C.; Hafez, H.M.; et al. Probiotics, Prebiotics, and Phytogenic Substances for Optimizing Gut Health in Poultry. Microorganisms 2022, 10, 395. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Yu, Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 2014, 5, 108–119. [Google Scholar] [CrossRef]
- Palamidi, I.; Paraskeuas, V.V.; Mountzouris, K.C. Mountzouris Dietary and phytogenic inclusion effects on the broiler chicken cecal ecosystem. Front. Anim. Sci. 2023, 3, 1094314. [Google Scholar] [CrossRef]
- Stanley, D.; Bajagai, Y.S. Feed Safety and the Development of Poultry Intestinal Microbiota. Animals 2022, 12, 2890. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.S.; Chatterjee, R.N.; Raju, M.V.L.N.; Prakash, B.; Rao, S.V.R.; Yadav, S.P.; Kannan, A. Gut Microbial Composition Differs Extensively among Indian Native Chicken Breeds Originated in Different Geographical Locations and a Commercial Broiler Line, but Breed-Specific, as Well as Across-Breed Core Microbiomes, Are Found. Microorganisms 2021, 9, 391. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Bai, X.; Wang, T.; Liu, J.; Miao, X.; Zeng, B.; Li, D. Gut Microbial Diversity Analysis of Different Native Chickens and Screening of Chicken-Derived Probiotics. Animals 2023, 13, 3672. [Google Scholar] [CrossRef]
- Kers, J.G.; Velkers, F.C.; Fischer, E.A.J.; Hermes, G.D.A.; Stegeman, J.A.; Smidt, H. Host and Environmental Factors Affecting the Intestinal Microbiota in Chickens. Front. Microbiol. 2018, 9, 235. [Google Scholar]
- Hassan, S.A.; Altemimi, A.B.; Hashmi, A.A.; Shahzadi, S.; Mujahid, W.; Ali, A.; Bhat, Z.F.; Naz, S.; Nawaz, A.; Abdi, G.; et al. Edible crickets as a possible way to curb protein-energy malnutrition: Nutritional status, food applications, and safety concerns. Food Chem. X 2024, 23, 101533. [Google Scholar]
- Krongdang, S.; Phokasem, P.; Venkatachalam, K.; Charoenphun, N. Edible Insects in Thailand: An Overview of Status, Properties, Processing, and Utilization in the Food Industry. Foods 2023, 12, 2162. [Google Scholar] [CrossRef]
- Stull, V.J.; Finer, E.; Bergmans, R.S.; Febvre, H.P.; Longhurst, C.; Manter, D.K.; Patz, J.A.; Weir, T.L. Impact of Edible Cricket Consumption on Gut Microbiota in Healthy Adults, a Double-blind, Randomized Crossover Trial. Sci. Rep. 2018, 17, 10762. [Google Scholar]
- van Huis, A.; Oonincx, D.G.A.B. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar]
- Zou, X.; Liu, M.; Li, X.; Pan, F.; Wu, X.; Fang, X.; Zhou, F.; Peng, W.; Tian, W. Applications of insect nutrition resources in animal production. J. Agric. Food Res. 2024, 15, 100966. [Google Scholar]
- Bungsrisawat, P.; Tumwasorn, S.; Loongyai, W.; Nakthong, S.; Sopannarath, P. Genetic parameters of some carcass and meat quality traits in Betong chicken (KU line). Agric. Nat. Resour. 2018, 52, 274–279. [Google Scholar]
- Horowitz, W.; Latimer, G.W. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Meijerink, N.; Kers, J.G.; Velkers, F.C.; van Haarlem, D.A.; Lamot, D.M.; de Oliveira, J.E.; Smidt, H.; Stegeman, J.A.; Rutten, V.P.M.G.; Jansen, C.A. Early Life Inoculation With Adult-Derived Microbiota Accelerates Maturation of Intestinal Microbiota and Enhances NK Cell Activation in Broiler Chickens. Front. Vet. Sci. 2020, 7, 584561. [Google Scholar] [CrossRef] [PubMed]
- Feye, K.; Baxter, M.; Tellez-Isaias, G.; Kogut, M.; Ricke, S. Influential factors on the composition of the conventionally raised broiler gastrointestinal microbiomes. Poult. Sci. 2020, 99, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Greene, G.; Koolman, L.; Whyte, P.; Burgess, C.; Bolton, D. The Gut Microbiota of Broilers Reared with and without Antibiotic Treatment. Microorganisms 2023, 11, 876. [Google Scholar] [CrossRef]
- Stanley, D.; Geier, M.S.; Denman, S.E.; Haring, V.R.; Crowley, T.M.; Hughes, R.J.; Moore, R.J. Identification of chicken intestinal microbiota correlated with the efficiency of energy extraction from feed. Vet. Microbiol. 2013, 164, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Li, F.; Kong, F.; Cui, Z.; Li, D.; Wang, Y.; Zhu, Q.; Shu, G.; Tian, Y.; Zhang, Y.; et al. Altitude-adaption of gut microbiota in Tibetan chicken. Poult. Sci. 2022, 101, 101998. [Google Scholar] [CrossRef] [PubMed]
- Burrows, P.; Godoy-Santos, F.; Lawther, K.; Richmond, A.; Corcionivoschi, N.; Huws, S. Decoding the chicken gastrointestinal microbiome. BMC Microbiol. 2025, 25, 35. [Google Scholar] [CrossRef]
- Gong, J.; Forster, R.J.; Yu, H.; Chambers, J.R.; Wheatcroft, R.; Sabour, P.M.; Chen, S. Molecular analysis of bacterial populations in the ileum of broiler chickens and comparison with bacteria in the cecum. FEMS Microbiol. Ecol. 2002, 41, 171–179. [Google Scholar] [CrossRef]
- Clavijo, V.; Flórez, M.J.V. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: A review. Poult. Sci. 2018, 97, 1006–1021. [Google Scholar] [CrossRef]
- Carrasco, J.M.D.; Casanova, N.A.; Miyakawa, M.E.F. Microbiota, Gut Health and Chicken Productivity: What Is the Connection? Microorganisms 2019, 7, 374. [Google Scholar] [CrossRef]
- Gasco, L.; Finke, M.; van Huis, A. Can diets containing insects promote animal health? J. Insects Food Feed. 2018, 4, 1–4. [Google Scholar]
- Malematja, E.; Manyelo, T.; Sebola, N.; Mabelebele, M. The role of insects in promoting the health and gut status of poultry. Comp. Clin. Pathol. 2023, 32, 501–513. [Google Scholar]
- Magara, H.J.O.; Niassy, S.; Ayieko, M.A.; Mukundamago, M.; Egonyu, J.P.; Tanga, C.M.; Kimathi, E.K.; Ongere, J.O.; Fiaboe, K.K.M.; Hugel, S.; et al. Edible Crickets (Orthoptera) Around the World: Distribution, Nutritional Value, and Other Benefits—A Review. Front. Nutr. 2021, 7, 537915. [Google Scholar]
- Siddiqui, S.A.; Zhao, T.; Fitriani, A.; Rahmadhia, S.N.; Alirezalu, K.; Fernando, I. Acheta domesticus (house cricket) as human foods—An approval of the European Commission—A systematic review. Food Front. 2024, 5, 435–473. [Google Scholar]
- Ververis, E.; Boué, G.; Poulsen, M.; Pires, S.M.; Niforou, A.; Thomsen, S.T.; Tesson, V.; Federighi, M.; Naska, A. A systematic review of the nutrient composition, microbiological and toxicological profile of Acheta domesticus (house cricket). J. Food Compos. Anal. 2022, 114, 104859. [Google Scholar]
- Lopez-Santamarina, A.; Mondragon, A.d.C.; Lamas, A.; Miranda, J.M.; Franco, C.M.; Cepeda, A. Animal-Origin Prebiotics Based on Chitin: An Alternative for the Future? A Critical Review. Foods 2020, 12, 782. [Google Scholar] [CrossRef]
- Zong, X.; Fu, J.; Xu, B.; Wang, Y.; Jin, M. Interplay between gut microbiota and antimicrobial peptides. Anim. Nutr. 2020, 6, 389–396. [Google Scholar] [PubMed]
- Zeiger, K.; Popp, J.; Becker, A.; Hankel, J.; Visscher, C.; Klein, G.; Meemken, D. Lauric acid as feed additive—An approach to reducing Campylobacter spp. in broiler meat. PLoS ONE 2017, 12, e0175693. [Google Scholar]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. Chapter Three—The Role of Short-Chain Fatty Acids in Health and Disease, in Advances in Immunology; Alt, F.W., Ed.; Academic Press: Cambridge, UK, 2014; pp. 91–119. [Google Scholar]
- ONoor, S.; Ridgway, K.; Scovell, L.; Kemsley, E.K.; Lund, E.K.; Jamieson, C.; Johnson, I.T.; Narbad, A. Ulcerative colitis and irritable bowel patients exhibit distinct abnormalities of the gut microbiota. BMC Gastroenterol. 2010, 10, 134. [Google Scholar]
- Chung, Y.W.; Gwak, H.-J.; Moon, S.; Rho, M.; Ryu, J.-H. Functional dynamics of bacterial species in the mouse gut microbiome revealed by metagenomic and metatranscriptomic analyses. PLoS ONE 2020, 15, e0227886. [Google Scholar]
- Gu, X.; Sim, J.X.; Lee, W.L.; Cui, L.; Chan, Y.F.; Chang, E.D.; Teh, Y.E.; Zhang, A.-N.; Armas, F.; Chandra, F.; et al. Gut Ruminococcaceae levels at baseline correlate with risk of antibiotic-associated diarrhea. iScience 2021, 25, 103644. [Google Scholar] [CrossRef] [PubMed]
- Brisbin, J.T.; Gong, J.; Orouji, S.; Esufali, J.; Mallick, A.I.; Parvizi, P.; Shewen, P.E.; Sharif, S. Oral treatment of chickens with lactobacilli influences elicitation of immune responses. Clin. Vaccine Immunol. 2011, 18, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Derave, W.; De Courten, B.; Baba, S.P. An update on carnosine and anserine research. Amino Acids 2019, 51, 1–4. [Google Scholar] [CrossRef] [PubMed]


| Group | Description | Code | Number |
|---|---|---|---|
| 1 | Betong Chicken (KU line) control | TC | 11 |
| Betong Chicken (KU line) test fed on crickets | TT | 12 | |
| 2 | White feather, black bone control | WC | 12 |
| White feather, black bone test fed on crickets | WT | 12 | |
| 3 | Black feather, black bone control | BC | 4 |
| Black feather, black bone test fed on crickets | BT | 4 |
| Items | Starter Diet | Finisher Diet | ||
|---|---|---|---|---|
| Control Group | Test Group | Control Group | Test Group | |
| Ingredients (% Fresh Matter) | ||||
| Maize | 62.05 | 64.35 | 60.04 | 66.11 |
| Defatted soybean meal | 30.7 | 17.22 | 27.618 | 13.2 |
| Cricket powder | 0 | 15 | 0 | 15 |
| Palm oil | 3.92 | 0 | 5.27 | 0 |
| Cellulose | 0 | 0 | 3.8 | 2.1 |
| Calcium carbonate | 1.33 | 1.35 | 1.53 | 1.59 |
| Dicalcium phosphate | 0.71 | 0.75 | 0.29 | 0.29 |
| Vitamin and mineral premix 1 | 0.25 | 0.25 | 0.25 | 0.25 |
| L-Lysine | 0.25 | 0.29 | 0.24 | 0.31 |
| Salt | 0.24 | 0.24 | 0.24 | 0.24 |
| DL-Methionine | 0.21 | 0.2 | 0.332 | 0.5 |
| Sodium bicarbonate | 0.20 | 0.20 | 0.20 | 0.20 |
| L-Threonine | 0.07 | 0.08 | 0.12 | 0.14 |
| Choline chloride | 0.07 | 0.07 | 0.07 | 0.07 |
| Calculated chemical composition (% fresh matter) | ||||
| Metabolizable energy (kcal/kg) | 3112 | 3112 | 3100 | 3100 |
| Crude protein | 19.5 | 19.5 | 18.0 | 18.0 |
| Ether extract | 6.51 | 6.23 | 7.73 | 6.23 |
| Crude fiber | 3.18 | 4.74 | 6.59 | 6.61 |
| Calcium | 0.90 | 0.90 | 0.80 | 0.80 |
| Available phosphorus | 0.45 | 0.45 | 0.30 | 0.30 |
| Lysine | 1.20 | 1.19 | 1.17 | 1.17 |
| Methionine | 0.51 | 0.50 | 0.61 | 0.77 |
| Methionine + Cysteine | 0.84 | 0.89 | 0.91 | 0.91 |
| Threonine | 0.80 | 0.80 | 0.79 | 0.79 |
| Analyzed chemical composition (% fresh matter) | ||||
| Moisture | 10.1 | 10.4 | 10.4 | 10.2 |
| Crude protein | 19.8 | 19.7 | 18.3 | 18.4 |
| Ether extract | 6.52 | 6.53 | 7.97 | 7.05 |
| Crude ash | 5.11 | 5.15 | 5.29 | 5.22 |
| Crude fiber | 3.23 | 4.84 | 6.21 | 6.23 |
| Strains of Chicken | Feed Group | Sample Type | n | Total Raw Reads | Total Retained Reads |
|---|---|---|---|---|---|
| Betong Chicken (T) | Control (C) | Cecum | 11 | 15,787 ± 1603 | 12,736 ± 1384 |
| Ileum | 11 | 18,090 ± 10,472 | 12,401 ± 6126 | ||
| Fed on crickets (T) | Cecum | 12 | 15,907 ± 1496 | 13,591 ± 1407 | |
| Ileum | 12 | 13,406 ± 1783 | 10,610 ± 2190 | ||
| White feather (W), black bone | Control (C) | Cecum | 12 | 16,516 ± 2594 | 13,185 ± 2048 |
| Ileum | 12 | 13,016 ± 3154 | 9063 ± 2544 | ||
| Fed on crickets (T) | Cecum | 12 | 15,287 ± 1861 | 12,485 ± 1494 | |
| Ileum | 12 | 12,149 ± 1875 | 8415 ± 1802 | ||
| Black feather (B), black bone | Control (C) | Cecum | 4 | 16,621 ± 2405 | 13,469 ± 2052 |
| Ileum | 4 | 20,280 ± 11,772 | 14,699 ± 7338 | ||
| Fed on crickets (T) | Cecum | 4 | 16,658 ± 884 | 13,118 ± 620 | |
| Ileum | 4 | 13,644 ± 1004 | 10,837 ± 1206 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
T-Thienprasert, N.P.; Jaithon, T.; Klomkliew, P.; Chanchaem, P.; Suwanasopee, T.; Koonawootrittriron, S.; Kovitvadhi, A.; Chundang, P.; Pongprayoon, P.; Kityakarn, S.; et al. Comparative Analysis of the Gut Microbiota of Thai Indigenous Chicken Fed House Crickets. Animals 2025, 15, 1070. https://doi.org/10.3390/ani15071070
T-Thienprasert NP, Jaithon T, Klomkliew P, Chanchaem P, Suwanasopee T, Koonawootrittriron S, Kovitvadhi A, Chundang P, Pongprayoon P, Kityakarn S, et al. Comparative Analysis of the Gut Microbiota of Thai Indigenous Chicken Fed House Crickets. Animals. 2025; 15(7):1070. https://doi.org/10.3390/ani15071070
Chicago/Turabian StyleT-Thienprasert, Nattanan Panjaworayan, Titiradsadakorn Jaithon, Pavit Klomkliew, Prangwalai Chanchaem, Thanathip Suwanasopee, Skorn Koonawootrittriron, Attawit Kovitvadhi, Pipatpong Chundang, Prapasiri Pongprayoon, Sutasinee Kityakarn, and et al. 2025. "Comparative Analysis of the Gut Microbiota of Thai Indigenous Chicken Fed House Crickets" Animals 15, no. 7: 1070. https://doi.org/10.3390/ani15071070
APA StyleT-Thienprasert, N. P., Jaithon, T., Klomkliew, P., Chanchaem, P., Suwanasopee, T., Koonawootrittriron, S., Kovitvadhi, A., Chundang, P., Pongprayoon, P., Kityakarn, S., Luksirikul, P., & Payungporn, S. (2025). Comparative Analysis of the Gut Microbiota of Thai Indigenous Chicken Fed House Crickets. Animals, 15(7), 1070. https://doi.org/10.3390/ani15071070









