Salinity Tolerance in Freshwater Drum (Aplodinotus grunniens): Investigating Biochemical, Antioxidant, Digestive Enzyme, and Gene Expression Responses to Acute Salinity Stress
Simple Summary
Abstract
1. Introduction
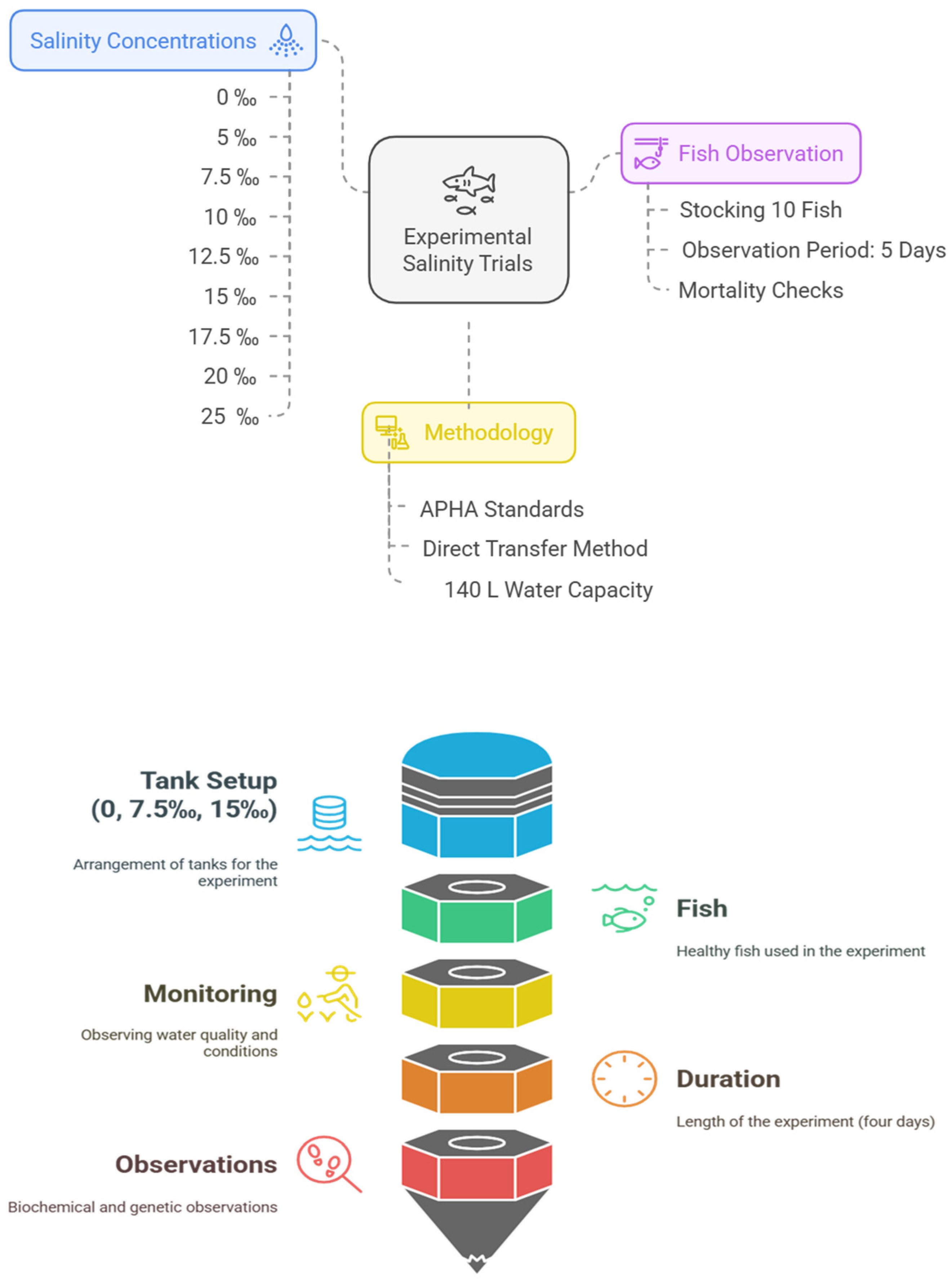
2. Materials and Methods
2.1. Ethics Statement
2.2. Fish Husbandry and Experimental System
2.3. Sample Collection
2.4. Liver Function and Antioxidant Enzyme Activities
2.5. Digestive Enzyme Activity Assay
- Amylase: The quantity of enzyme capable of hydrolyzing 10 mg of starch within 30 min at 37 °C in the presence of 1 mg of tissue protein [40].
- Lipase: The amount of enzyme that can process 1 µmol of substrate per minute in a reaction containing 1 g of tissue protein at 37 °C [41].
- Pepsin: The enzyme quantity that can break down protein to yield an equivalent of 1 µg of amino acids per minute per 1 mg of tissue protein at 37 °C [41].
2.6. Extraction of RNA and Purification Measurement
2.7. cDNA Synthesis, Primer Sequences, and Real-Time PCR Analysis (qRT-PCR)
2.8. Statistical Analysis
3. Results
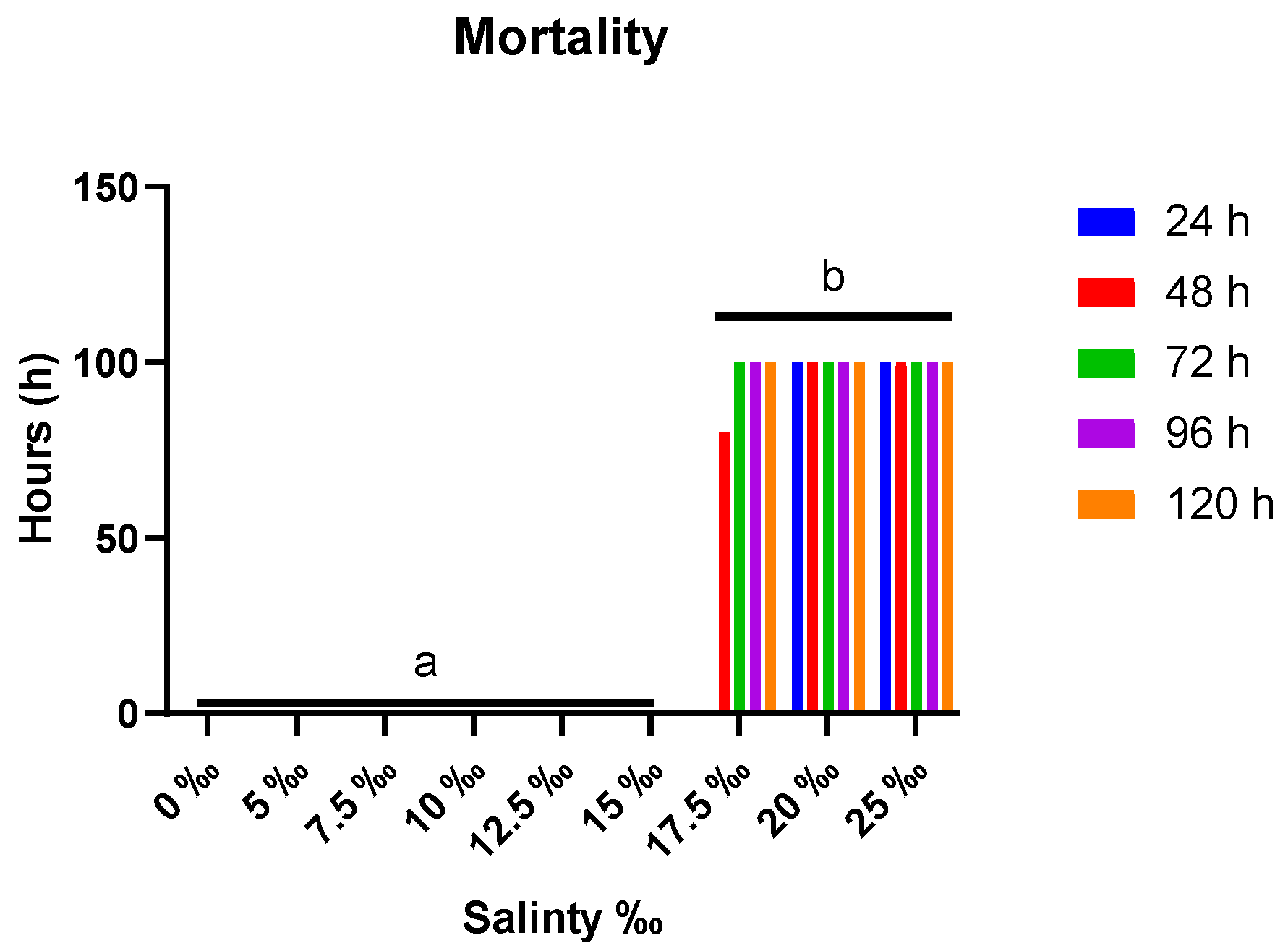
3.1. Observation of Mortalities
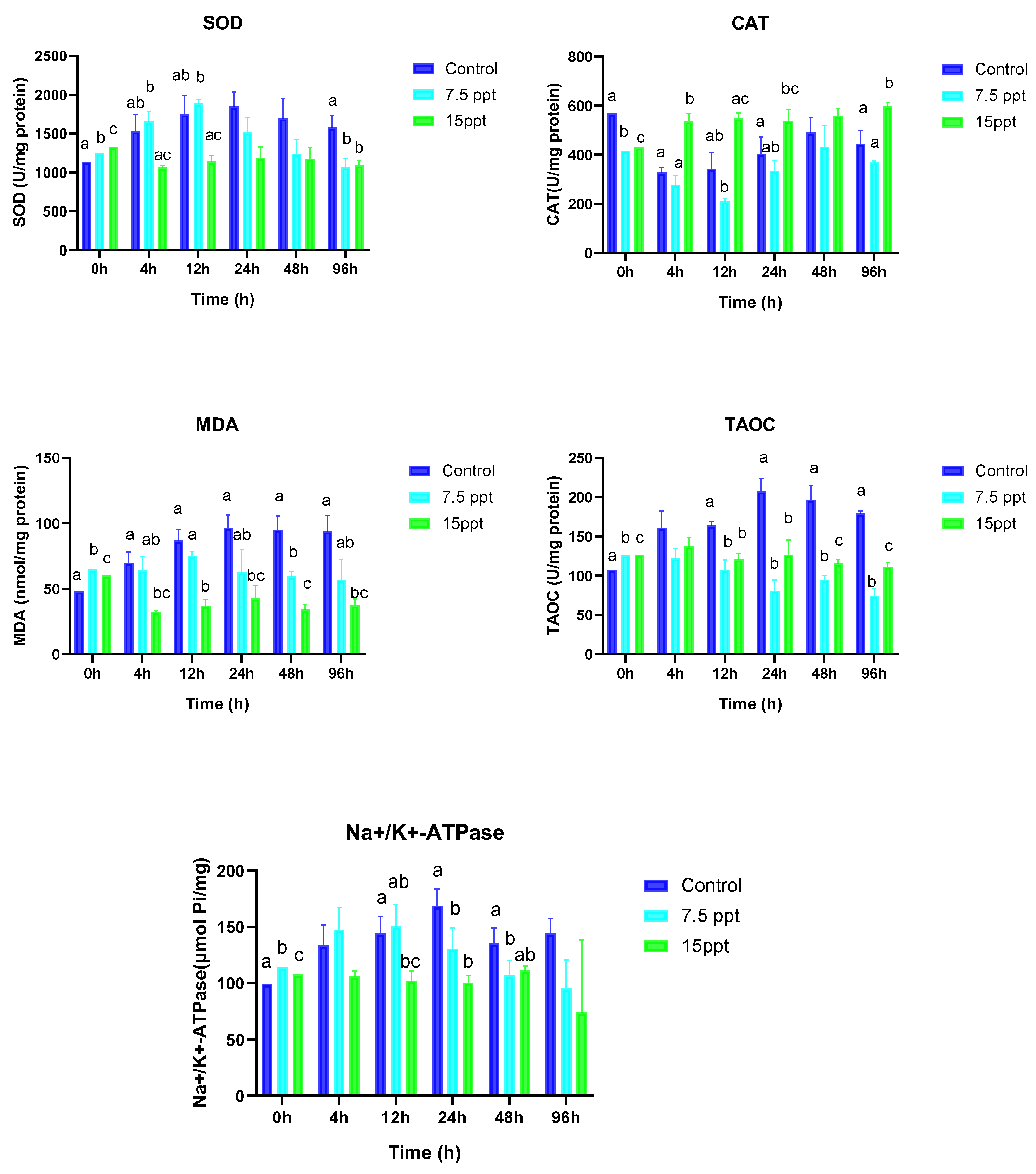
3.2. Liver Function Enzyme Activities
3.3. Antioxidant Enzyme Activities and Na+/K+-ATPase
3.4. Digestive Lipase Activities, Amylase, and Pepsin
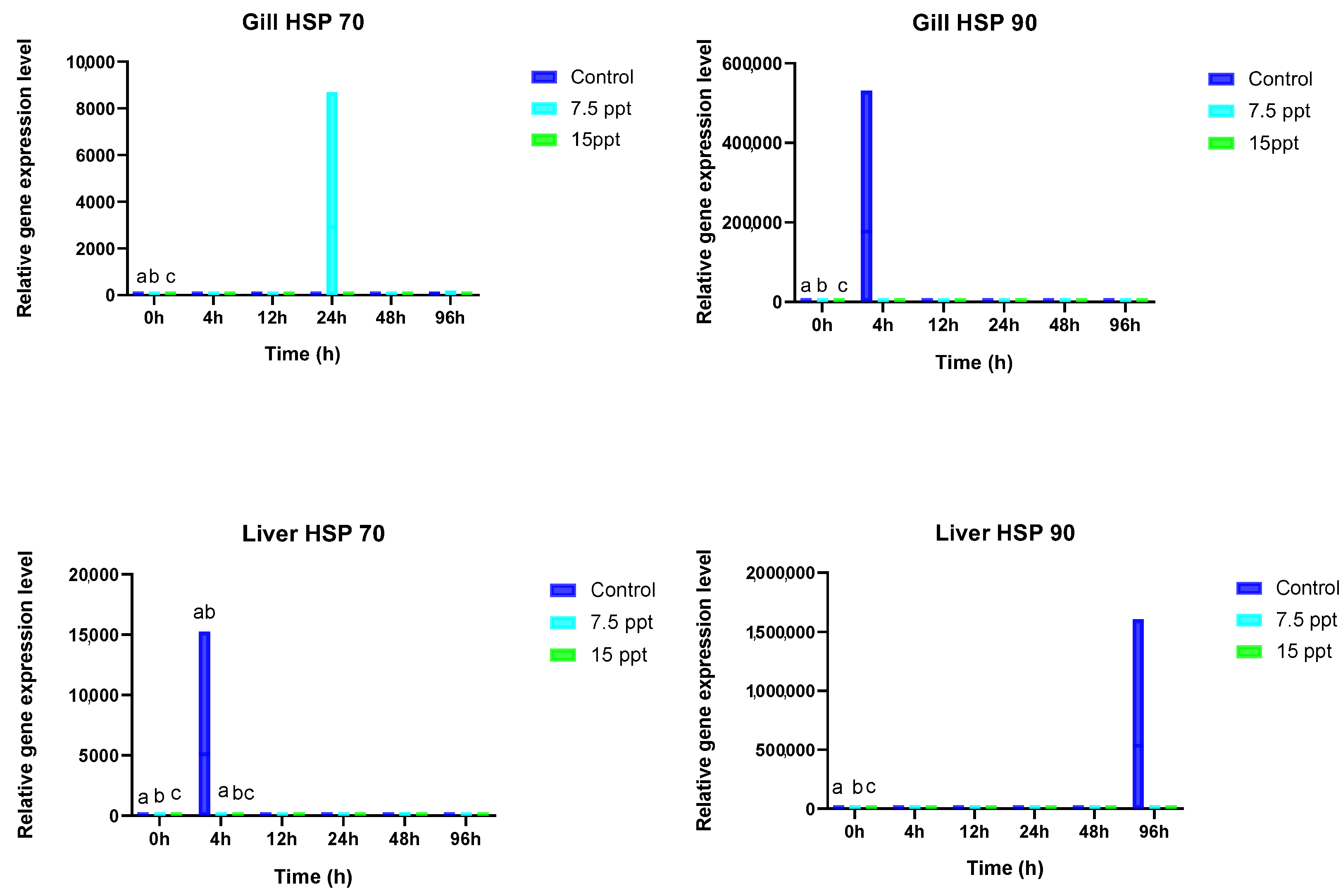
3.5. Gill and Liver HSP 70 and HSP 90
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fitzsimmons, K.; Janjua, R.; Ashraf, M. Aquaculture Handbook, Fish Farming and Nutrition in Pakistan; USDA and American Soybean Association: Lahore, Pakistan, 2015; pp. 969–978. [Google Scholar]
- Sadeghi, N.; Rahimi, H. Unlocking the Water Potential of Agriculture; Food and Agriculture Organization: Rome, Italy, 2003. [Google Scholar]
- Velasco, J.; Gutiérrez-Cánovas, C.; Botella-Cruz, M.; Sánchez-Fernández, D.; Arribas, P.; Carbonell, J.A.; Millán, A.; Pallarés, S. Effects of Salinity Changes on Aquatic Organisms in a Multiple Stressor Context. Philos. Trans. R. Soc. B 2019, 374, 20180011. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Bi, S.; Chi, C.; Dong, Y.; Xia, S.; Liu, Z.; Zhou, L.; Sun, X.; Geng, Y.; Wu, B. Effects of High Salinity Stress on the Survival, Gill Tissue, Enzyme Activity and Free Amino Acid Content in Razor Clam Sinonovacula constricta. Front. Mar. Sci. 2022, 9, 839614. [Google Scholar] [CrossRef]
- Portz, D.E.; Woodley, C.M.; Cech, J.J. Stress-Associated Impacts of Short-Term Holding on Fishes. Rev. Fish Biol. Fish. 2006, 16, 125–170. [Google Scholar] [CrossRef]
- Bachman, P.M.; Rand, G.M. Effects of Salinity on Native Estuarine Fish Species in South Florida. Ecotoxicology 2008, 17, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Jeong, E.-H.; Jeon, Y.-H.; Kim, S.K.; Hur, Y.B. Salinity-Mediated Changes in Hematological Parameters, Stress, Antioxidant Responses, and Acetylcholinesterase of Juvenile Olive Flounders (Paralichthys olivaceus). Environ. Toxicol. Pharmacol. 2021, 83, 103597. [Google Scholar] [CrossRef]
- Rosemore, B.J.; Welsh, C.A. The Effects of Rearing Density, Salt Concentration, and Incubation Temperature on Japanese Medaka (Oryzias Latipes) Embryo Development. Zebrafish 2012, 9, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Komoroske, L.M.; Jeffries, K.M.; Connon, R.E.; Dexter, J.; Hasenbein, M.; Verhille, C.; Fangue, N.A. Sublethal Salinity Stress Contributes to Habitat Limitation in an Endangered Estuarine Fish. Evol. Appl. 2016, 9, 963–981. [Google Scholar] [CrossRef]
- Evans, T.G.; Kültz, D. The Cellular Stress Response in Fish Exposed to Salinity Fluctuations. J. Exp. Zool. Part Ecol. Integr. Physiol. 2020, 333, 421–435. [Google Scholar] [CrossRef]
- Salati, A.; Baghbanzadeh, A.; Soltani, M.; Peyghan, R.; Riazi, G. Effect of Different Levels of Salinity on Gill and Kidney Function in Common Carp Cyprinus carpio (Pisces: Cyprinidae). Ital. J. Zool. 2011, 78, 298–303. [Google Scholar] [CrossRef]
- Marx, M.T.S.; Souza, C.d.F.; Almeida, A.P.G.; Descovi, S.N.; Bianchini, A.E.; Martos-Sitcha, J.A.; Martínez-Rodríguez, G.; Antoniazzi, A.Q.; Baldisserotto, B. Expression of Ion Transporters and Na+/K+-ATPase and H+-ATPase Activities in the Gills and Kidney of Silver Catfish (Rhamdia quelen) Exposed to Different pHs. Fishes 2022, 7, 261. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Saad, M.F.; Shukry, M.; El-Keredy, A.M.; Nasif, O.; Van Doan, H.; Dawood, M.A. Physiological and Ion Changes of Nile Tilapia (Oreochromis niloticus) under the Effect of Salinity Stress. Aquac. Rep. 2021, 19, 100567. [Google Scholar] [CrossRef]
- Tang, C.-H.; Lai, D.-Y.; Lee, T.-H. Effects of Salinity Acclimation on Na+/K+–ATPase Responses and FXYD11 Expression in the Gills and Kidneys of the Japanese Eel (Anguilla japonica). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2012, 163, 302–310. [Google Scholar] [CrossRef]
- Armesto, P.; Cousin, X.; Salas-Leiton, E.; Asensio, E.; Manchado, M.; Infante, C. Molecular Characterization and Transcriptional Regulation of the Renin–Angiotensin System Genes in Senegalese Sole (Solea senegalensis Kaup, 1858): Differential Gene Regulation by Salinity. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 184, 6–19. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kang, J.-C. Oxidative Stress, Neurotoxicity, and Non-Specific Immune Responses in Juvenile Red Sea Bream, Pagrus major, Exposed to Different Waterborne Selenium Concentrations. Chemosphere 2015, 135, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J.; Svendsen, O. Oxidants and Antioxidants in Disease: Oxidative Stress in Farm Animals. Vet. J. 2007, 173, 502–511. [Google Scholar] [CrossRef]
- Tapia-Paniagua, S.T.; Reyes-Becerril, M.; Ascencio-Valle, F.; Esteban, M.Á.; Clavijo, E.; Balebona, M.C.; Moriñigo, M.A. Modulation of the Intestinal Microbiota and Immune System of Farmed Sparus Aurata by the Administration of the Yeast Debaryomyces hansenii L2 in Conjunction with Inulin. J. Aquac. Res. Dev. 2011, 1, 10–4172. [Google Scholar]
- Mohankumar, K.; Ramasamy, P. White Spot Syndrome Virus Infection Decreases the Activity of Antioxidant Enzymes in Fenneropenaeus indicus. Virus Res. 2006, 115, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Eissa, N. Probiotic Effect on Molecular Antioxidant Profiles in Yellow Perch, Perca flavescens. Glob. J. Fish. Aquac. Res. 2014, 1, 16–29. [Google Scholar]
- Zhang, X.-Y.; Hu, C.-G.; Yao, J.-L. Tetraploidization of Diploid Dioscorea Results in Activation of the Antioxidant Defense System and Increased Heat Tolerance. J. Plant Physiol. 2010, 167, 88–94. [Google Scholar] [CrossRef]
- Baharloei, M.; Heidari, B.; Zamani, H.; Hadavi, M. Effects of Pro-Tex® on the Expression of Hsp70 Gene and Immune Response Parameters in the Persian Sturgeon Fingerlings, Acipenser persicus, Infected with Aeromonas hydrophila. J. Appl. Ichthyol. 2020, 36, 393–401. [Google Scholar] [CrossRef]
- Basu, N.; Nakano, T.; Grau, E.; Iwama, G. The Effects of Cortisol on Heat Shock Protein 70 Levels in Two Fish Species. Gen. Comp. Endocrinol. 2001, 124, 97–105. [Google Scholar] [CrossRef]
- Sinha, A.K.; Diricx, M.; Chan, L.P.; Liew, H.J.; Kumar, V.; Blust, R.; De Boeck, G. Expression Pattern of Potential Biomarker Genes Related to Growth, Ion Regulation and Stress in Response to Ammonia Exposure, Food Deprivation and Exercise in Common Carp (Cyprinus carpio). Aquat. Toxicol. 2012, 122, 93–105. [Google Scholar] [CrossRef]
- Basu, N.; Todgham, A.; Ackerman, P.; Bibeau, M.; Nakano, K.; Schulte, P.; Iwama, G.K. Heat Shock Protein Genes and Their Functional Significance in Fish. Gene 2002, 295, 173–183. [Google Scholar] [CrossRef]
- Boone, A.N.; Vijayan, M.M. Constitutive Heat Shock Protein 70 (HSC70) Expression in Rainbow Trout Hepatocytes: Effect of Heat Shock and Heavy Metal Exposure. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 132, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.-L.; Terlecky, S.R.; Plant, C.P.; Dice, J.F. A Role for a 70-Kilodalton Heat Shock Protein in Lysosomal Degradation of Intracellular Proteins. Science 1989, 246, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.R.; Martin, R.L.; Hansen, W.J.; Beckmann, R.P.; Welch, W.J. The Constitutive and Stress Inducible Forms of Hsp 70 Exhibit Functional Similarities and Interact with One Another in an ATP-Dependent Fashion. J. Cell Biol. 1993, 120, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Airaksinen, S.; Råbergh, C.; Sistonen, L.; Nikinmaa, M. Effects of Heat Shock and Hypoxia on Protein Synthesis in Rainbow Trout (Oncorhynchus mykiss) Cells. J. Exp. Biol. 1998, 201, 2543–2551. [Google Scholar] [CrossRef]
- Hamer, B.; Hamer, D.P.; Müller, W.; Batel, R. Stress-70 Proteins in Marine Mussel Mytilus galloprovincialis as Biomarkers of Environmental Pollution: A Field Study. Environ. Int. 2004, 30, 873–882. [Google Scholar] [CrossRef]
- Mu, C.; Zhang, S.; Yu, G.; Chen, N.; Li, X.; Liu, H. Overexpression of Small Heat Shock Protein LimHSP16.45 in Arabidopsis Enhances Tolerance to Abiotic Stresses. PLoS ONE 2013, 8, e82264. [Google Scholar] [CrossRef] [PubMed]
- Saurabh, S.; Sahoo, P. Lysozyme: An Important Defence Molecule of Fish Innate Immune System. Aquac. Res. 2008, 39, 223–239. [Google Scholar] [CrossRef]
- Rice, E.W.; Bridgewater, L. American Public Health Association. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2012; Volume 10. [Google Scholar]
- Bringolf, R.B.; Kwak, T.J.; Cope, W.G.; Larimore, M.S. Salinity Tolerance of Flathead Catfish: Implications for Dispersal of Introduced Populations. Trans. Am. Fish. Soc. 2005, 134, 927–936. [Google Scholar] [CrossRef]
- Jiang, W.; Feng, L.; Liu, Y.; Jiang, J.; Zhou, X. Myo-inositol Prevents Oxidative Damage, Inhibits Oxygen Radical Generation and Increases Antioxidant Enzyme Activities of Juvenile Jian Carp (Cyprinus carpio Var. Jian). Aquac. Res. 2009, 40, 1770–1776. [Google Scholar] [CrossRef]
- Zhang, X.-D.; Zhu, Y.-F.; Cai, L.-S.; Wu, T.-X. Effects of Fasting on the Meat Quality and Antioxidant Defenses of Market-Size Farmed Large Yellow Croaker (Pseudosciaena crocea). Aquaculture 2008, 280, 136–139. [Google Scholar] [CrossRef]
- Wang, S.-H.; Chen, J.-C. The Protective Effect of Chitin and Chitosan against Vibrio alginolyticus in White Shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2005, 19, 191–204. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Gisbert, E.; Fernández Monzón, I.; Alvarez-González, C. Prolonged Feed Deprivation Does Not Permanently Compromise Digestive Function in Migrating European Glass Eels Anguilla anguilla. J. Fish Biol. 2011, 78, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Decker, L.A. Worthington Enzyme Manual: Enzymes, Enzyme Reagents, Related Biochemicals; Worthington Biochemical Co.: Lakewood, NJ, USA, 1977. [Google Scholar]
- Gawlicka, A.K.; Parent, B.; Horn, M.H.; Ross, N.W.; Opstad, I.; Torrissen, O.J. Activity of Digestive Enzymes in Yolk-Sac Larvae of Atlantic Halibut (Hippoglossus hippoglossus): Indication of Readiness for First Feeding. Aquaculture 2000, 184, 303–314. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Benli, A.Ç.K.; Özkul, A. Acute Toxicity and Histopathological Effects of Sublethal Fenitrothion on Nile Tilapia, Oreochromis niloticus. Pestic. Biochem. Physiol. 2010, 97, 32–35. [Google Scholar] [CrossRef]
- Rand, G.M. Fundamentals of Aquatic Toxicology: Effects, Environmental Fate and Risk Assessment; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Lawson, E.; Anetekhai, M. Salinity Tolerance and Preference of Hatchery Reared Nile Tilapia, Oreochromis niloticus (Linneaus 1758). Asian J. Agric. Sci. 2011, 3, 104–110. [Google Scholar]
- Hassan, M.; Zakariah, M.; Wahab, W.; Muhammad, S.; Idris, N.; Jasmani, S. Histopathological and Behavioral Changes in Oreochromis sp. after Exposure to Different Salinities. J. Fish. Livest. Prod. 2013, 1, 2–5. [Google Scholar]
- Iwama, G.; Takemura, A.; Takano, K. Oxygen Consumption Rates of Tilapia in Fresh Water, Sea Water, and Hypersaline Sea Water. J. Fish Biol. 1997, 51, 886–894. [Google Scholar] [CrossRef]
- Rand, G.M.; Clark, J.R. Hazard/Risk Assessment of Pyridaben: I. Aquatic Toxicity and Environmental Chemistry. Ecotoxicology 2000, 9, 157–168. [Google Scholar] [CrossRef]
- Kefford, B.J.; Papas, P.J.; Metzeling, L.; Nugegoda, D. Do Laboratory Salinity Tolerances of Freshwater Animals Correspond with Their Field Salinity? Environ. Pollut. 2004, 129, 355–362. [Google Scholar] [CrossRef]
- Ghosh, A.; Ghosh, S.; Sarkar, N. On the Salinity Tolerance of Fry and Fingerlings of Indian Major Carps. J. Inland Fish. Soc. India 1973, 5, 215–217. [Google Scholar]
- Tsuzuki, M.Y.; Sugai, J.K.; Maciel, J.C.; Francisco, C.J.; Cerqueira, V.R. Survival, Growth and Digestive Enzyme Activity of Juveniles of the Fat Snook (Centropomus parallelus) Reared at Different Salinities. Aquaculture 2007, 271, 319–325. [Google Scholar] [CrossRef]
- Bœuf, G.; Payan, P. How Should Salinity Influence Fish Growth? Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 130, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Kilambi, R.; Zdinak, A. The Effects of Acclimation on the Salinity Tolerance of Grass Carp, Ctenopharyngodon idella (Cuv. and Val.). J. Fish Biol. 1980, 16, 171–175. [Google Scholar] [CrossRef]
- Pan, C.H.; Chien, Y.-H.; Hunter, B. The Resistance to Ammonia Stress of Penaeus monodon Fabricius Juvenile Fed Diets Supplemented with Astaxanthin. J. Exp. Mar. Biol. Ecol. 2003, 297, 107–118. [Google Scholar] [CrossRef]
- Al-Khshali, M.S.; Al Hilali, H.A. Some Physiological Changes (ALP, AST and ALT) of Common Carp (Cyprinus carpio) Caused by High Salinity. Biochem. Cell. Arch. 2019, 19, 4605–4610. [Google Scholar]
- Al-Khashal, M.S.; Al-Shawi, S.A.S. Effect of Salt Stress on ALT and AST Enzymes Activity and Cortisol Level in Adults of Carassius auratus. Pak. J. Nutr. 2013, 12, 97–100. [Google Scholar] [CrossRef]
- Roche, H.; Chaar, K.; Peres, G. The Effect of a Gradual Decrease in Salinity on the Significant Constituents of Tissue in the Sea Bass (Dicentrarchus labrax Pisces). Comp. Biochem. Physiol. A Physiol. 1989, 93, 785–789. [Google Scholar] [CrossRef]
- Sultan, F. Effect of Salt Acclimatization on Some Nutritional and Physiological Aspects in Juvenile Acanthopagrus latus. Ph.D. Thesis, University of Basrah, Basrah, Iraq, 2007. [Google Scholar]
- Blewett, T.A.; Binning, S.A.; Weinrauch, A.M.; Ivy, C.M.; Rossi, G.S.; Borowiec, B.G.; Lau, G.Y.; Overduin, S.L.; Aragao, I.; Norin, T. Physiological and Behavioural Strategies of Aquatic Animals Living in Fluctuating Environments. J. Exp. Biol. 2022, 225, jeb242503. [Google Scholar] [CrossRef]
- Kim, J.-H.; Park, H.-J.; Kim, K.-W.; Hwang, I.-K.; Kim, D.-H.; Oh, C.W.; Lee, J.S.; Kang, J.-C. Growth Performance, Oxidative Stress, and Non-Specific Immune Responses in Juvenile Sablefish, Anoplopoma fimbria, by Changes of Water Temperature and Salinity. Fish Physiol. Biochem. 2017, 43, 1421–1431. [Google Scholar] [CrossRef]
- Wu, J.H.; Xu, C.; Shan, C.Y.; Tan, R.X. Antioxidant Properties and PC12 Cell Protective Effects of APS-1, a Polysaccharide from Aloe vera var. Chinensis. Life Sci. 2006, 78, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Li, Y.; Hou, Y.; Qiu, H.; Zhou, Q. Effect of Dietary Vitamin C on the Growth Performance, Antioxidant Ability and Innate Immunity of Juvenile Yellow Catfish (Pelteobagrus fulvidraco R Ichardson). Aquac. Res. 2017, 48, 149–160. [Google Scholar] [CrossRef]
- Leite, T.; Branco, P.; Ferreira, M.T.; Santos, J.M. Activity, Boldness and Schooling in Freshwater Fish Are Affected by River Salinization. Sci. Total Environ. 2022, 819, 153046. [Google Scholar] [CrossRef]
- Issac, P.K.; Guru, A.; Velayutham, M.; Pachaiappan, R.; Arasu, M.V.; Al-Dhabi, N.A.; Choi, K.C.; Harikrishnan, R.; Arockiaraj, J. Oxidative Stress Induced Antioxidant and Neurotoxicity Demonstrated in Vivo Zebrafish Embryo or Larval Model and Their Normalization Due to Morin Showing Therapeutic Implications. Life Sci. 2021, 283, 119864. [Google Scholar] [CrossRef]
- Yin, F.; Peng, S.; Sun, P.; Shi, Z. Effects of Low Salinity on Antioxidant Enzymes Activities in Kidney and Muscle of Juvenile Silver Pomfret Pampus argenteus. Acta Ecol. Sin. 2011, 31, 55–60. [Google Scholar] [CrossRef]
- MaMartinez-Alvarez, R.M.; Hidalgo, M.C.; Domezain, A.; Morales, A.E.; García-Gallego, M.; Sanz, A. Physiological Changes of Sturgeon Acipenser naccarii Caused by Increasing Environmental Salinity. J. Exp. Biol. 2002, 205, 3699–3706. [Google Scholar] [CrossRef]
- Choi, C.Y.; An, K.W.; An, M.I. Molecular Characterization and mRNA Expression of Glutathione Peroxidase and Glutathione S-Transferase during Osmotic Stress in Olive Flounder (Paralichthys olivaceus). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2008, 149, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, W.-N.; Wang, A.; Wang, J.; Sun, R. Effects of Dietary Vitamin E Supplementation on Antioxidant Enzyme Activities in Litopenaeus vannamei (Boone, 1931) Exposed to Acute Salinity Changes. Aquaculture 2007, 265, 351–358. [Google Scholar] [CrossRef]
- Ross, S.W.; Dalton, D.A.; Kramer, S.; Christensen, B.L. Physiological (Antioxidant) Responses of Estuarine Fishes to Variability in Dissolved Oxygen. Comp. Biochem. Physiol. Toxicol. Pharmacol. 2001, 130, 289–303. [Google Scholar] [CrossRef]
- Zhao, F.; Zhuang, P.; Zhang, L.Z.; Huang, X.R.; Zhang, T.; Feng, G.P. Responses of Antioxidases in Different Tissues of Acipenser schrenckii to Increased Salinity in Water. Mar. Fish. Res. 2008, 29, 65–69. [Google Scholar]
- Tian, L.; Tan, P.; Yang, L.; Zhu, W.; Xu, D. Effects of Salinity on the Growth, Plasma Ion Concentrations, Osmoregulation, Non-Specific Immunity, and Intestinal Microbiota of the Yellow Drum (Nibea albiflora). Aquaculture 2020, 528, 735470. [Google Scholar] [CrossRef]
- Gaweł, S.; Wardas, M.; Niedworok, E.; Wardas, P. Malondialdehyde (MDA) as a Lipid Peroxidation Marker. Wiadomosci Lek. 2004, 57, 453–455. [Google Scholar]
- Lepage, G.; Munoz, G.; Champagne, J.; Roy, C.C. Preparative Steps Necessary for the Accurate Measurement of Malondialdehyde by High-Performance Liquid Chromatography. Anal. Biochem. 1991, 197, 277–283. [Google Scholar] [CrossRef]
- Liu, X.; Xi, Q.; Yang, L.; Li, H.; Jiang, Q.; Shu, G.; Wang, S.; Gao, P.; Zhu, X.; Zhang, Y.-L. The Effect of Dietary Panax Ginseng Polysaccharide Extract on the Immune Responses in White Shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2011, 30, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Wang, L.-N.; Zhang, D.; Liu, W.; Xu, W. Berberine Attenuates Oxidative Stress and Hepatocytes Apoptosis via Protecting Mitochondria in Blunt Snout Bream Megalobrama amblycephala Fed High-Fat Diets. Fish Physiol. Biochem. 2017, 43, 65–76. [Google Scholar] [CrossRef]
- Devasena, T.; Lalitha, S.; Padma, K. Lipid Peroxidation, Osmotic Fragility and Antioxidant Status in Children with Acute Post-Streptococcal Glomerulonephritis. Clin. Chim. Acta Int. J. Clin. Chem. 2001, 308, 155–161. [Google Scholar] [CrossRef]
- Chen, W.; Miraji, S.M.; Tian, Y.; Ma, X.; Jin, W.; Wen, H.; Xu, G.; Xu, P.; Cheng, H. Effect of Different Salinities on the Biochemical Properties and Meat Quality of Adult Freshwater Drum (Aplodinotus grunniens) During Temporary Rearing. Antioxidants 2024, 13, 1273. [Google Scholar] [CrossRef]
- Kurhaluk, N. Formation of an Antioxidant Profile in the Sea Trout (Salmo trutta m. trutta L.) from the Slupia River. Zoology 2019, 133, 54–65. [Google Scholar] [CrossRef]
- Moskowitz, J.M.; Safety, E.R. Ex Parte: Submitted to FCC Dockets Nos. 03-137, 13-84, 17-79 and 17-84. Ph.D. Thesis, University of California, Berkeley, CA, USA, 2019. [Google Scholar]
- Ma, H.; Wei, P.; Li, X.; Liu, S.; Tian, Y.; Zhang, Q.; Liu, Y. Effects of Photoperiod on Growth, Digestive, Metabolic and Non-Special Immunity Enzymes of Takifugu rubripes Larvae. Aquaculture 2021, 542, 736840. [Google Scholar] [CrossRef]
- Dawood, M.A.; Gewaily, M.; Sewilam, H. Combined Effects of Water Salinity and Ammonia Exposure on the Antioxidative Status, Serum Biochemistry, and Immunity of Nile Tilapia (Oreochromis niloticus). Fish Physiol. Biochem. 2023, 49, 1461–1477. [Google Scholar] [CrossRef]
- Oruc, E. Oxidative Stress Responses and Recovery Patterns in the Liver of Oreochromis niloticus Exposed to Chlorpyrifos-Ethyl. Bull. Environ. Contam. Toxicol. 2012, 88, 678–684. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Yin, S. Effects of Salinity Change on Two Superoxide Dismutases (SODs) in Juvenile Marbled Eel Anguilla marmorata. PeerJ 2016, 4, e2149. [Google Scholar] [CrossRef]
- Saoud, I.; Kreydiyyeh, S.; Chalfoun, A.; Fakih, M. Influence of Salinity on Survival, Growth, Plasma Osmolality and Gill Na+–K+–ATPase Activity in the Rabbitfish Siganus rivulatus. J. Exp. Mar. Biol. Ecol. 2007, 348, 183–190. [Google Scholar] [CrossRef]
- Sangiao-Alvarellos, S.; Arjona, F.J.; Míguez, J.M.; del Río, M.P.M.; Soengas, J.L.; Mancera, J.M. Growth Hormone and Prolactin Actions on Osmoregulation and Energy Metabolism of Gilthead Sea Bream (Sparus auratus). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006, 144, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Paumard, P.; Vaillier, J.; Coulary, B.; Schaeffer, J.; Soubannier, V.; Mueller, D.M.; Brèthes, D.; di Rago, J.; Velours, J. The ATP Synthase Is Involved in Generating Mitochondrial Cristae Morphology. EMBO J. 2002, 21, 221–230. [Google Scholar] [CrossRef]
- Lee, T.; Hwang, P.; Shieh, Y.; Lin, C. The Relationship Betweendeep-Hole’mitochondria-Rich Cells and Salinity Adaptation in the Euryhaline Teleost, Oreochromis mossambicus. Fish Physiol. Biochem. 2000, 23, 133–140. [Google Scholar] [CrossRef]
- Fielder, D.S.; Allan, G.L.; Pepperall, D.; Pankhurst, P.M. The Effects of Changes in Salinity on Osmoregulation and Chloride Cell Morphology of Juvenile Australian Snapper, Pagrus auratus. Aquaculture 2007, 272, 656–666. [Google Scholar] [CrossRef]
- Liu, J.; Ai, T.; Yang, J.; Shang, M.; Jiang, K.; Yin, Y.; Gao, L.; Jiang, W.; Zhao, N.; Ju, J. Effects of Salinity on Growth, Digestive Enzyme Activity, and Antioxidant Capacity of Spotbanded Scat (Selenotoca multifasciata) Juveniles. Fishes 2024, 9, 309. [Google Scholar] [CrossRef]
- Pan, L.; Tang, X.; Liu, H.; Tian, J. Effects of Salinity on Plasma Osmolality and Gill Na+-K+-ATPase Activity of Juvinile Japanese Flounder Paralichthys olivaceus. Oceanol. Limnol. Sin. 2006, 37, 6. [Google Scholar]
- Gheisvandi, N.; Hajimoradloo, A.; Ghorbani, R.; Hoseinifar, S.H. The Effects of Gradual or Abrupt Changes of Salinity on Digestive Enzymes Activity of Caspian Kutum, Rutilus kutum (Kamensky, 1901) Larvae. J. Appl. Ichthyol. 2015, 31, 1107–1112. [Google Scholar] [CrossRef]
- Navarro-Guillén, C.; Yúfera, M.; Perera, E. Biochemical Features and Modulation of Digestive Enzymes by Environmental Temperature in the Greater Amberjack, Seriola dumerili. Front. Mar. Sci. 2022, 9, 960746. [Google Scholar] [CrossRef]
- Hieu, D.Q.; Hang, B.T.B.; Huong, D.T.T.; Kertaoui, N.E.; Farnir, F.; Phuong, N.T.; Kestemont, P. Salinity Affects Growth Performance, Physiology, Immune Responses and Temperature Resistance in Striped Catfish (Pangasianodon hypophthalmus) during Its Early Life Stages. Fish Physiol. Biochem. 2021, 47, 1995–2013. [Google Scholar] [CrossRef]
- Hamed, S.S.; Jiddawi, N.S.; Poj, B. Effect of Salinity Levels on Growth, Feed Utilization, Body Composition and Digestive Enzymes Activities of Juvenile Silver Pompano Trachinotus blochii. Int. J. Fish. Aquat. Stud. 2016, 4, 279–283. [Google Scholar]
- Fang, L.S.; Chiou, S.F. Effect of Salinity on the Activities of Digestive Proteases from the Tilapia Fish, Oreochromis niloticus in Different Culture Environments. Comp. Biochem. Physiol. A Physiol. 1989, 93, 439–443. [Google Scholar]
- Squires, E.; Haard, N.; Feltham, L. Gastric Proteases of the Greenland Cod Gadus ogac. I. Isolation and Kinetic Properties. Biochem. Cell Biol. Biochim. Biol. Cell. 1986, 64, 205–214. [Google Scholar] [CrossRef]
- Mozanzadeh, M.T.; Safari, O.; Oosooli, R.; Mehrjooyan, S.; Najafabadi, M.Z.; Hoseini, S.J.; Saghavi, H.; Monem, J. The Effect of Salinity on Growth Performance, Digestive and Antioxidant Enzymes, Humoral Immunity and Stress Indices in Two Euryhaline Fish Species: Yellowfin Seabream (Acanthopagrus latus) and Asian Seabass (Lates calcarifer). Aquaculture 2021, 534, 736329. [Google Scholar] [CrossRef]
- Bolasina, S.N.; Tagawa, M.; Yamashita, Y. Changes on Cortisol Level and Digestive Enzyme Activity in Juveniles of Japanese Flounder, Paralichthys olivaceus, Exposed to Different Salinity Regimes. Aquaculture 2007, 266, 255–261. [Google Scholar] [CrossRef]
- Liu, Z.-F.; Gao, X.-Q.; Yu, J.-X.; Qian, X.-M.; Xue, G.-P.; Zhang, Q.-Y.; Liu, B.-L.; Hong, L. Effects of Different Salinities on Growth Performance, Survival, Digestive Enzyme Activity, Immune Response, and Muscle Fatty Acid Composition in Juvenile American Shad (Alosa sapidissima). Fish Physiol. Biochem. 2017, 43, 761–773. [Google Scholar] [CrossRef]
- Chiu, Y.N.; Benitez, L.V. Studies on the Carbohydrases in the Digestive Tract of the Milkfish Chanos Chanos. Mar. Biol. 1981, 61, 247–254. [Google Scholar] [CrossRef]
- Soltan, N.M.; Soaudy, M.R.; Abdella, M.M.; Hassaan, M.S. Partial Dietary Fishmeal Replacement with Mixture of Plant Protein Sources Supplemented with Exogenous Enzymes Modify Growth Performance, Digestibility, Intestinal Morphology, Haemato-Biochemical and Immune Responses for Nile Tilapia, Oreochromis niloticus. Anim. Feed Sci. Technol. 2023, 299, 115642. [Google Scholar] [CrossRef]
- Abolfathi, M.; Hajimoradloo, A.; Ghorbani, R.; Zamani, A. Effect of Starvation and Refeeding on Digestive Enzyme Activities in Juvenile Roach, Rutilus rutilus caspicus. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2012, 161, 166–173. [Google Scholar] [CrossRef]
- Psochiou, E.; Mamuris, Z.; Panagiotaki, P.; Kouretas, D.; Moutou, K.A. The Response of Digestive Proteases to Abrupt Salinity Decrease in the Euryhaline Sparid Sparus aurata L. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 147, 156–163. [Google Scholar] [CrossRef]
- Iwama, G.K.; Thomas, P.T.; Forsyth, R.B.; Vijayan, M.M. Heat Shock Protein Expression in Fish. Rev. Fish Biol. Fish. 1998, 8, 35–56. [Google Scholar] [CrossRef]
- Morimoto, R.I. Regulation of the Heat Shock Transcriptional Response: Cross Talk between a Family of Heat Shock Factors, Molecular Chaperones, and Negative Regulators. Genes Dev. 1998, 12, 3788–3796. [Google Scholar] [CrossRef]
- Deane, E.E.; Woo, N.Y. Differential Gene Expression Associated with Euryhalinity in Sea Bream (Sparus sarba). Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2004, 287, R1054–R1063. [Google Scholar] [CrossRef]
- Larsen, P.F.; Nielsen, E.E.; Koed, A.; Thomsen, D.S.; Olsvik, P.A.; Loeschcke, V. Interpopulation Differences in Expression of Candidate Genes for Salinity Tolerance in Winter Migrating Anadromous Brown Trout (Salmo trutta L.). BMC Genet. 2008, 9, 12. [Google Scholar] [CrossRef]
- Tine, M.; Bonhomme, F.; McKenzie, D.J.; Durand, J.-D. Differential Expression of the Heat Shock Protein Hsp70 in Natural Populations of the Tilapia, Sarotherodon melanotheron, Acclimatised to a Range of Environmental Salinities. BMC Ecol. 2010, 10, 11. [Google Scholar] [CrossRef]
- Sun, M.; Jiang, K.; Zhang, F.; Zhang, D.; Shen, A.; Jiang, M.; Shen, X.; Ma, L. Effects of Various Salinities on Na+-K+-ATPase, Hsp70 and Hsp90 Expression Profiles in Juvenile Mitten Crabs, Eriocheir sinensis. Genet. Mol. Res. 2012, 11, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Lai, B.-T.; Chin, N.W.; Stanek, A.E.; Keh, W.; Lanks, K.W. Quantitation and Intracellular Localization of the 85K Heat Shock Protein by Using Monoclonal and Polyclonal Antibodies. Mol. Cell. Biol. 1984, 4, 2802–2810. [Google Scholar] [PubMed]
- Ramaglia, V.; Buck, L.T. Time-Dependent Expression of Heat Shock Proteins 70 and 90 in Tissues of the Anoxic Western Painted Turtle. J. Exp. Biol. 2004, 207, 3775–3784. [Google Scholar] [CrossRef] [PubMed]
- Padmini, E.; Rani, M.U. Impact of Seasonal Variation on HSP70 Expression Quantitated in Stressed Fish Hepatocytes. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2008, 151, 278–285. [Google Scholar] [CrossRef]
- Antonopoulou, E.; Kentepozidou, E.; Feidantsis, K.; Roufidou, C.; Despoti, S.; Chatzifotis, S. Starvation and Re-Feeding Affect Hsp Expression, MAPK Activation and Antioxidant Enzymes Activity of European Sea Bass (Dicentrarchus labrax). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2013, 165, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Werner, I. The Influence of Salinity on the Heat-Shock Protein Response of Potamocorbula amurensis (Bivalvia). Mar. Environ. Res. 2004, 58, 803–807. [Google Scholar] [CrossRef]


| Gene | Primers |
|---|---|
| β-actin | F:5′-AGGCTGTGCTGTCCCTGTAT-3′ |
| R:5′-GCTGTGGTGGTGAAGGAGTAC-3′ | |
| HSP 70 | F:5′-ATTGCTGAAGCCTACCTCGG-3′ |
| R:5′-GTGCCTCCACCGAGATCAAA-3′ | |
| HSP 90 | F:5′-CTTTGCCACGTTCTGGAAGC-3′ |
| R:5′-CCAGACCTCCAACAGCGAAA-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amankwah, J.F.; Jin, W.; Ma, X.; Xu, P.; Wen, H.; Amuneke, K.E.; Munganga, B.P.; Li, K.; Liu, J.; Li, H. Salinity Tolerance in Freshwater Drum (Aplodinotus grunniens): Investigating Biochemical, Antioxidant, Digestive Enzyme, and Gene Expression Responses to Acute Salinity Stress. Animals 2025, 15, 1015. https://doi.org/10.3390/ani15071015
Amankwah JF, Jin W, Ma X, Xu P, Wen H, Amuneke KE, Munganga BP, Li K, Liu J, Li H. Salinity Tolerance in Freshwater Drum (Aplodinotus grunniens): Investigating Biochemical, Antioxidant, Digestive Enzyme, and Gene Expression Responses to Acute Salinity Stress. Animals. 2025; 15(7):1015. https://doi.org/10.3390/ani15071015
Chicago/Turabian StyleAmankwah, Justice Frimpong, Wu Jin, Xueyan Ma, Pao Xu, Haibo Wen, Kennedy Emeka Amuneke, Brian Pelekelo Munganga, Kang Li, Jingwei Liu, and Hongxia Li. 2025. "Salinity Tolerance in Freshwater Drum (Aplodinotus grunniens): Investigating Biochemical, Antioxidant, Digestive Enzyme, and Gene Expression Responses to Acute Salinity Stress" Animals 15, no. 7: 1015. https://doi.org/10.3390/ani15071015
APA StyleAmankwah, J. F., Jin, W., Ma, X., Xu, P., Wen, H., Amuneke, K. E., Munganga, B. P., Li, K., Liu, J., & Li, H. (2025). Salinity Tolerance in Freshwater Drum (Aplodinotus grunniens): Investigating Biochemical, Antioxidant, Digestive Enzyme, and Gene Expression Responses to Acute Salinity Stress. Animals, 15(7), 1015. https://doi.org/10.3390/ani15071015








