A Quadruplex RT-qPCR for the Detection of Porcine Sapelovirus, Porcine Kobuvirus, Porcine Teschovirus, and Porcine Enterovirus G
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reference Strains
2.2. Design of Primers and Probes
2.3. Clinical Samples
2.4. Extraction of Nucleic Acid
2.5. Construction of Standard Plasmids
2.6. Optimization of Reaction System and Reaction Procedure
2.7. Generation of Standard Curves
2.8. Analytical Specificity Analysis
2.9. Analytical Sensitivity Analysis
2.10. Repeatability Analysis
2.11. Assessment of the Developed Assay Using Clinical Samples
3. Results
3.1. Preparation of the Standard Plasmids
3.2. Attainment of the Optimal Reaction Parameters
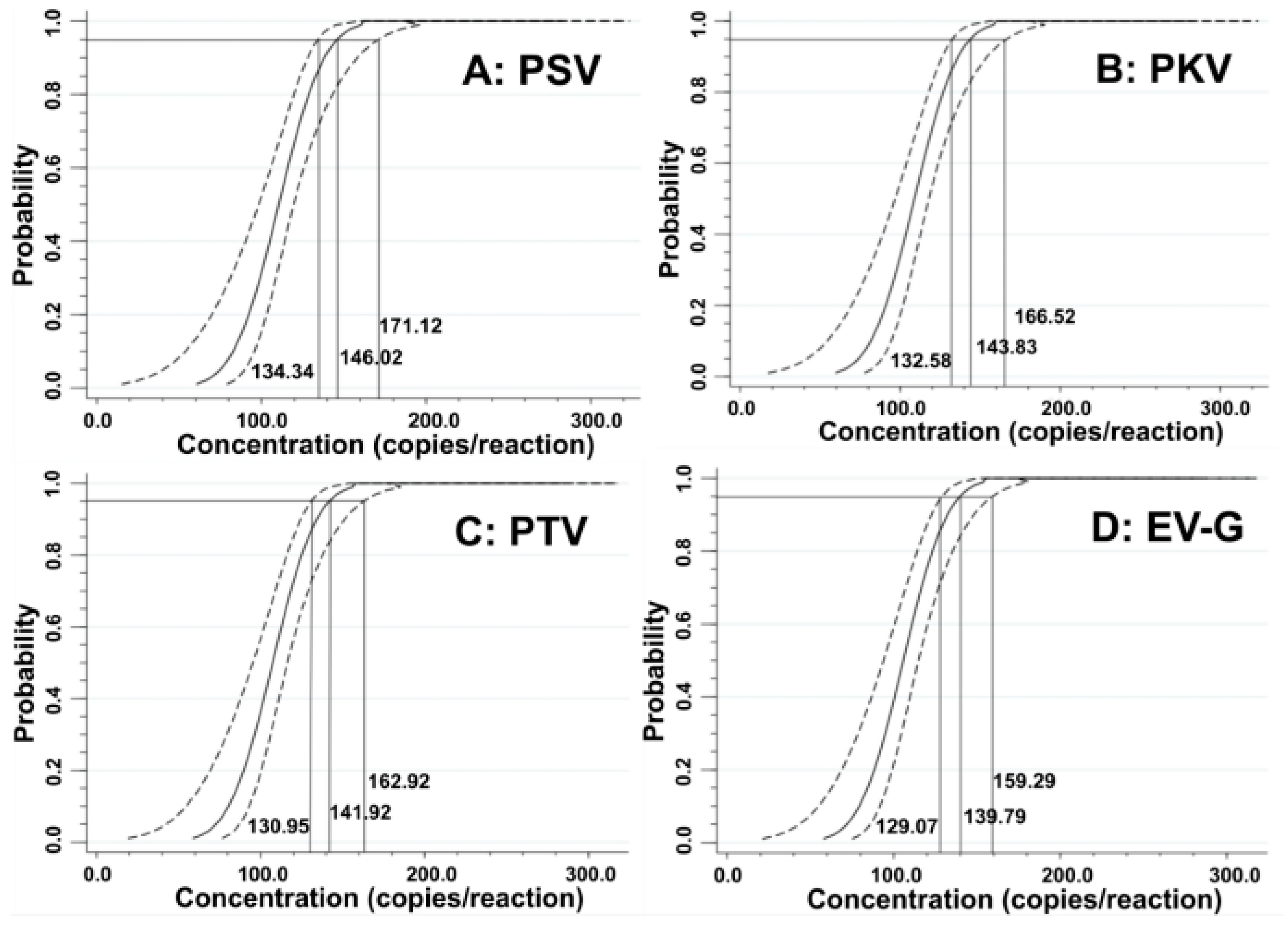
3.3. Generation of the Standard Curves
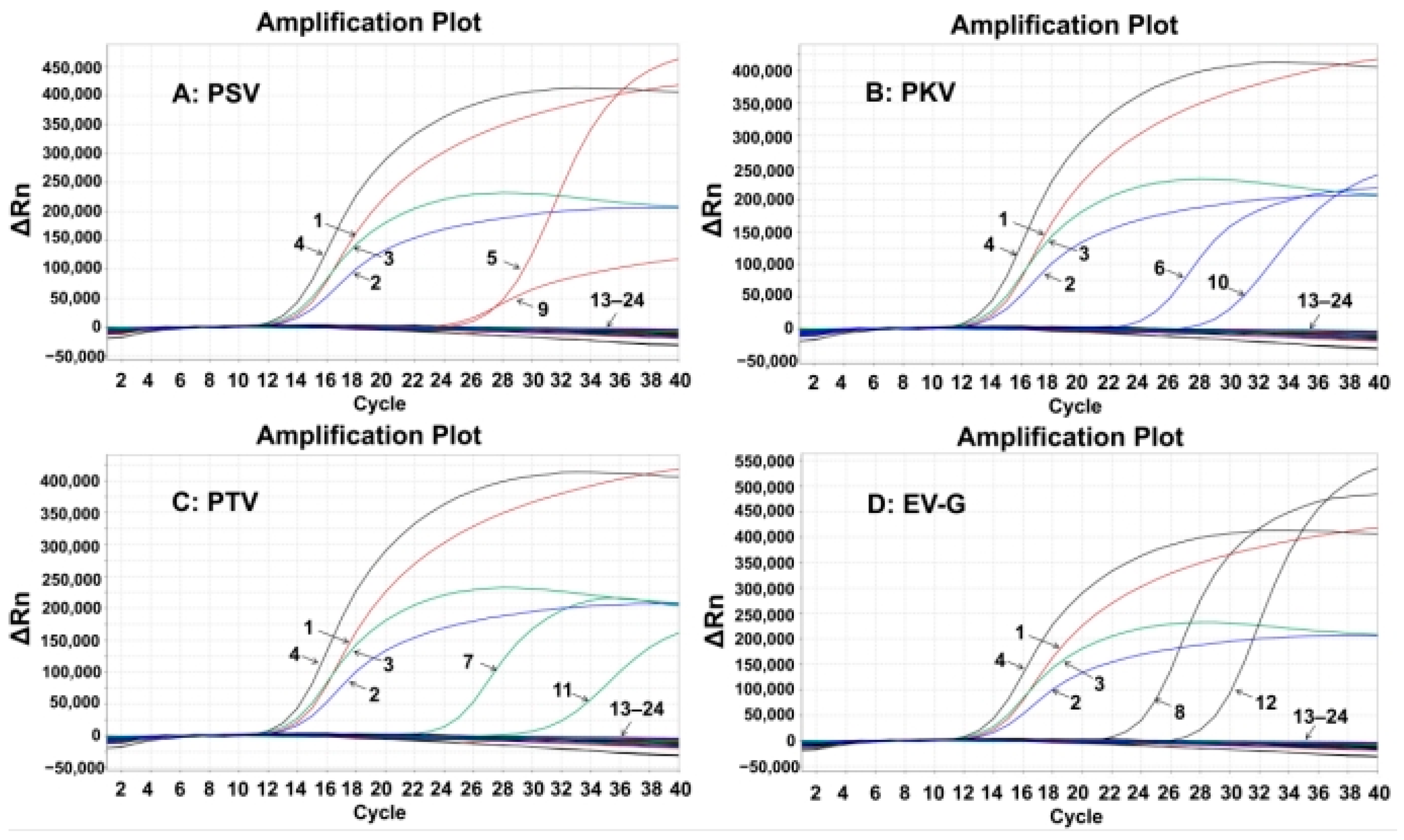
3.4. Specificity of the Quadruplex RT-qPCR
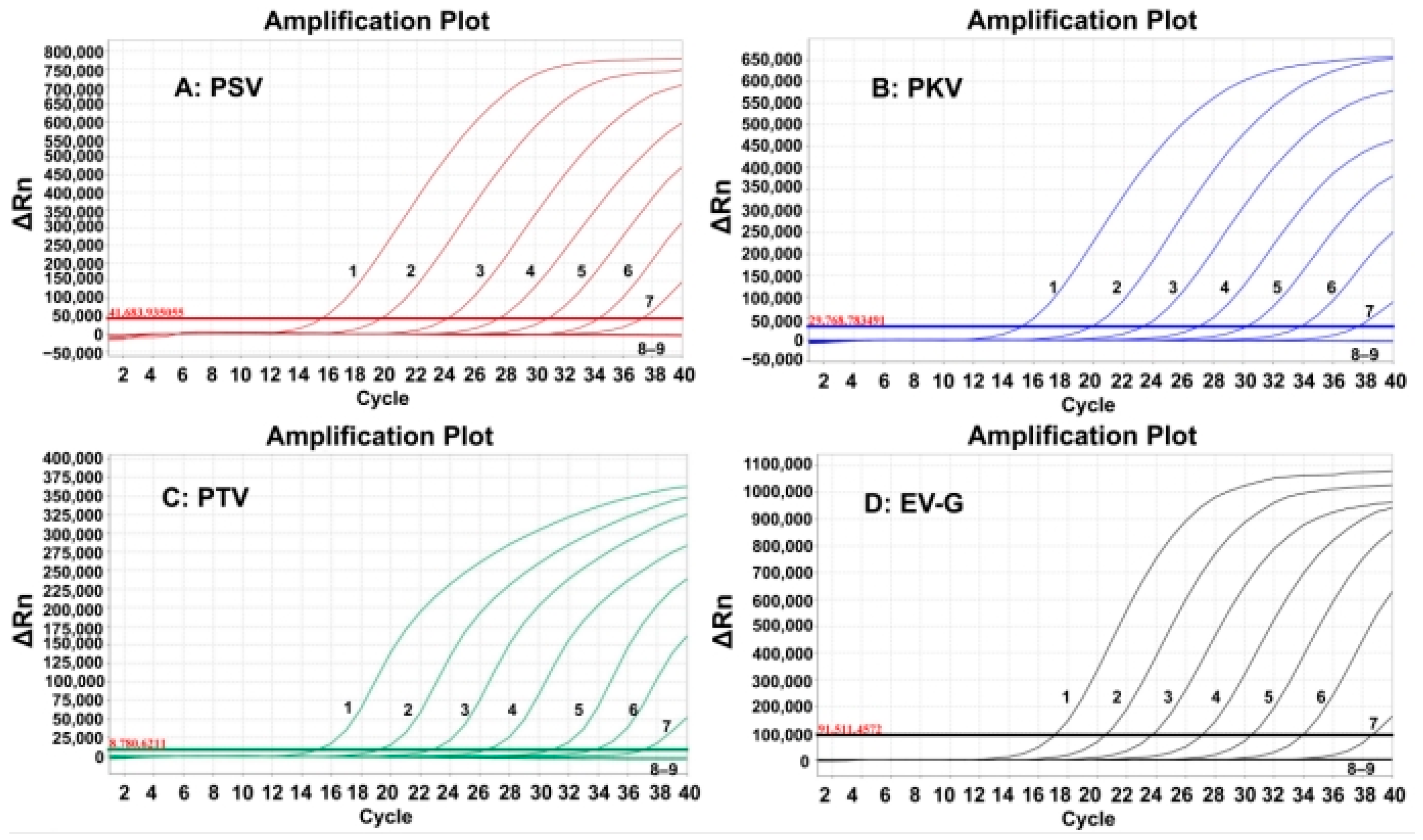
3.5. Sensitivity of the Quadruplex RT-qPCR
3.6. Repeatability of the Quadruplex RT-qPCR
3.7. Assessment Results of the Clinical Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zell, R.; Delwart, E.; Gorbalenya, A.E.; Hovi, T.; King, A.M.Q.; Knowles, N.J.; Lindberg, A.M.; Pallansch, M.A.; Palmenberg, A.C.; Reuter, G.; et al. ICTV Virus Taxonomy Profile: Picornaviridae. J. Gen. Virol. 2017, 98, 2421–2422. [Google Scholar] [CrossRef] [PubMed]
- Son, K.Y.; Kim, D.S.; Kwon, J.; Choi, J.S.; Kang, M.I.; Belsham, G.J.; Cho, K.O. Full-Length Genomic Analysis of Korean Porcine Sapelovirus Strains. PLoS ONE 2014, 9, e107860. [Google Scholar] [CrossRef]
- Boros, Á.; László, Z.; Pankovics, P.; Marosi, A.; Albert, M.; Cságola, A.; Bíró, H.; Fahsbender, E.; Delwart, E.; Reuter, G. High Prevalence, Genetic Diversity and a Potentially Novel Genotype of Sapelovirus A (Picornaviridae) in Enteric and Respiratory Samples in Hungarian Swine Farms. J. Gen. Virol. 2020, 101, 609–621. [Google Scholar] [CrossRef]
- Kumari, S.; Ray, P.K.; Singh, R.; Desingu, P.A.; Varshney, R.; Saikumar, G. Pathological and Molecular Investigation of Porcine Sapelovirus Infection in Naturally Affected Indian Pigs. Microb. Pathog. 2019, 127, 320–325. [Google Scholar] [CrossRef]
- Chen, J.; Suo, X.; Cao, L.; Yuan, C.; Shi, L.; Duan, Y.; Zheng, H.; Wang, Q. Virome Analysis for Identification of a Novel Porcine Sapelovirus Isolated in Western China. Microbiol. Spectr. 2022, 10, e0180122. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, L.; Jin, T.; Cheng, Y.; Zhang, X.; Jiao, S.; Huang, T.; Zhang, Y.; Yan, Y.; Gu, J.; et al. Characterization and Epidemiological Survey of Porcine Sapelovirus in China. Vet. Microbiol. 2019, 232, 13–21. [Google Scholar] [CrossRef]
- Arruda, P.H.; Arruda, B.L.; Schwartz, K.J.; Vannucci, F.; Resende, T.; Rovira, A.; Sundberg, P.; Nietfeld, J.; Hause, B.M. Detection of a Novel Sapelovirus in Central Nervous Tissue of Pigs with Polioencephalomyelitis in the USA. Transbound. Emerg. Dis. 2017, 64, 311–315. [Google Scholar] [CrossRef]
- Lu, S.J.; Ma, M.Y.; Yan, X.G.; Zhao, F.J.; Hu, W.Y.; Ding, Q.W.; Ren, H.J.; Xiang, Y.Q.; Zheng, L.L. Development and Application of a Low-Priced Duplex Quantitative PCR Assay Based on SYBR Green I for the Simultaneous Detection of Porcine Deltacoronavirus and Porcine Sapelovirus. Vet. Med. 2023, 68, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, Y.M.; Zhang, W.; Werid, G.M.; Zhang, H.; Feng, Y.; Pan, Y.; Zhang, L.; Li, C.; Lin, H.; Chen, H.; et al. Isolation, Characterization, and Molecular Detection of Porcine Sapelovirus. Viruses 2022, 14, 349. [Google Scholar] [CrossRef]
- Liu, X.; Oka, T.; Wang, Q. Genomic Characterization of a US Porcine Kobuvirus Strain. Arch. Microbiol. 2015, 197, 1033–1040. [Google Scholar] [CrossRef]
- Khamrin, P.; Maneekarn, N.; Okitsu, S.; Ushijima, H. Epidemiology of Human and Animal Kobuviruses. Virusdisease 2014, 25, 195–200. [Google Scholar]
- Werid, G.M.; Ibrahim, Y.M.; Chen, H.; Fu, L.; Wang, Y. Molecular Detection and Genetic Characterization of Potential Zoonotic Swine Enteric Viruses in Northern China. Pathogens 2022, 11, 417. [Google Scholar] [CrossRef]
- Zang, Y.; Feng, B.; Huang, Z.; Zhao, D.; Qi, W.; Qiu, Y.; Qiu, M.; Li, C.; Lin, H.; Zheng, W.; et al. Epidemiologic and Genomic Characterizations of Porcine Kobuviruses in Diarrheic and Healthy Pigs. Animals 2023, 13, 3129. [Google Scholar] [CrossRef]
- Wu, S.; Gou, F.; Meng, J.; Jin, X.; Liu, W.; Ding, W.; Xu, W.; Gu, C.; Hu, X.; Cheng, G.; et al. Porcine Kobuvirus Enhances Porcine Epidemic Diarrhea Virus Pathogenicity and Alters the Number of Intestinal Lymphocytes in Piglets. Vet. Microbiol. 2024, 293, 110100. [Google Scholar]
- Oba, M.; Naoi, Y.; Ito, M.; Masuda, T.; Katayama, Y.; Sakaguchi, S.; Omatsu, T.; Furuya, T.; Yamasato, H.; Sunaga, F.; et al. Metagenomic Identification and Sequence Analysis of a Teschovirus A-Related Virus in Porcine Feces in Japan, 2014-2016. Infect. Genet. Evol. 2018, 66, 210–216. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, M.; Wu, M.; Ghonaim, A.H.; Fan, S.; He, Q. Isolation and Genetic Characteristics of a Neurotropic Teschovirus Variant Belonging to Genotype 1 in Northeast China. Arch. Virol. 2021, 166, 1355–1370. [Google Scholar]
- Zhang, B.; Guo, R.; Xiao, L.; Zhong, C.; Yuan, X.; Huang, J.; Zhu, X.; Zhou, J.; Fan, B.; Xue, T.; et al. Analysis on the Genome of a Teschovirus Type 1 Isolates with Swine Diarrhea. Heliyon 2023, 9, e14710. [Google Scholar]
- Liang, W.; Wu, X.; Ding, Z.; Zhong, S.; Qian, X.; Ye, P.; Liu, H.; Chen, Z.; Zhang, J.; Cao, H.; et al. Identification of a Novel Porcine Teschovirus 2 Strain as Causative Agent of Encephalomyelitis in Suckling Piglets with High Mortality in China. BMC Vet. Res. 2023, 19, 2. [Google Scholar]
- Carnero, J.; Prieto, C.; Polledo, L.; Martínez-Lobo, F.J. Detection of Teschovirus Type 13 from Two Swine Herds Exhibiting Nervous Clinical Signs in Growing Pigs. Transbound. Emerg. Dis. 2018, 65, e489–e493. [Google Scholar] [PubMed]
- Yang, T.; Li, R.; Yao, Q.; Zhou, X.; Liao, H.; Ge, M.; Yu, X. Prevalence of Porcine Teschovirus Genotypes in Hunan, China: Identification of Novel Viral Species and Genotypes. J. Gen. Virol. 2018, 99, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.; Kattoor, J.J.; Sircar, S.; VinodhKumar, O.R.; Thomas, P.; Ghosh, S.; Malik, Y.S. Detection and Molecular Characterization of Porcine Teschoviruses in India: Identification of New Genotypes. Indian J. Microbiol. 2024, 64, 963–972. [Google Scholar] [PubMed]
- Bhat, S.; Ansari, M.I.; Kattoor, J.J.; Sircar, S.; Dar, P.S.; Deol, P.; Vinodh Kumar, O.R.; Thomas, P.; Ghosh, S.; El Zowalaty, M.E.; et al. Emerging Porcine Enterovirus G Infections, Epidemiological, Complete Genome Sequencing, Evolutionary and Risk Factor Analysis in India. Virology 2024, 590, 109906. [Google Scholar]
- Xiao, D.; Zhang, L.; Li, S.; Liang, Y.; Wu, R.; Wen, Y.; Yan, Q.; Du, S.; Zhao, Q.; Han, X.; et al. Characterization, Phylogenetic Analysis, and Pathogenicity of a Novel Genotype 2 Porcine Enterovirus G. Virus Res. 2023, 335, 199185. [Google Scholar]
- Mi, X.; Yang, C.; Lu, Y.; Wang, H.; Qin, Q.; Chen, R.; Chen, Z.; Luo, Y.; Chen, Y.; Wei, Z.; et al. Isolation, Identification, and Evaluation of the Pathogenicity of a Porcine Enterovirus G Isolated from China. Front. Vet. Sci. 2021, 8, 712679. [Google Scholar]
- Van Dung, N.; Anh, P.H.; Van Cuong, N.; Hoa, N.T.; Carrique-Mas, J.; Hien, V.B.; Sharp, C.; Rabaa, M.; Berto, A.; Campbell, J.; et al. Large-Scale Screening and Characterization of Enteroviruses and Kobuviruses Infecting Pigs in Vietnam. J. Gen. Virol. 2016, 97, 378–388. [Google Scholar] [PubMed]
- Ibrahim, Y.M.; Zhang, W.; Wang, X.; Werid, G.M.; Fu, L.; Yu, H.; Wang, Y. Molecular Characterization and Pathogenicity Evaluation of Enterovirus G Isolated from Diarrheic Piglets. Microbiol. Spectr. 2023, 11, e0264323. [Google Scholar]
- Imai, R.; Rongduo, W.; Kaixin, L.; Borjigin, S.; Matsumura, H.; Masuda, T.; Ozawa, T.; Oba, M.; Makino, S.; Nagai, M.; et al. Novel Recombinant Porcine Enterovirus G Viruses Lacking Structural Proteins Are Maintained in Pig Farms in Japan. J. Vet. Med. Sci. 2023, 85, 252–265. [Google Scholar]
- Prodělalová, J. The Survey of Porcine Teschoviruses, Sapeloviruses and Enteroviruses B Infecting Domestic Pigs and Wild Boars in the Czech Republic between 2005 and 2011. Infect. Genet. Evol. 2012, 12, 1447–1451. [Google Scholar]
- Stäubli, T.; Rickli, C.I.; Torgerson, P.R.; Fraefel, C.; Lechmann, J. Porcine Teschovirus, Sapelovirus, and Enterovirus in Swiss Pigs: Multiplex RT-PCR Investigation of Viral Frequencies and Disease Association. J. Vet. Diagn. Investig. 2021, 33, 864–874. [Google Scholar]
- Hawkins, S.F.C.; Guest, P.C. Multiplex Analyses Using Real-Time Quantitative PCR. Methods Mol. Biol. 2017, 1546, 125–133. [Google Scholar]
- Kralik, P.; Ricchi, M. A Basic Guide to Real Time PCR in Microbial Diagnostics: Definitions, Parameters, and Everything. Front. Microbiol. 2017, 8, 108. [Google Scholar]
- Ma, Y.; Shi, K.; Chen, Z.; Shi, Y.; Zhou, Q.; Mo, S.; Wei, H.; Hu, L.; Mo, M. Simultaneous Detection of Porcine Respiratory Coronavirus, Porcine Reproductive and Respiratory Syndrome Virus, Swine Influenza Virus, and Pseudorabies Virus via Quadruplex One-Step RT-qPCR. Pathogens 2024, 13, 341. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Sun, Z.H.; Che, Y.L.; Chen, R.J.; Wu, X.M.; Wu, R.J.; Wang, L.B.; Zhou, L.J. High Prevalence, Genetic Diversity, and Recombination of Porcine Sapelovirus in Pig Farms in Fujian, Southern China. Viruses 2023, 15, 1751. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Y.; Chen, J.; Zhang, X.; Shi, H.; Shi, D.; Gao, J.; Feng, L. Development of TaqMan Real-Time Reverse Transcription-Polymerase Chain Reaction for the Detection and Quantitation of Porcine Kobuvirus. J. Virol. Methods 2016, 234, 132–136. [Google Scholar]
- Zhang, C.; Wang, Z.; Hu, F.; Liu, Y.; Qiu, Z.; Zhou, S.; Cui, S.; Wang, M. The Survey of Porcine Teschoviruses in Field Samples in China with a Universal Rapid Probe Real-Time RT-PCR Assay. Trop. Anim. Health Prod. 2013, 45, 1057–1061. [Google Scholar]
- Smoľak, D.; Šalamúnová, S.; Jacková, A.; Haršányová, M.; Budiš, J.; Szemes, T.; Vilček, Š. Analysis of RNA Virome in Rectal Swabs of Healthy and Diarrheic Pigs of Different Age. Comp. Immunol. Microbiol. Infect. Dis. 2022, 90–91, 101892. [Google Scholar]
- Zhou, H.; Shi, K.; Long, F.; Zhao, K.; Feng, S.; Yin, Y.; Xiong, C.; Qu, S.; Lu, W.; Li, Z. A Quadruplex qRT-PCR for Differential Detection of Four Porcine Enteric Coronaviruses. Vet. Sci. 2022, 9, 634. [Google Scholar] [CrossRef]
- He, J.; Shi, K.; Shi, Y.; Yin, Y.; Feng, S.; Long, F.; Qu, S.; Song, X. Development of a Quadruplex RT-qPCR for the Detection of Porcine Astrovirus, Porcine Sapovirus, Porcine Norovirus, and Porcine Rotavirus A. Pathogens 2024, 13, 1052. [Google Scholar] [CrossRef]
- Lan, D.; Ji, W.; Yang, S.; Cui, L.; Yang, Z.; Yuan, C.; Hua, X. Isolation and Characterization of the First Chinese Porcine Sapelovirus Strain. Arch. Virol. 2011, 156, 1567–1574. [Google Scholar]
- Liu, J.; Li, B.; Tao, J.; Cheng, J.; Shi, Y.; Qiao, C.; Shen, X.; Liu, H. Development of an Indirect ELISA Method Based on the VP1 Protein for Detection of IgG Antibodies against Porcine Sapelovirus. Can. J. Vet. Res. 2023, 87, 176–183. [Google Scholar]
- Reuter, G.; Boldizsár, A.; Kiss, I.; Pankovics, P. Candidate New Species of Kobuvirus in Porcine Hosts. Emerg. Infect. Dis. 2008, 14, 1968–1970. [Google Scholar] [PubMed]
- Yu, J.; Jin, M.; Zhang, Q.; Li, H.; Li, D.; Xu, Z.; Li, J.; Cui, S.; Yang, S.; Liu, N.; et al. Candidate Porcine Kobuvirus, China. Emerg. Infect. Dis. 2009, 15, 823–825. [Google Scholar]
- Wei, R.; Shang, R.; Cheng, K.; Wang, S.; Yuan, X.; Wu, J.; Yu, Z. Phylogenetic Analysis and Molecular Characterization of the Co-Infection of the New Variant of the Porcine Epidemic Diarrhea Virus and the Novel Porcine Kobuvirus Isolated from Piglets with Diarrhea. Braz. J. Microbiol. 2023, 54, 2527–2534. [Google Scholar]
- Cui, Y.; Li, J.; Guo, J.; Pan, Y.; Tong, X.; Liu, C.; Wang, D.; Xu, W.; Shi, Y.; Ji, Y.; et al. Evolutionary Origin, Genetic Recombination, and Phylogeography of Porcine Kobuvirus. Viruses 2023, 15, 240. [Google Scholar] [CrossRef]
- Sytiuk, M.P.; Bezymennyy, M.V.; Halka, I.V.; Uhovskyy, V.V.; Muzykina, L.M.; Lavalley, M.; Nychyk, S.A.; Nedosekov, V.V.; Howard, M.W.; Bortz, E. Seroprevalence of Enzootic Teschen Disease in the Wild Boar Population in Ukraine. Vector Borne Zoonotic Dis. 2022, 22, 138–147. [Google Scholar]
- Wang, B.; Tian, Z.J.; Gong, D.Q.; Li, D.Y.; Wang, Y.; Chen, J.Z.; An, T.Q.; Peng, J.M.; Tong, G.Z. Isolation of Serotype 2 Porcine Teschovirus in China: Evidence of Natural Recombination. Vet. Microbiol. 2010, 146, 138–143. [Google Scholar] [PubMed]
- Li, Y.; Chen, S.; Shi, Y.; Huang, H.; Wang, W.; Zheng, M.; Zhao, C.; Zhang, X.; Lei, X.; Sun, W.; et al. Construction and Characterization of the Full-Length cDNA of an Infectious Clone of Emerging Porcine Teschovirus-2. Pathog. Dis. 2022, 80, ftac033. [Google Scholar]
- Yang, T.; Lu, Y.; Zhang, L.; Li, X. Identification of Novel Genotypes Belonging to the Species Teschovirus A from Indigenous Pigs in Western Jiangxi, China. Arch. Virol. 2020, 165, 993–1001. [Google Scholar]
- Vilar, M.J.; Peralta, B.; García-Bocanegra, I.; Simon-Grifé, M.; Bensaid, A.; Casal, J.; Segalés, J.; Pina-Pedrero, S. Distribution and Genetic Characterization of Enterovirus G and Sapelovirus A in Six Spanish Swine Herds. Virus Res. 2016, 215, 42–49. [Google Scholar]
- Janetanakit, T.; Chaiyawong, S.; Charoenkul, K.; Tangwangvivat, R.; Chamsai, E.; Udom, K.; Jairak, W.; Amonsin, A. Distribution and Genetic Diversity of Enterovirus G (EV-G) on Pig Farms in Thailand. BMC Vet. Res. 2021, 17, 277. [Google Scholar]
- Hong, D.; Bian, J.; Zeng, L.; Huang, S.; Qin, Y.; Chen, Y.; Wei, Z.; Huang, W.; Ouyang, K. A Novel VP1-Based Enzyme-Linked Immunosorbent Assay Revealed Widespread Enterovirus G Infections in Guangxi, China. J. Virol. Methods 2024, 325, 114873. [Google Scholar]
- Patel, S.K.; Agrawal, A.; Pathak, M.; Singh, A.; Varshney, R.; Rana, J.; Saikumar, G. Detection of Porcine Enteric Picornaviruses from Faecal Samples of Indian Pigs. Virusdisease 2022, 33, 102–107. [Google Scholar]
- Donin, D.G.; de Arruda Leme, R.; Alfieri, A.F.; Alberton, G.C.; Alfieri, A.A. First Report of Porcine Teschovirus (PTV), Porcine Sapelovirus (PSV) and Enterovirus G (EV-G) in Pig Herds of Brazil. Trop. Anim. Health Prod. 2014, 46, 523–528. [Google Scholar]
- Sawant, P.M.; Atre, N.; Kulkarni, A.; Gopalkrishna, V. Detection and Molecular Characterization of Porcine Enterovirus G15 and Teschovirus from India. Pathog. Dis. 2020, 78, ftaa039. [Google Scholar]
- Puente, H.; Arguello, H.; Cortey, M.; Gómez-García, M.; Mencía-Ares, O.; Pérez-Perez, L.; Díaz, I.; Carvajal, A. Detection and Genetic Characterization of Enteric Viruses in Diarrhoea Outbreaks from Swine Farms in Spain. Porc. Health Manag. 2023, 9, 29. [Google Scholar]
- Capai, L.; Piorkowski, G.; Maestrini, O.; Casabianca, F.; Masse, S.; de Lamballerie, X.; Charrel, R.N.; Falchi, A. Detection of Porcine Enteric Viruses (Kobuvirus, Mamastrovirus and Sapelovirus) in Domestic Pigs in Corsica, France. PLoS ONE 2022, 17, e0260161. [Google Scholar] [CrossRef]
- Shi, K.; Li, B.; Shi, Y.; Feng, S.; Yin, Y.; Long, F.; Pan, Y.; Wei, Y. Phylogenetic and Evolutionary Analysis of Porcine Epidemic Diarrhea Virus in Guangxi Province, China, during 2020 and 2024. Viruses 2024, 16, 1126. [Google Scholar] [CrossRef]
- Khamrin, P.; Maneekarn, N.; Hidaka, S.; Kishikawa, S.; Ushijima, K.; Okitsu, S.; Ushijima, H. Molecular Detection of Kobuvirus Sequences in Stool Samples Collected from Healthy Pigs in Japan. Infect. Genet. Evol. 2010, 10, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Okitsu, S.; Khamrin, P.; Thongprachum, A.; Hidaka, S.; Kongkaew, S.; Kongkaew, A.; Maneekarn, N.; Mizuguchi, M.; Hayakawa, S.; Ushijima, H. Sequence Analysis of Porcine Kobuvirus VP1 Region Detected in Pigs in Japan and Thailand. Virus Genes 2012, 44, 253–257. [Google Scholar] [CrossRef] [PubMed]

| Primer/Probe | Sequence (5′ → 3′) | Gene | Tm/°C | Product (bp) |
|---|---|---|---|---|
| PSV-F | GACTGGGCCTATACTACCTGATA | 5′ UTR | 57.2 | 140 |
| PSV-R | AGGTACACACGGGCTCTCTG | 60.6 | ||
| PSV-P | ROX-TGGCCGCCTGTAACTAGTATAGTCAGT-BHQ2 | 63.6 | ||
| PKV-F | CGTGCTGAGTAATGGGATAGG | 5′ UTR | 57.0 | 149 |
| PKV-R | TGCACTTCAGAGGTCAGAGAA | 57.9 | ||
| PKV-P | VIC-ATGAGTAGAGCATGGACTGCGGTG-BHQ1 | 63.4 | ||
| PTV-F | GGACTGCRTTGCATATCCCTA | 5′ UTR | 58.3 | 137 |
| PTV-R | GACTATACAAAGTACAGACGGCCA | 58.9 | ||
| PTV-P | CY5-CTGTATGGGAATGCAGGACTGG-BHQ3 | 59.9 | ||
| EV-G-F | GGACCTAGTAGTGATAGGCTGTA | 5′ UTR | 57.1 | 113 |
| EV-G-R | CTGAGCGAAACGCCAAGA | 57.2 | ||
| EV-G-P | FAM-GCCGAAGATGAACCCGTCCGTTAT-BHQ1 | 63.8 |
| Ingredient | Volume (µL) | Final Concentration (nM) |
|---|---|---|
| 2× One-Step RT-PCR Buffer III | 10.0 | / |
| Ex Taq HS (5 U/µL) | 0.4 | / |
| PrimeScript RT Enzyme Mix II | 0.4 | / |
| PSV-F | 0.3 | 300 |
| PSV-R | 0.3 | 200 |
| PSV-P | 0.2 | 200 |
| PKV-F | 0.2 | 200 |
| PKV-R | 0.2 | 200 |
| PKV-P | 0.2 | 200 |
| PTV-F | 0.2 | 200 |
| PTV-R | 0.2 | 200 |
| PTV-P | 0.2 | 200 |
| EV-G-F | 0.2 | 200 |
| EV-G-R | 0.2 | 200 |
| EV-G- P | 0.2 | 200 |
| Total Nucleic Acid | 2.0 | / |
| Nuclease-Free Distilled Water | Up to 20.0 | / |
| RNA | Copies/Reaction | Number of Samples | Quadruplex RT-qPCR | |
|---|---|---|---|---|
| Ct Value ( ± SD) | Hit Rate (%) | |||
| PSV | 500 | 36 | 34.93 ± 0.13 | 100 |
| 250 | 36 | 35.49 ± 0.12 | 100 | |
| 125 | 36 | 35.89 ± 0.13 | 75.0 | |
| 62.5 | 36 | ND | 0 | |
| PKV | 500 | 36 | 34.86 ± 0.11 | 100 |
| 250 | 36 | 35.41 ± 0.13 | 100 | |
| 125 | 36 | 35.83 ± 0.15 | 77.8 | |
| 62.5 | 36 | ND | 0 | |
| PTV | 500 | 36 | 34.73 ± 0.10 | 100 |
| 250 | 36 | 35.37 ± 0.13 | 100 | |
| 125 | 36 | 35.76 ± 0.15 | 80.6 | |
| 62.5 | 36 | ND | 0 | |
| EV-G | 500 | 36 | 34.67 ± 0.14 | 100 |
| 250 | 36 | 35.33 ± 0.10 | 100 | |
| 125 | 36 | 34.73 ± 0.13 | 83.3 | |
| 62.5 | 36 | ND | 0 | |
| Plasmid | Concentration (Copies/μL) | Ct Values of Intra-Assay | Ct Values of Inter-Assay | ||||
|---|---|---|---|---|---|---|---|
| SD | CV (%) | SD | CV (%) | ||||
| p-PSV | 1.0 × 106 | 15.92 | 0.25 | 1.57% | 15.45 | 0.12 | 0.78% |
| 1.0 × 103 | 25.89 | 0.08 | 0.31% | 25.88 | 0.10 | 0.39% | |
| 1.0 × 101 | 33.94 | 0.15 | 0.44% | 33.56 | 0.11 | 0.33% | |
| p-PKV | 1.0 × 106 | 15.85 | 0.19 | 1.20% | 15.26 | 0.17 | 1.11% |
| 1.0 × 103 | 26.17 | 0.13 | 0.50% | 26.04 | 0.08 | 0.31% | |
| 1.0 × 101 | 33.65 | 0.25 | 0.74% | 33.83 | 0.07 | 0.21% | |
| p-PTV | 1.0 × 106 | 15.43 | 0.15 | 0.97% | 15.58 | 0.07 | 0.45% |
| 1.0 × 103 | 26.59 | 0.09 | 0.34% | 26.52 | 0.09 | 0.34% | |
| 1.0 × 101 | 33.11 | 0.09 | 0.27% | 33.02 | 0.11 | 0.33% | |
| p-EV-G | 1.0 × 106 | 15.45 | 0.23 | 1.49% | 14.93 | 0.21 | 1.41% |
| 1.0 × 103 | 25.57 | 0.08 | 0.31% | 25.36 | 0.13 | 0.51% | |
| 1.0 × 101 | 32.39 | 0.12 | 0.37% | 32.37 | 0.30 | 0.93% | |
| Region | Number | Positive Sample | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PSV | PKV | PTV | EV-G | S + K | S + T | S + E | K + T | K + E | T + E | S + K + T | S + T + E | S + K + E | K + T + E | S + K + T + E | ||
| Nanning | 197 | 7 | 76 | 15 | 22 | 1 | 4 | 3 | 12 | 18 | 10 | 1 | 2 | 1 | 9 | 1 |
| Liuzhou | 240 | 9 | 23 | 33 | 14 | 6 | 4 | 4 | 9 | 10 | 6 | 4 | 3 | 3 | 5 | 3 |
| Guilin | 23 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Wuzhou | 54 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Beihai | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fangchenggang | 88 | 6 | 24 | 8 | 22 | 0 | 1 | 1 | 0 | 6 | 2 | 1 | 4 | 1 | 1 | 1 |
| Qinzhou | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Guigang | 221 | 68 | 125 | 64 | 136 | 31 | 40 | 66 | 30 | 88 | 58 | 11 | 40 | 31 | 27 | 11 |
| Yulin | 124 | 10 | 27 | 21 | 18 | 4 | 2 | 2 | 3 | 10 | 6 | 0 | 0 | 2 | 3 | 0 |
| Baise | 482 | 137 | 77 | 158 | 202 | 19 | 64 | 101 | 73 | 68 | 134 | 19 | 61 | 19 | 68 | 19 |
| Hezhou | 93 | 38 | 12 | 29 | 57 | 1 | 20 | 35 | 1 | 6 | 27 | 0 | 20 | 0 | 1 | 0 |
| Hechi | 39 | 2 | 0 | 5 | 2 | 0 | 1 | 1 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 0 |
| Laibin | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Chongzuo | 92 | 1 | 31 | 9 | 20 | 0 | 0 | 0 | 9 | 20 | 6 | 0 | 0 | 0 | 6 | 0 |
| Total | 1823 | 278 (15.25%) | 396 (21.72%) | 343 (18.82%) | 494 (27.10%) | 62 (3.40%) | 136 (7.46%) | 213 (11.68%) | 137 (7.51%) | 226 (12.39%) | 251 (13.76%) | 36 (1.97%) | 131 (7.18%) | 57 (3.12%) | 120 (6.58%) | 35 (1.91%) |
| The Developed Assay | The Reference Assay | Total | Diagnostic Sensitivity (95% CI) | Diagnostic Specificity (95% CI) | ||
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| PSV | Positive | 272 | 6 | 278 | 99.27% (97.38–99.80%) | 99.61% (99.16–99.82%) |
| Negative | 2 | 1543 | 1545 | |||
| Total | 274 | 1549 | 1823 | |||
| PKV | Positive | 389 | 7 | 396 | 98.98% (97.41–99.60%) | 99.51% (98.99–99.76%) |
| Negative | 4 | 1423 | 1427 | |||
| Total | 393 | 1430 | 1823 | |||
| PTV | Positive | 337 | 6 | 343 | 99.12% (97.44–99.70%) | 99.60% (99.12–99.81%) |
| Negative | 3 | 1477 | 1480 | |||
| Total | 340 | 1483 | 1823 | |||
| EV-G | Positive | 482 | 12 | 494 | 98.77% (97.34–99.44%) | 99.10% (98.44–99.49%) |
| Negative | 6 | 1323 | 1329 | |||
| Total | 488 | 1335 | 1823 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Shi, K.; Shi, Y.; Feng, S.; Yin, Y.; Lu, W.; Long, F.; Wei, Z.; Wei, Y. A Quadruplex RT-qPCR for the Detection of Porcine Sapelovirus, Porcine Kobuvirus, Porcine Teschovirus, and Porcine Enterovirus G. Animals 2025, 15, 1008. https://doi.org/10.3390/ani15071008
Li B, Shi K, Shi Y, Feng S, Yin Y, Lu W, Long F, Wei Z, Wei Y. A Quadruplex RT-qPCR for the Detection of Porcine Sapelovirus, Porcine Kobuvirus, Porcine Teschovirus, and Porcine Enterovirus G. Animals. 2025; 15(7):1008. https://doi.org/10.3390/ani15071008
Chicago/Turabian StyleLi, Biao, Kaichuang Shi, Yuwen Shi, Shuping Feng, Yanwen Yin, Wenjun Lu, Feng Long, Zuzhang Wei, and Yingyi Wei. 2025. "A Quadruplex RT-qPCR for the Detection of Porcine Sapelovirus, Porcine Kobuvirus, Porcine Teschovirus, and Porcine Enterovirus G" Animals 15, no. 7: 1008. https://doi.org/10.3390/ani15071008
APA StyleLi, B., Shi, K., Shi, Y., Feng, S., Yin, Y., Lu, W., Long, F., Wei, Z., & Wei, Y. (2025). A Quadruplex RT-qPCR for the Detection of Porcine Sapelovirus, Porcine Kobuvirus, Porcine Teschovirus, and Porcine Enterovirus G. Animals, 15(7), 1008. https://doi.org/10.3390/ani15071008







