Farming Practices, Biosecurity Gaps, and Genetic Insights into African Swine Fever Virus in the Iringa and Ruvuma Regions of Tanzania
Simple Summary
Abstract
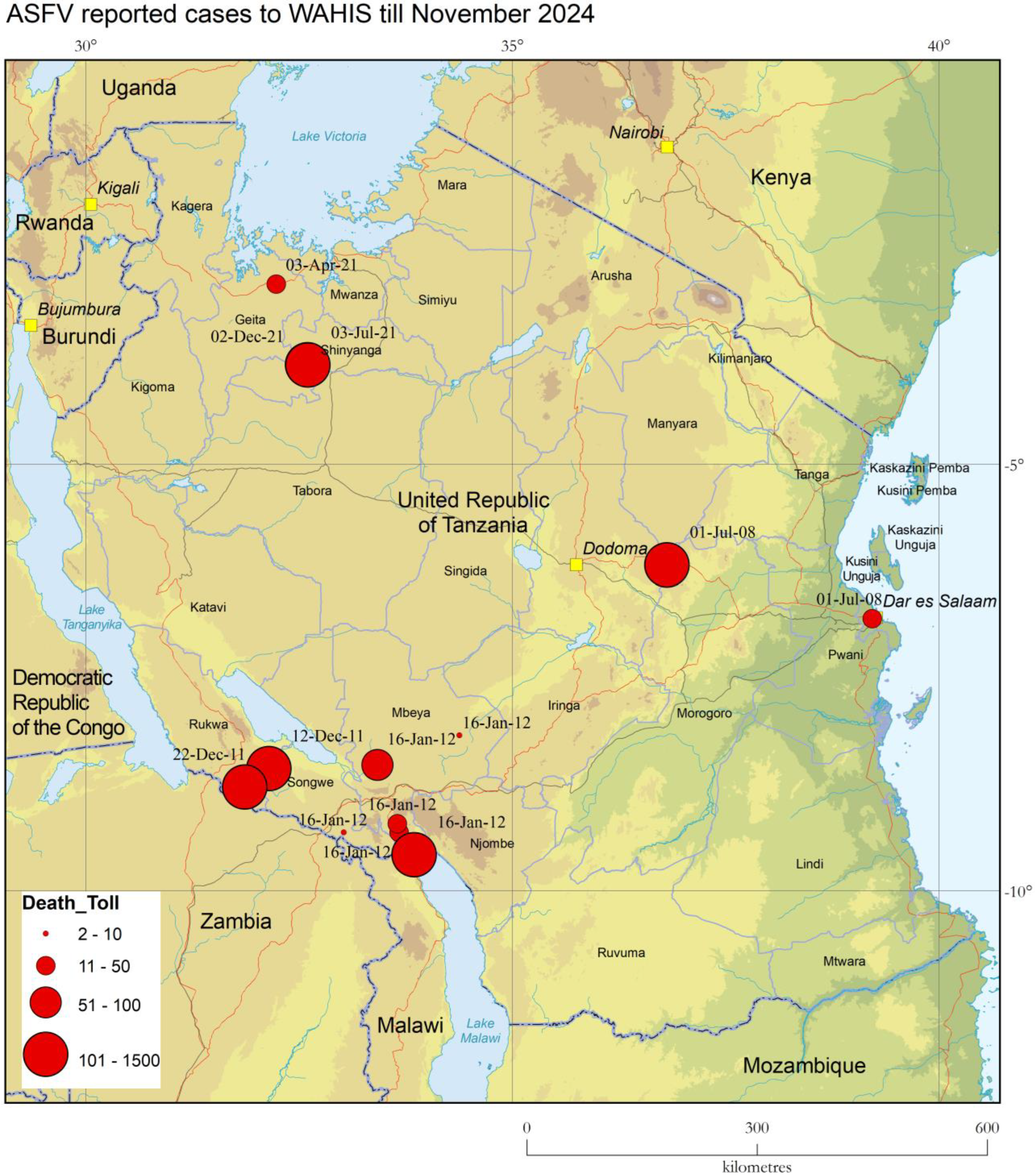
1. Introduction to ASF and Current Disease Situation in Tanzania
2. Materials and Methods
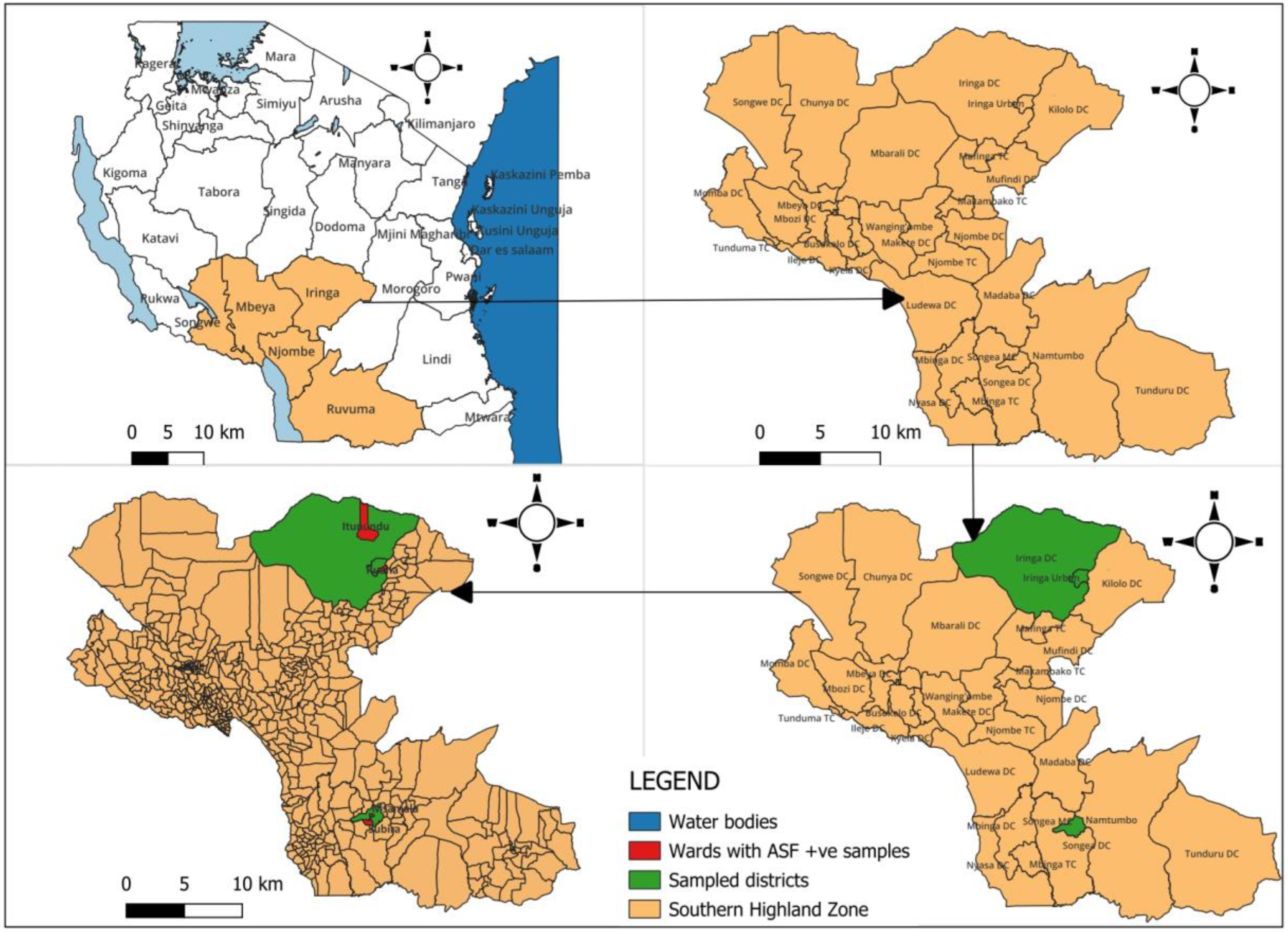
2.1. Sampling Strategy
2.2. Data Collection and Biosecurity Assessment
2.3. Laboratory Analysis
2.4. Detection of Co-Infections and Molecular Characterization
2.4.1. Detection of ASFV and Other Pathogens
- (1)
- An in-house multiplex assay was employed to screen for Erysipelothrix rhusiopathiae and Salmonella spp., in addition to ASFV, as per protocols developed by the APHL.
- (2)
- A second one for PCV-2, PCV-3, PPV1, and PrV screening using the methodology described by Chen et al. (2023) [21].
2.4.2. Molecular Characterization of ASFV Genes
- B646L Gene (p72 Protein): the C-terminus of the B646L gene was amplified and sequenced to determine ASFV genotype following the method outlined by Bastos et al. (2003) [22].
- E183L Gene (p54 Protein): the complete E183L gene was amplified according to the protocol established by Gallardo et al. (2009) [23].
- CD2v Protein Sequences: the CD2v fragment was amplified using the methodology of Malogolovkin et al. (2015) [24].
- Central Hypervariable Region of B602L Gene (CVR): the CVR of the B602L gene was analyzed following the protocol by Nix et al. (2006) [25].
- Intergenic Region (ECOA): the intergenic region between the I73R and I329L genes was amplified to evaluate genetic variation [26].
2.5. Data Analysis
3. Results
3.1. PCR Results
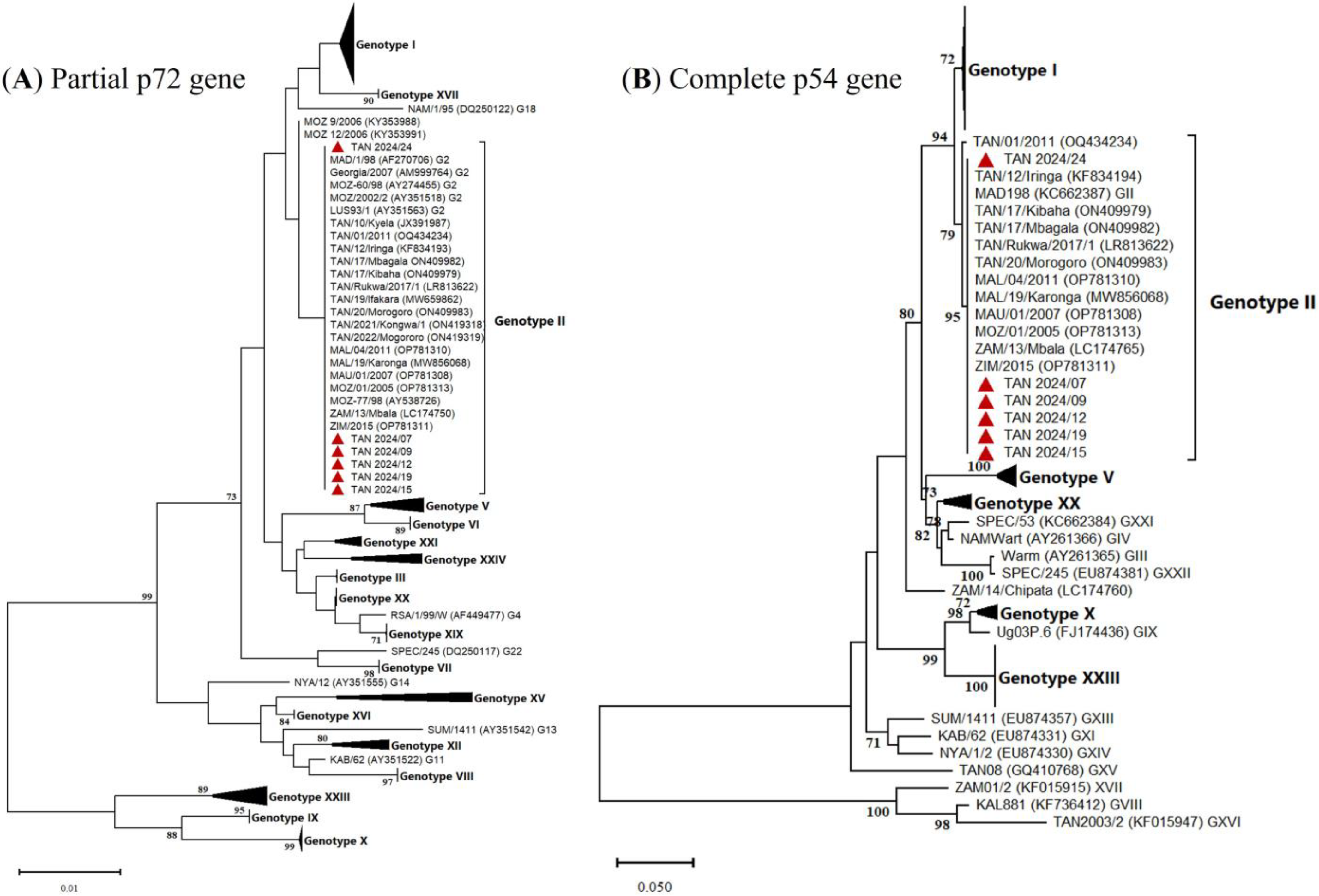
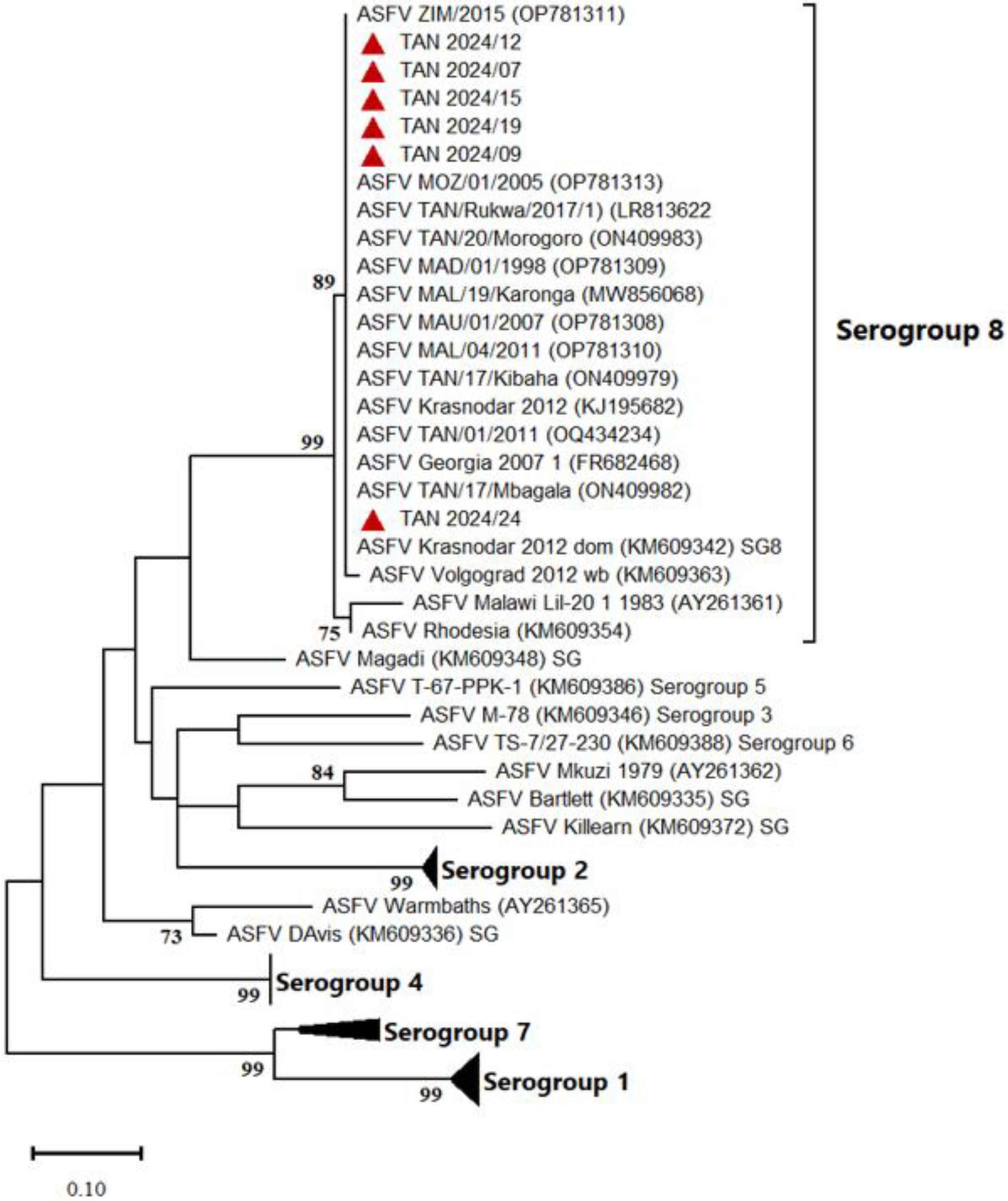
3.2. Molecular Characterization of Tanzanian ASFV Isolates
3.3. Questionnaire
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dixon, L.K.; Sun, H.; Roberts, H. African swine fever. Antivir. Res. 2019, 165, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Vizcaino, J.M.; Laddomada, A.; Arias, M.L. Diseases of Swine, 11th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2019; Volume 25, pp. 443–452. [Google Scholar]
- Vu, H.L.X.; McVey, D.S. Recent progress on gene-deleted live-attenuated African swine fever virus vaccines. NPJ Vaccines 2024, 9, 60. [Google Scholar] [CrossRef]
- Auer, A.; Cattoli, G.; Padungtod, P.; Lamien, C.E.; Oh, Y.; Jayme, S.; Rozstalnyy, A. Challenges in the Application of African Swine Fever Vaccines in Asia. Animals 2024, 14, 2473. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.; Borca, M.; Dixon, L.; Revilla, Y.; Rodriguez, F.; Escribano, J.M.; ICTV Report Consortium. ICTV virus taxonomy profile: Asfarviridae. J. Gen. Virol. 2018, 99, 613–614. [Google Scholar] [CrossRef]
- Gaudreault, N.N.; Madden, D.W.; Wilson, W.C.; Trujillo, J.D.; Richt, J.A. African swine fever virus: An emerging DNA arbovirus. Front. Vet. Sci. 2020, 7, 215. [Google Scholar] [CrossRef]
- Bisimwa, P.N.; Ongus, J.R.; Tiambo, C.K.; Machuka, E.M.; Bisimwa, E.B.; Steinaa, L.; Pelle, R. First detection of African swine fever (ASF) virus genotype X and serogroup 7 in symptomatic pigs in the Democratic Republic of Congo. Virol. J. 2020, 17, 135. [Google Scholar]
- Goatley, L.C.; Freimanis, G.L.; Tennakoon, C.; Bastos, A.; Heath, L.; Netherton, C.L. African swine fever virus NAM P1/95 is a mixture of genotype I and genotype VIII viruses. Microbiol. Resour. Announc. 2024, 13, e0006724. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- United Republic of Tanzania (URT). National Sample Census of Agriculture 2019/20—National Report; United Republic of Tanzania (URT): Dodoma, Tanzania, 2021. [Google Scholar]
- Montgomery, R.E. On a form of swine fever occurring in British East Africa (Kenya Colony). J. Comp. Pathol. Ther. 1921, 34, 159–191. [Google Scholar] [CrossRef]
- Loretu, K.; Kapaga, A.M.; Kipuyo, G.M.; Msami, H.M.; Hyera, J.M. An outbreak of African swine fever in Tanzania and its effect on the emerging pig industry. In Proceedings of the 6th Tanzania Veterinary Association Scientific Conference, Arusha, Tanzania, 6–8 December 1988. [Google Scholar]
- Wambura, P.N.; Masambu, J.; Msami, H. Molecular epidemiology and diagnosis of African swine fever outbreaks in Tanzania. Vet. Res. Commun. 2006, 30, 667–672. [Google Scholar] [CrossRef]
- Misinzo, G.; Kasanga, C.; Mpelumbe-Ngeleja, C.A.R.; Masambu, J.; Kitambi, A.; Van Doorsselaere, J. African swine fever Virus, Tanzania, 2010–2012. Emerg. Infect. Dis. 2012, 18, 2081–2083. [Google Scholar] [CrossRef]
- Misinzo, G.; Kwavi, D.E.; Sikombe, C.D.; Makange, M.; Peter, E.; Muhairwa, A.P.; Madege, M.J. Molecular characterization of African swine fever virus from domestic pigs in northern Tanzania during an outbreak in 2013. Trop. Anim. Health Prod. 2014, 46, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Hakizimana, J.N.; Yona, C.; Kamana, O.; Nauwynck, H.; Misinzo, G. African Swine Fever Virus Circulation between Tanzania and Neighboring Countries: A Systematic Review and Meta-Analysis. Viruses 2021, 13, 306. [Google Scholar] [CrossRef]
- Chang’a, J.S.; Mayenga, C.; Settypalli, T.B.K.; Achenbach, J.E.; Mwanandota, J.J.; Magidanga, B.; Cattoli, G.; Jeremiah, M.; Kamigwe, A.; Guo, S.; et al. Symptomatic and asymptomatic cases of African swine fever in Tanzania. Transbound. Emerg. Dis. 2019, 66, 2402–2410. [Google Scholar] [CrossRef]
- Yona, C. Studies on Epidemiology and Socio-Economic Impact Associated with African Swine Fever 2015–2017 Outbreaks in Tanzania. Master’s Thesis, Sokoine University of Agriculture, Morogoro, Tanzania, 2017. [Google Scholar]
- Fasina, F.O. Epidemiology of African Swine Fever in Nigeria. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2013. [Google Scholar]
- King, D.P.; Reid, S.M.; Hutchings, G.H.; Grierson, S.S.; Wilkinson, P.J.; Dixon, L.K.; Bastos, A.D.; Drew, T.W. Development of a TaqMan® PCR assay with internal amplification control for the detection of African swine fever virus. J. Virol. Methods 2003, 107, 53–61. [Google Scholar] [CrossRef]
- Auer, A.; Settypalli, T.B.K.; Mouille, B.; Angot, A.; De Battisti, C.; Lamien, C.E.; Cattoli, G. Comparison of the sensitivity, specificity, correlation and inter-assay agreement of eight diagnostic in vitro assays for the detection of African swine fever virus. Transbound. Emerg. Dis. 2022, 69, e3231–e3238. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, S.; Tan, J.; Zhang, L.; Qiu, S.; Hao, Z.; Wang, N.; Deng, Z.; Wang, A.; Yang, Q.; et al. Establishment and application of multiplex real-time PCR for simultaneous detection of four viruses associated with porcine reproductive failure. Front. Microbiol. 2023, 14, 1092273. [Google Scholar] [CrossRef] [PubMed]
- Bastos, A.D.S.; Penrith, M.L.; Crucière, C.; Edrich, J.L.; Hutchings, G.; Roger, F.; Couacy-Hymann, E.; Thomson, G.R. Genotyping field strains of African swine fever virus by partial p72 gene characterisation. Arch. Virol. 2003, 148, 693–706. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, C.; Mwaengo, D.; Macharia, J.M.; Arias, M.; Taracha, E.A.; Soler, A.; Okoth, E.; Martin, E.; Kasiti, J.; Bishop, R.P. Enhanced discrimination of African swine fever virus isolates through nucleotide sequencing of the p54 gene. Virus Genes 2009, 38, 85–95. [Google Scholar] [CrossRef]
- Malogolovkin, A.; Burmakina, G.; Tulman, E.R.; Delhon, G.; Diel, D.G.; Salnikov, N.; Kutish, G.F.; Kolbasov, D.; Rock, D.L. Comparative analysis of African swine fever virus genotypes and serogroups. J. Gen. Virol. 2015, 96, 408–419. [Google Scholar] [CrossRef]
- Nix, R.J.; Gallardo, C.; Hutchings, G.; Blanco, E.; Dixon, L.K. Molecular epidemiology of African swine fever virus studied by analysis of four variable genome regions. Arch. Virol. 2006, 151, 2475–2494. [Google Scholar] [CrossRef]
- Gallardo, C.; Fernández-Pinero, J.; Pelayo, V.; Gazaev, I.; Markowska-Daniel, I.; Pridotkas, G.; Nieto, R.; Fernández-Pacheco, P.; Bokhan, S.; Nevolko, O.; et al. Genetic variation among African swine fever genotype II viruses, eastern and central Europe. Emerg. Infect. Dis. 2014, 20, 1544–1547. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pandolfi, F.; Edwards, S.A.; Maes, D.; Kyriazakis, I. Connecting different data sources to assess the interconnections between biosecurity, health, welfare, and performance in commercial pig farms in Great Britain. Front. Vet. Sci. 2018, 5, 41. [Google Scholar] [CrossRef]
- Swai, E.S.; Lyimo, C.J. Impact of African swine fever epidemics in smallholder pig production units in Rombo district of Kilimanjaro, Tanzania. Livest. Res. Rural. Dev. 2014, 26, 32. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). Progressive Management Pathway for Terrestrial Animal Biosecurity (FAO-PMP-TAB): Framework for Strengthening Biosecurity in Terrestrial Animal Production Systems; FAO: Rome, Italy, 2022. [Google Scholar]
- Stadler, J.; Lillie-Jaschniski, K.; Zwickl, S.; Zoels, S.; Theuns, S.; Ritzmann, M.; Vereecke, N. Cross-correlation between biosecurity measures and the detection of viral and bacterial agents on German farms with respiratory disease. Transbound. Emerg. Dis. 2024, 2024, 6205899. [Google Scholar] [CrossRef]
- Hakizimana, J.N.; Kamwendo, G.; Chulu, J.L.C.; Kamana, O.; Nauwynck, H.J.; Misinzo, G. Genetic profile of African swine fever virus responsible for the 2019 outbreak in northern Malawi. BMC Vet. Res. 2020, 16, 316. [Google Scholar] [CrossRef]
- Njau, E.P.; Domelevo Entfellner, J.B.; Machuka, E.M.; Bochere, E.N.; Cleaveland, S.; Shirima, G.M.; Kusiluka, L.J.; Upton, C.; Bishop, R.P.; Pelle, R.; et al. The first genotype II African swine fever virus isolated in Africa provides insight into the current Eurasian pandemic. Sci. Rep. 2021, 11, 13081. [Google Scholar] [CrossRef]
- Ankhanbaatar, U.; Auer, A.; Ulziibat, G.; Settypalli, T.B.K.; Gombo-Ochir, D.; Basan, G.; Takemura, T.; Tseren-Ochir, E.O.; Ouled Ahmed, H.; Meki, I.K.; et al. Comparison of the Whole-Genome Sequence of the African Swine Fever Virus from a Mongolian Wild Boar with Genotype II Viruses from Asia and Europe. Pathogens 2023, 12, 1143. [Google Scholar] [CrossRef]
- Daigle, J.; Onyilagha, C.; Truong, T.; Le, V.P.; Nga, B.T.T.; Nguyen, T.L.; Clavijo, A.; Ambagala, A. Rapid and highly sensitive portable detection of African swine fever virus. Transbound. Emerg. Dis. 2020, 67, 2179–2186. [Google Scholar] [CrossRef]
- Elnagar, A.; Blome, S.; Beer, M.; Hoffmann, B. Point-of-Care Testing for Sensitive Detection of the African Swine Fever Virus Genome. Viruses 2022, 14, 2827. [Google Scholar] [CrossRef]
- Franzo, G.; Zerbo, H.L.; Ouoba, B.L.; Dji-Tombo, A.D.; Kindo, M.G.; Sawadogo, R.; Chang’a, J.; Bitanyi, S.; Kamigwe, A.; Mayenga, C.; et al. A Phylogeographic Analysis of Porcine Parvovirus 1 in Africa. Viruses 2023, 15, 207. [Google Scholar] [CrossRef]
- Franzo, G.; Settypalli, T.B.K.; Agusi, E.R.; Meseko, C.; Minoungou, G.; Ouoba, B.L.; Habibata, Z.L.; Wade, A.; de Barros, J.L.; Tshilenge, C.; et al. Porcine circovirus-2 in Africa: Identification of continent-specific clusters and evidence of independent viral introductions from Europe, North America and Asia. Transbound. Emerg. Dis. 2022, 69, e1142–e1152. [Google Scholar] [CrossRef] [PubMed]
- Luka, P.D.; Adedeji, A.J.; Jambol, A.R.; Ifende, I.V.; Luka, H.G.; Choji, N.D.; Weka, R.; Settypalli, T.B.; Achenbach, J.E.; Cattoli, G.; et al. Coinfections of African swine fever virus, porcine circovirus 2 and 3, and porcine parvovirus 1 in swine in Nigeria. Arch. Virol. 2022, 167, 2715–2722. [Google Scholar] [CrossRef] [PubMed]
- Chang’a, J.S.; Bitanyi, S.S.; Kamigwe, A.; Magidanga, B.; Guo, S.; Makoroma, P.; Francis, G.; Jumbe, J.; Jeremiah, M.; Nyakilinga, D.; et al. Molecular characterization of porcine circovirus-2 and -3 in pigs in Tanzania. J. Vet. Med. Sci. 2023, 85, 691–694. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chenais, E.; Depner, K.; Ebata, A.; Penrith, M.-L.; Pfeiffer, D.U.; Price, C.; Ståhl, K.; Fischer, K. Exploring the hurdles that remain for control of African swine fever in smallholder farming settings. Transbound. Emerg. Dis. 2022, 69, e3370–e3378. [Google Scholar] [CrossRef]
- Penrith, M.-L.; Depner, K.; Jori, F.; Dione, M.; Alders, R.; Chenais, E. Editorial: African Swine Fever in Smallholder and Traditional Pig Farming Systems: Research, Challenges and Solutions. Front. Vet. Sci. 2022, 9, 878928. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Montenegro, M.; Perez, A. Space–Time Dynamics of African Swine Fever Spread in the Philippines. Microorganisms 2023, 11, 1492. [Google Scholar] [CrossRef]
- Jiang, D.; Ma, T.; Hao, M.; Ding, F.; Sun, K.; Wang, Q.; Kang, T.; Wang, D.; Zhao, S.; Li, M.; et al. Quantifying risk factors and potential geographic extent of African swine fever across the world. PLoS ONE 2022, 17, e0267128. [Google Scholar] [CrossRef]
- Plowright, W.; Thomson, G.R.; Neser, J.A. African swine fever. In Infectious Disease of Livestock, with Special Reference to Southern Africa; Oxford University Press: Cape Town, South Africa, 1994; Volume 1, pp. 568–599. [Google Scholar]
- Ungur, A.; Cazan, C.D.; Panait, L.-C.; Coroian, M.; Cătoi, C. What is the real influence of climatic and environmental factors in the outbreaks of African swine fever? Animals 2022, 12, 781. [Google Scholar] [CrossRef]
- Olesen, A.S.; Lohse, L.; Boklund, A.; Halasa, T.; Belsham, G.J.; Rasmussen, T.B.; Bøtner, A. Short time window for transmissibility of African swine fever virus from a contaminated environment. Transbound. Emerg. Dis. 2018, 65, 1024–1032. [Google Scholar] [CrossRef]


| Category | Details |
|---|---|
| Estimated Domestic Pig Population | 1.5 million (of 3.2 million nationally) |
| ASF Outbreak Years | 2010, 2011, 2014, 2015, 2016–2020, 2022 |
| Pig Population Density | Mbeya: 7.2 pigs/km2, Ruvuma: 6 pigs/km2 |
| Main ASF Virus Genotype | Genotype II |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Auer, A.; Yohana, A.S.; Settypalli, T.B.K.; Sallu, R.; Chang’a, J.; Bitanyi, S.; Kiambi, S.G.; Meki, I.K.; Dundon, W.G.; Metlin, A.; et al. Farming Practices, Biosecurity Gaps, and Genetic Insights into African Swine Fever Virus in the Iringa and Ruvuma Regions of Tanzania. Animals 2025, 15, 1007. https://doi.org/10.3390/ani15071007
Auer A, Yohana AS, Settypalli TBK, Sallu R, Chang’a J, Bitanyi S, Kiambi SG, Meki IK, Dundon WG, Metlin A, et al. Farming Practices, Biosecurity Gaps, and Genetic Insights into African Swine Fever Virus in the Iringa and Ruvuma Regions of Tanzania. Animals. 2025; 15(7):1007. https://doi.org/10.3390/ani15071007
Chicago/Turabian StyleAuer, Agathe, Anderson Samwel Yohana, Tirumala B. K. Settypalli, Raphael Sallu, Jelly Chang’a, Stella Bitanyi, Stella Gaichugi Kiambi, Irene K. Meki, William G. Dundon, Artem Metlin, and et al. 2025. "Farming Practices, Biosecurity Gaps, and Genetic Insights into African Swine Fever Virus in the Iringa and Ruvuma Regions of Tanzania" Animals 15, no. 7: 1007. https://doi.org/10.3390/ani15071007
APA StyleAuer, A., Yohana, A. S., Settypalli, T. B. K., Sallu, R., Chang’a, J., Bitanyi, S., Kiambi, S. G., Meki, I. K., Dundon, W. G., Metlin, A., Rozstalnyy, A., Mbata, G. H., Okachu, J. A., Magwisha, H., Hamis, S. A., Choga, J. T., Chalo, S. L., Kimutai, J., Misinzo, G., ... Lamien, C. E. (2025). Farming Practices, Biosecurity Gaps, and Genetic Insights into African Swine Fever Virus in the Iringa and Ruvuma Regions of Tanzania. Animals, 15(7), 1007. https://doi.org/10.3390/ani15071007







