Concurrent Persistent Truncus Arteriosus and Left Atrial Diverticulum in a Domestic Short-Haired Cat
Simple Summary
Abstract
1. Introduction
2. Detailed Case Description
2.1. History and Clinical Examination
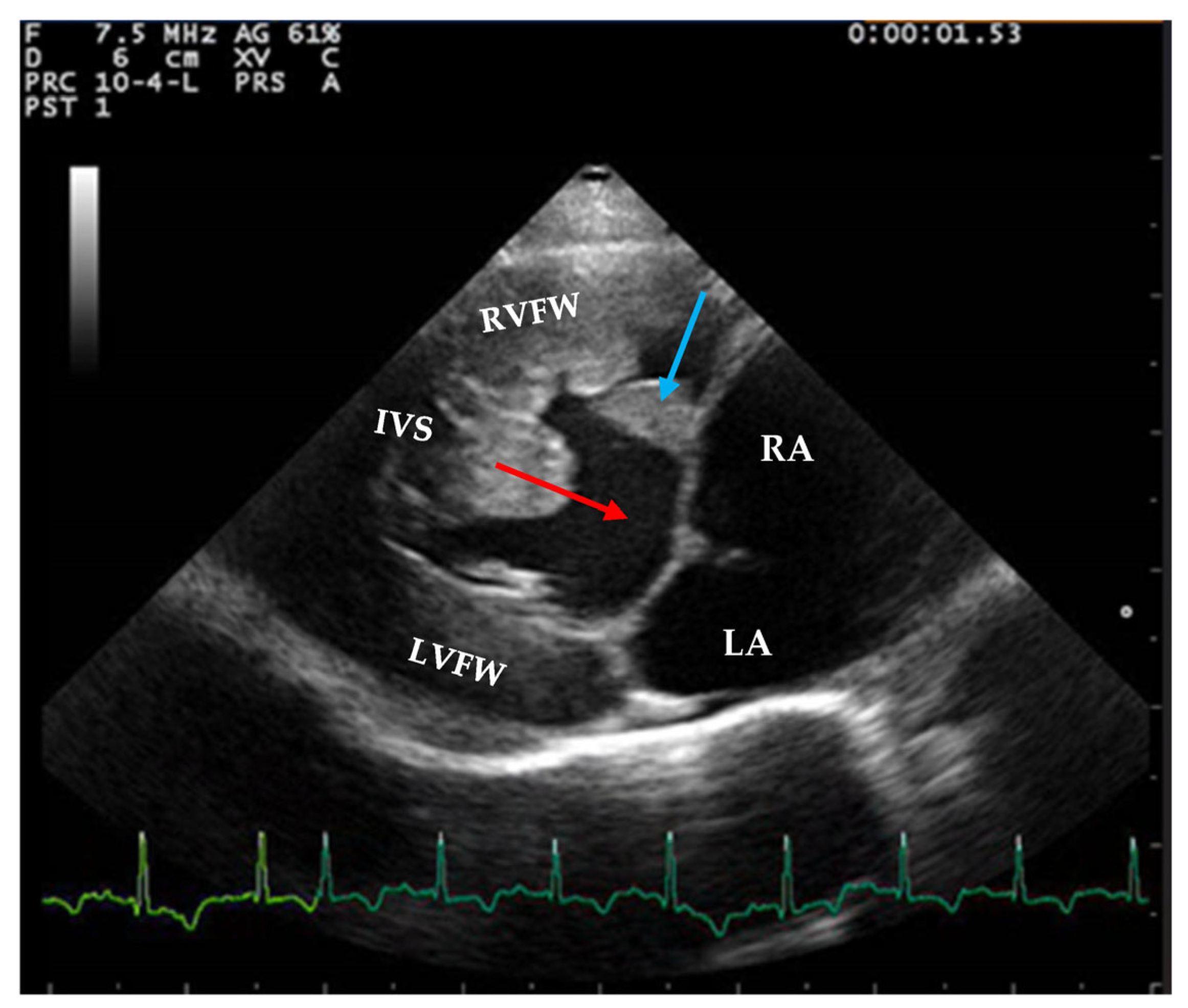
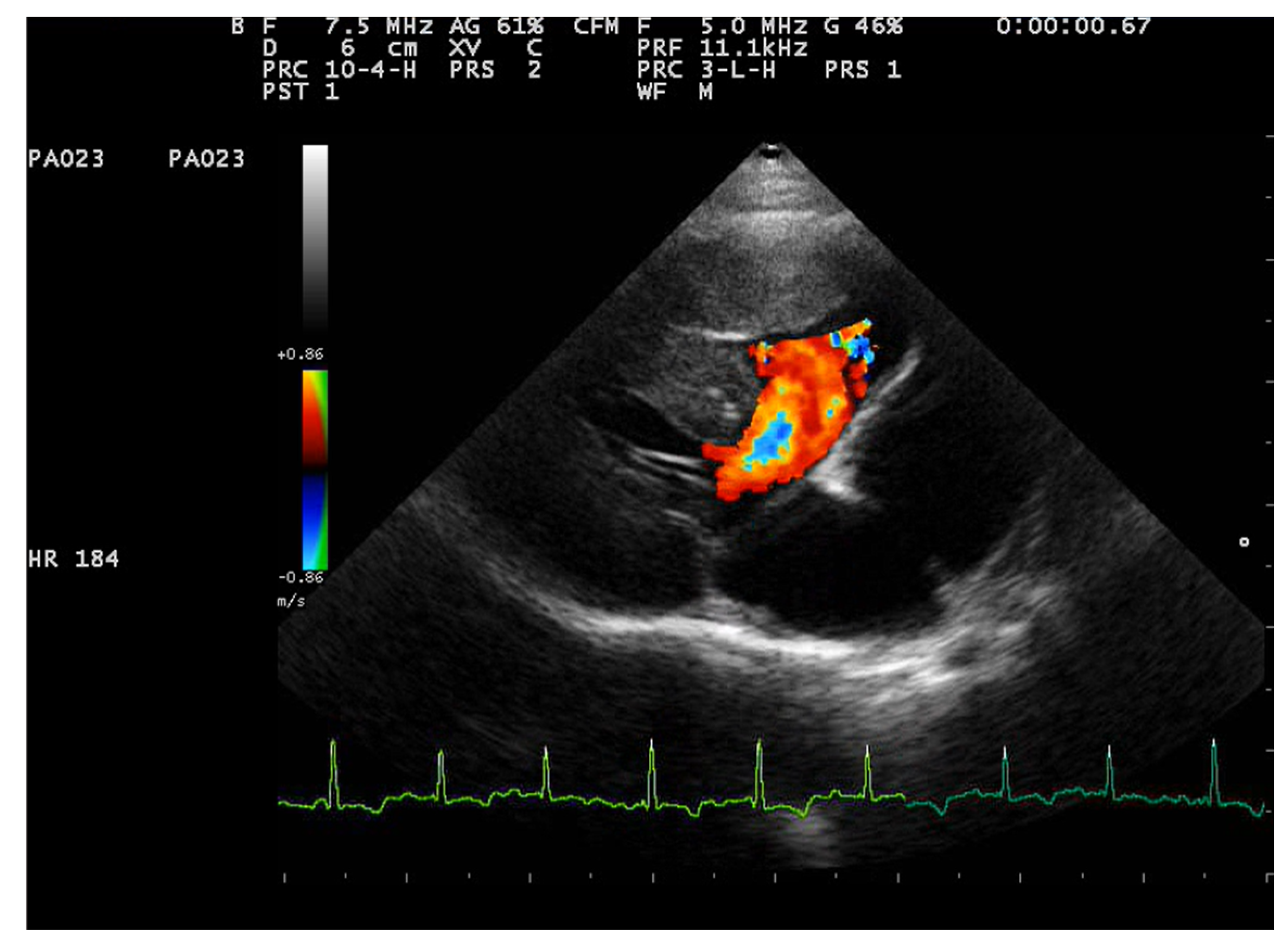
2.2. Echocardiographic Findings
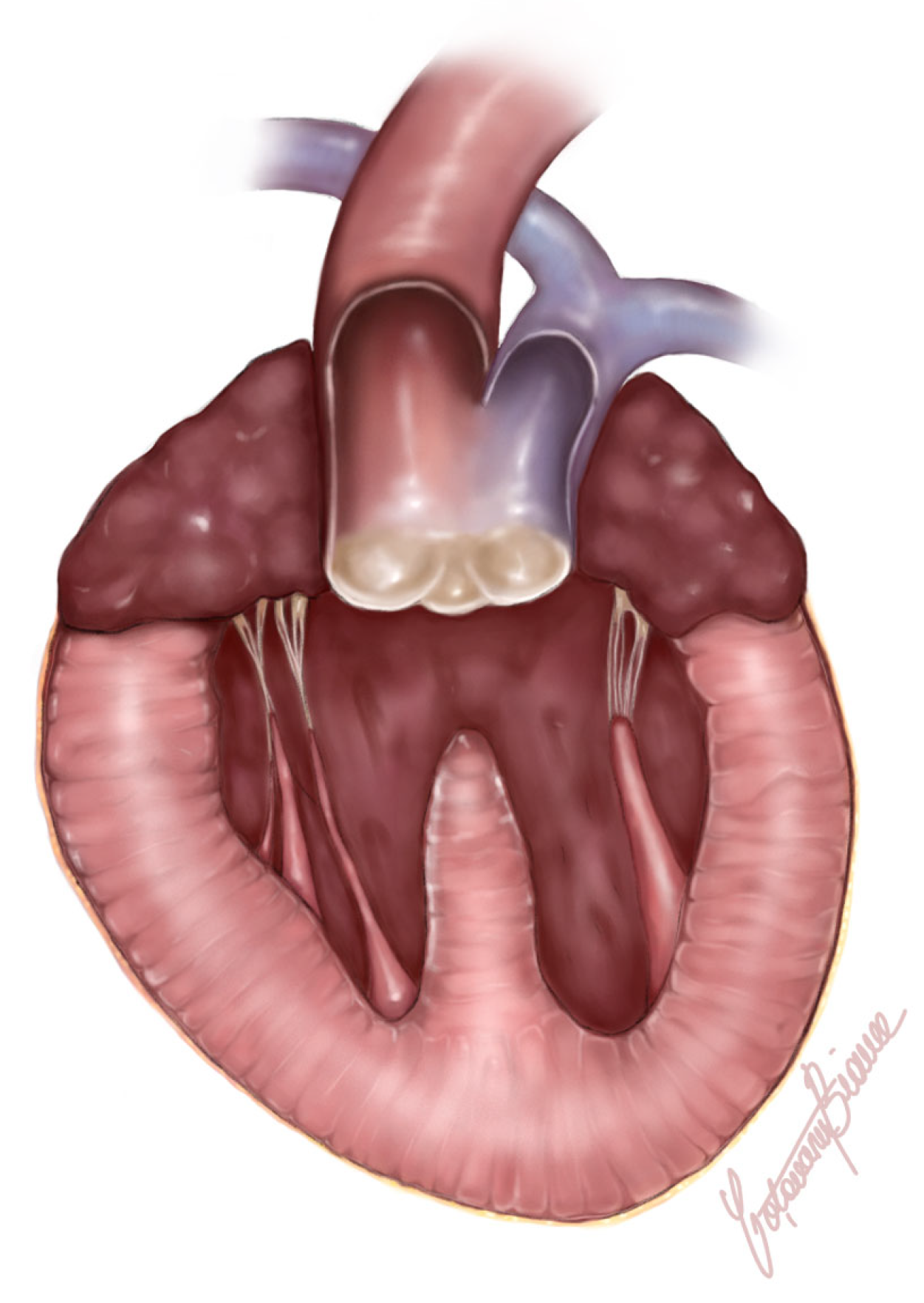
2.3. Postmortem and Histological Findings
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collett, R.W.; Edwards, J.E. Persistent truncus arteriosus: A classification according to anatomic types. Surg. Clin. N. Am. 1949, 29, 1245–1270. [Google Scholar] [CrossRef] [PubMed]
- Chuzel, T.; Bublot, I.; Couturier, L.; Nicolier, A.; Rivier, P.; Mai, W.; Cadoré, J.L. Persistent truncus arteriosus in a cat. J. Vet. Cardiol. 2007, 9, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Monge-Utrilla, O.; García-Rodríguez, M.B.; Cuesta-García, N.; Diez-Prieto, I. Atrioventricular dissociation in a cat with persistent Truncus arteriosus. Vet. Med. Sci. 2023, 9, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, A.F.T.; Lazard, M.; Bouhsina, N.; Fusellier-Tesson, M.S. What Is Your Diagnosis? J. Am. Vet. Med. Assoc. 2019, 254, 583–585. [Google Scholar] [CrossRef]
- Markovic, L.E.; Scansen, B.A.; Potter, B.M. Role of computed tomography angiography in the differentiation of feline truncus arteriosus communis from pulmonary atresia with ventricular septal defect. J. Vet. Cardiol. 2017, 19, 514–522. [Google Scholar] [CrossRef]
- Nicolle, A.P.; Tessier-Vetzel, D.; Begon, E.; Sampedrano, C.C.; Pouchelon, J.; Chetboul, V. Persistent Truncus Arteriosus in a 6-year-old Cat. J. Vet. Med. Ser. A 2005, 52, 350–353. [Google Scholar] [CrossRef]
- Nakao, S.; Atkinson, A.; Motomochi, T.; Fukunaga, D.; Dobrzynski, H. Common arterial trunk in a cat: A high-resolution morphological analysis with micro-computed tomography. J. Vet. Cardiol. 2021, 34, 8–15. [Google Scholar] [CrossRef]
- Kochi, M.; Sugimoto, K.; Kawamoto, S.; Inoue, M.; Machida, N. Persistent truncus arteriosus with an anomalous coronary artery in a cat. J. Vet. Cardiol. 2021, 35, 8–13. [Google Scholar] [CrossRef]
- Gao, C.; Wang, R.; Wang, G.; Wang, Y. Giant Left Atrial Diverticulum. J. Card. Surg. 2011, 26, 70. [Google Scholar] [CrossRef]
- Zimmerman, S.L. Left atrial diverticula. In Pearls and Pitfalls in Cardiovascular Imaging: Pseudolesions, Artifacts, and Other Difficult Diagnoses; Zimmerman, S.L., Fishman, E.K., Eds.; Cambridge University Press: Cambridge, UK, 2015; pp. 54–56. [Google Scholar]
- Schober, K.; Savino, S.; Yildiz, V. Right ventricular involvement in feline hypertrophic cardiomyopathy. J. Vet. Cardiol. 2016, 18, 297–309. [Google Scholar] [CrossRef]
- Schober, K.E.; Chetboul, V. Echocardiographic evaluation of left ventricular diastolic function in cats: Hemodynamic determinants and pattern recognition. J. Vet. Cardiol. 2015, 17, S102–S133. [Google Scholar] [CrossRef] [PubMed]
- Constantin, I.; Tăbăran, A.F. Dissection Techniques and Histological Sampling of the Heart in Large Animal Models for Cardiovascular Diseases. J. Vis. Exp. 2022, 184, e63809. [Google Scholar] [CrossRef]
- Prophet, E.B.; Mills, B.; Arrington, J.B.; Sobin, L.H. Laboratory Methods in Histotechnology; Armed Forces Institute of Pathology-American Registry of Pathology: Washington, DC, USA, 1992. [Google Scholar]
- Weerakkody, Y.; Tatco, V. Left Atrial Diverticulum. Reference Article. Available online: https://radiopaedia.org/ (accessed on 20 January 2025). [CrossRef]
- Shaher, R.; Anis, W.; Alley, R.; Mintzer, J. Congenital enlargement of the left atrium. J. Thorac. Cardiovasc. Surg. 1972, 63, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Chockalingam, A.; Alagesan, R.; Nandakumar, M.; Gnanavelu, G. Massive left atrial appendage aneurysm presenting as supraventricular tachycardia. Indian Heart J. 2003, 55, 379–381. [Google Scholar]
- De Ponti, R.; Lumia, D.; Marazzi, R.; Mameli, S.; Doni, L.A.; DE Venuto, G.; Fugazzola, C.; Salerno-Uriarte, J.A. Left atrial diverticula in patients undergoing atrial fibrillation ablation: Morphologic analysis and clinical impact. J. Cardiovasc. Electrophysiol. 2013, 24, 1232–1239. [Google Scholar] [CrossRef]
- Ding, X.; Zhu, M.; Wei, C.; Zhu, H.; Zhang, X.; Qu, X.; Li, Y.; Yuan, J. Double cardiac diverticula after pericardectomy. Int. J. Cardiol. 2015, 201, 449–453. [Google Scholar] [CrossRef]
- Halbertsma, F.J.J.; van Oort, A.; van der Staak, F. Cardiac diverticulum and omphalocele: Cantrell’s pentalogy or syndrome. Cardiol. Young 2002, 12, 71–74. [Google Scholar] [CrossRef]
- Ohlow, M.-A. Congenital Left Ventricular Aneurysms and Diverticula: An Entity in Search of an Identity; Science Press: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Constantin, I.; Tăbăran, A.; Cătoi, C.; Pop, R.; Scurtu, I. Left ventricular apical diverticulum with aortic thromboembolism in a cat. J. Comp. Pathol. 2024, 210, 92. [Google Scholar] [CrossRef]
- Pedro, B.; Sarcinella, F.; Linney, C.; Neves, J.; Mesquita, L. Congenital right atrial diverticulum in a kitten. J. Vet. Cardiol. 2024, 53, 1–5. [Google Scholar] [CrossRef]
- Kittleson, M.D. Myxomatous Atrioventricular Valve Degeneration in Dogs and Cats. January 2023. MSD Veterinary Manual. Available online: https://www.msdvetmanual.com/circulatory-system/various-heart-diseases-in-dogs-and-cats/myxomatous-atrioventricular-valve-degeneration-in-dogs-and-cats (accessed on 20 January 2025).
- Sisson, D.; Kvart, C.P. Acquired valvular heart disease in dogs and cats. In Textbook of Canine and Feline Cardiology, 2nd ed.; Fox, P.R., Sisson, D., Moise, N., Eds.; Saunders: Philadelphia, PA, USA, 1999; pp. 536–555. [Google Scholar]
- Constantin, I.; Pop, R.; Scurtu, I.; Popa, T.; Beteg, F.; Cătoi, C.; Tăbăran, A. Myxomatous degeneration of the mitral valve associated with tricuspid dysplasia and subaortic stenosis in a cat. J. Comp. Pathol. 2023, 203, 92–93. [Google Scholar] [CrossRef]
- Barone, R. Anatomie Comparée des Mammifères Domestiques: Angiologie, 2nd ed.; Vigot Maloine: Paris, France, 2012. [Google Scholar]
- Hill, A.J.; Iaizzo, P.A. Comparative Cardiac Anatomy. In Handbook of Cardiac Anatomy, Physiology, and Devices, 2nd ed.; Springer: New York, NY, USA, 2009; pp. 87–108. [Google Scholar]
- Adin, D.B.; Diley-Poston, L. Papillary muscle measurements in cats with normal echocardiograms and cats with concentric left ventricular hypertrophy. J. Vet. Intern. Med. 2007, 21, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Kittleson, M.D. Feline Hypertrophic Cardiomyopathy—Getting into the Thick of It. Available online: https://www.vin.com/apputil/project/defaultadv1.aspx?pId=12886&id=7054766 (accessed on 20 January 2025).
- Kittleson, M.D. Hypertrophic Cardiomyopathy in Dogs and Cats. January 2023. MSD Veterinary Manual. Available online: https://www.msdvetmanual.com/circulatory-system/cardiomyopathy-in-dogs-and-cats/hypertrophic-cardiomyopathy-in-dogs-and-cats (accessed on 20 January 2025).
- Kittleson, M.D.; Côté, E. The Feline Cardiomyopathies: 2. Hypertrophic cardiomyopathy. J. Feline Med. Surg. 2021, 23, 1028–1051. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, A.E.; Ariyurek, M.O.; Karcaaltincaba, M. Splenic Anomalies of Shape, Size, and Location: Pictorial Essay. Sci. World J. 2013, 2013, 321810. [Google Scholar] [CrossRef] [PubMed]
- Omeri, A.K.; Matsumoto, S.; Kiyonaga, M.; Takaji, R.; Yamada, Y.; Ando, Y.; Mori, H.; Uchida, H.; Iwashita, Y.; Ohta, M.; et al. Fusion anomaly of the pancreatic tail and spleen: A case report. J. Med. Case Rep. 2017, 11, 238. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constantin, I.; Cofaru, A.; Murariu, R.; Scurtu, I.C.; Tăbăran, F.-A. Concurrent Persistent Truncus Arteriosus and Left Atrial Diverticulum in a Domestic Short-Haired Cat. Animals 2025, 15, 899. https://doi.org/10.3390/ani15060899
Constantin I, Cofaru A, Murariu R, Scurtu IC, Tăbăran F-A. Concurrent Persistent Truncus Arteriosus and Left Atrial Diverticulum in a Domestic Short-Haired Cat. Animals. 2025; 15(6):899. https://doi.org/10.3390/ani15060899
Chicago/Turabian StyleConstantin, Irina, Alexandra Cofaru, Raluca Murariu, Iuliu Călin Scurtu, and Flaviu-Alexandru Tăbăran. 2025. "Concurrent Persistent Truncus Arteriosus and Left Atrial Diverticulum in a Domestic Short-Haired Cat" Animals 15, no. 6: 899. https://doi.org/10.3390/ani15060899
APA StyleConstantin, I., Cofaru, A., Murariu, R., Scurtu, I. C., & Tăbăran, F.-A. (2025). Concurrent Persistent Truncus Arteriosus and Left Atrial Diverticulum in a Domestic Short-Haired Cat. Animals, 15(6), 899. https://doi.org/10.3390/ani15060899







