Effects of Dandelion Flavonoid Extract on the Accumulation of Flavonoids in Layer Hen Meat, Slaughter Performance and Blood Antioxidant Indicators of Spent Laying Hens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diets, and Experimental Design
2.2. Main Materials and Reagents
2.3. Main Instruments
2.4. Blood Sampling
2.5. Determination of the Contents of Rutin and Quercetin in Chicken Muscle and Plasma
2.6. Determination of Total Phenols in Feed and Layer Hen Meat
2.7. Slaughter Performance Indicators and Chicken Sampling
2.8. Nutrient Testing in Muscle Tissue
2.9. Measurement of Blood Biochemical Indicators and Antioxidant Capacity
2.10. Statistical Analysis
3. Results
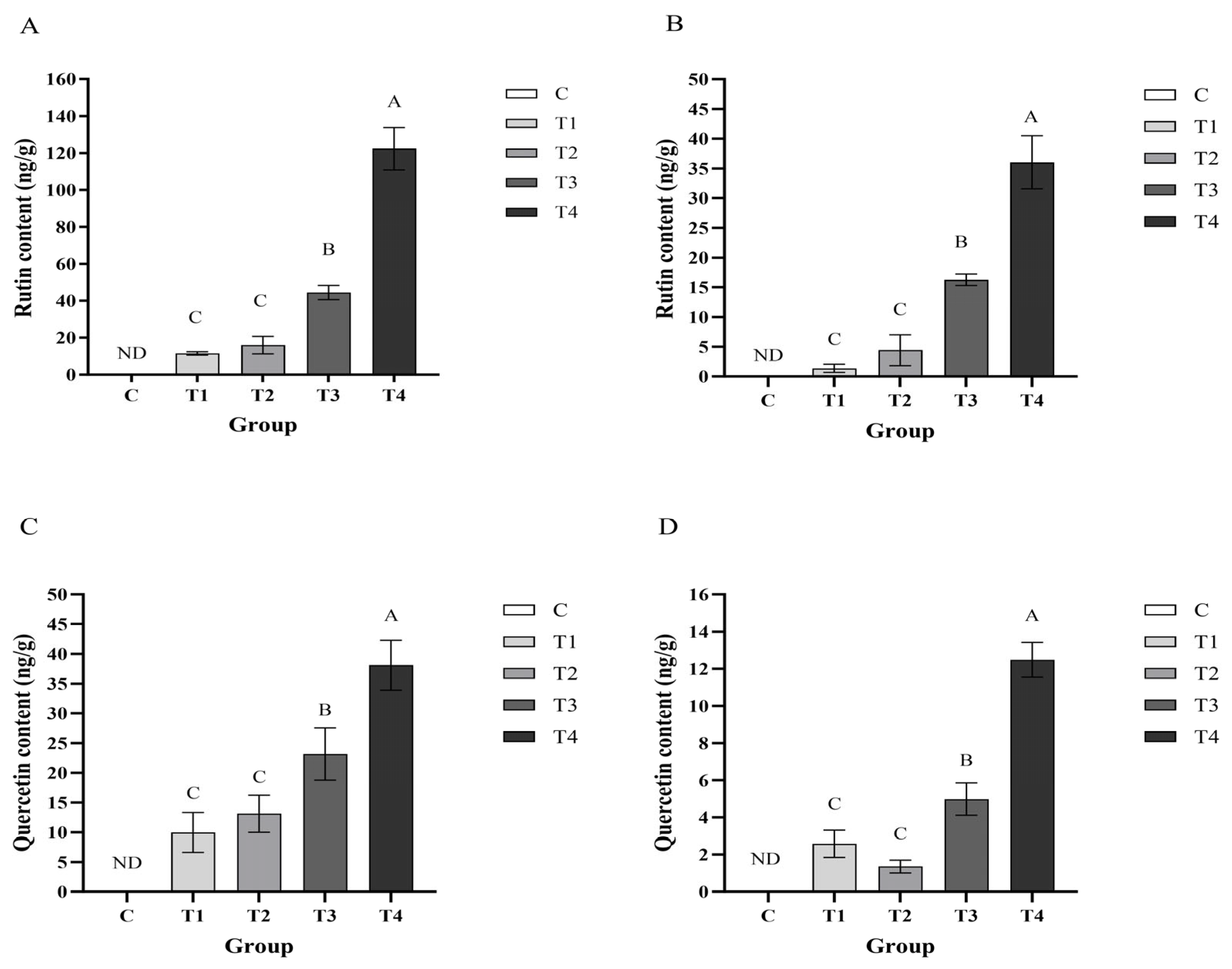
3.1. Content of Rutin and Quercetin in Layer Hen Meat and Plasma
3.2. Total Phenol Content and Correlation Between Feed and Layer Hen Meat
3.3. Slaughtering Performance and Basic Nutritional Composition of Muscle Tissue
3.4. Effects of DFE on Blood Biochemical Indicators
3.5. Effects of DFE on Antioxidant Capacity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bain, M.M.; Nys, Y.; Dunn, I.C. Increasing persistency in lay and stabilising egg quality in longer laying cycles. What are the challenges? Br. Poult. Sci. 2016, 57, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Al-Rahman, N.N.A.; Al-Jabari, Q.H.; Aljumaily, T.K. Evaluation of Different Induce Forced Resting Events in Productive Performance and some Characteristics of Egg Quality of Commercial Laying Hens. IOP Conf. Ser. Earth Environ. 2025, 1449, 012022. [Google Scholar] [CrossRef]
- Han, G.P.; Kim, D.Y.; Lee, E.C.; Urriola, P.E.; Kil, D.Y. Effect of dietary supplementation of taurine on productive performance, egg quality, and liver health in aged laying hens. Anim. Feed Sci. Technol. 2023, 304, 115734. [Google Scholar] [CrossRef]
- Konieczka, P.; Czauderna, M.; Smulikowska, S. The enrichment of chicken meat with omega-3 fatty acids by dietary fish oil or its mixture with rapeseed or flaxseed—Effect of feeding duration: Dietary fish oil, flaxseed, and rapeseed and n-3 enriched broiler meat. Anim. Feed Sci. Technol. 2017, 223, 42–52. [Google Scholar] [CrossRef]
- Iaffaldano, B.J.; Zhang, Y.; Cardina, J.; Cornish, K. Genome size variation among common dandelion accessions informs their mode of reproduction and suggests the absence of sexual diploids in North America. Plant Syst. Evol. 2017, 303, 719–725. [Google Scholar] [CrossRef]
- Jianhao, W.; Jialin, S.; Meiqi, L.; Xiaozhuang, Z.; Lingyang, K.; Lengleng, M.; Shan, J.; Xiubo, L.; Wei, M. Botany, Traditional Use, Phytochemistry, Pharmacology and Quality Control of Taraxaci herba: Comprehensive Review. Pharmaceuticals 2024, 17, 1113. [Google Scholar] [CrossRef]
- Zhuang, X.; Shi, W.; Shen, T.; Cheng, X.; Wan, Q.; Fan, M.; Hu, D. Research Updates and Advances on Flavonoids Derived from Dandelion and Their Antioxidant Activities. Antioxidants 2024, 13, 1449. [Google Scholar] [CrossRef]
- Ürüşan, H. Effects of Dandelion (Taraxacum officinale) Supplementation on Productive Performance, Egg Quality Traits, Serum Biochemical Parameters and Liver Fat Rate in Laying Hens. Indian J. Anim. Res. 2023, 57, 1018–1024. [Google Scholar] [CrossRef]
- Fan, M.; Zhang, X.; Song, H.; Zhang, Y. Dandelion (Taraxacum Genus): A review of chemical constituents and pharmacological effects. Molecules 2023, 28, 5022. [Google Scholar] [CrossRef]
- Oni, A.I.; Adeleye, O.O.; Adebowale, T.O.; Oke, O.E. The role of phytogenic feed additives in stress mitigation in broiler chickens. J. Anim. Physiol. Anim. Nutr. 2024, 108, 81–98. [Google Scholar] [CrossRef]
- Olagaray, K.; Bradford, B. Plant flavonoids to improve productivity of ruminants–A review. Anim. Feed Sci. Technol. 2019, 251, 21–36. [Google Scholar] [CrossRef]
- Yan, Q.; Xing, Q.; Liu, Z.; Zou, Y.; Liu, X.; Xia, H. The phytochemical and pharmacological profile of dandelion. Biomed. Pharmacother. 2024, 179, 117334. [Google Scholar] [CrossRef]
- Jyoti, S.; Jaspreet, K.; Sawinder, K.; Prasad, R.; Umi, L.; Kartik, S.; Vishesh, B. Dandelion (Taraxacum officinale): A Promising Source of Nutritional and Therapeutic Compounds. Recent Adv. Food Nutr. Agric. 2024, 16, 41–56. [Google Scholar]
- Subramanian, K.N.; Padmanaban, G.; Sarma, P.S. Folin-Ciocalteu reagent for the estimation of siderochromes. Anal. Biochem. 1965, 12, 106–112. [Google Scholar] [CrossRef] [PubMed]
- GB/T 19478-2018; Operating procedure of livestock and poultry slaughtering—Chicken. National Standardization Administration and China National Standardization Committee: Beijing, China, 2018.
- Ishii, K.; Furuta, T.; Kasuya, Y. High-performance liquid chromatographic determination of quercetin in human plasma and urine utilizing solid-phase extraction and ultraviolet detection. J. Chromatogr. B 2003, 794, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Erlund, I.; Kosonen, T.; Alfthan, G.; Mäenpää, J.; Perttunen, K.; Kenraali, J.; Parantainen, J.; Aro, A. Pharmacokinetics of quercetin from quercetin aglycone and rutin in healthy volunteers. Eur. J. Clin. Pharmacol. 2000, 56, 545–553. [Google Scholar] [CrossRef]
- Iriti, M.; Varoni, E.M.; Vitalini, S. Bioactive Compounds in Health and Disease–Focus on Rutin. Bioact. Compd. Health Dis. 2023, 6, 235–242. [Google Scholar] [CrossRef]
- Ader, P.; Wessmann, A.; Wolffram, S. Bioavailability and metabolism of the flavonol quercetin in the pig. Free Radic. Biol. Med. 2000, 28, 1056–1067. [Google Scholar] [CrossRef]
- Graf, B.A.; Ameho, C.; Dolnikowski, G.G.; Milbury, P.E.; Chen, C.-Y.; Blumberg, J.B. Rat gastrointestinal tissues metabolize quercetin. J. Nutr. 2006, 136, 39–44. [Google Scholar] [CrossRef]
- Wu, T.; Yang, F.; Jiao, T.; Zhao, S. Effects of Dietary Oregano Essential Oil on Cecal Microorganisms and Muscle Fatty Acids of Luhua Chickens. Animals 2022, 12, 3215. [Google Scholar] [CrossRef]
- Hassan, M.S.; Abdel-Moneim, M.A.; El-Chaghaby, G.A. Antioxidant Activity of Marjoram Extract and its Effect on the Antioxidative Properties of Broilers’ Chicken Meat. Egypt. J. Nutr. 2017, 32, 131–149. [Google Scholar]
- Jung, S.; Choe, J.H.; Kim, B.; Yun, H.; Kruk, Z.A.; Jo, C. Effect of dietary mixture of gallic acid and linoleic acid on antioxidative potential and quality of breast meat from broilers. Meat Sci. 2010, 86, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.N.; Yang, B.; Yang, S.; Chen, Y.; Zhao, M.; Ashraf, M.; Jiang, Y. Identification of phenolic compounds and appraisal of antioxidant and antityrosinase activities from litchi (Litchi sinensis Sonn.) seeds. Food Chem. 2009, 116, 1–7. [Google Scholar] [CrossRef]
- Goi, I.; Brenes, A.; Centeno, C.; Viveros, A.; Saura-Calixto, F.; Rebolé, A.; Arija, I.; Estevez, R. Effect of Dietary Grape Pomace and Vitamin E on Growth Performance, Nutrient Digestibility, and Susceptibility to Meat Lipid Oxidation in Chickens. Poult. Sci. 2007, 86, 508–516. [Google Scholar]
- Simitzis, P.; Deligeorgis, S.; Bizelis, J.; Dardamani, A.; Theodosiou, I.; Fegeros, K. Effect of dietary oregano oil supplementation on lamb meat characteristics. Meat Sci. 2008, 79, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Hussain, Y.; Santarcangelo, C.; Baldi, A.; Di Minno, A.; Khan, H.; Xiao, J.; Daglia, M. Natural polyphenols for the preservation of meat and dairy products. Molecules 2022, 27, 1906. [Google Scholar] [CrossRef]
- Tavaniello, S.; Maiorano, G.; Siwek, M.; Knaga, S.; Witkowski, A.; Di Memmo, D.; Bednarczyk, M. Growth performance, meat quality traits, and genetic mapping of quantitative trait loci in 3 generations of Japanese quail populations (Coturnix japonica). Poult. Sci. 2014, 93, 2129–2140. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Fabian, D.; Babeľová, J.; Vlčková, R.; Alwasel, S.; Harrath, A.H. Body fat affects mouse reproduction, ovarian hormone release, and response to follicular stimulating hormone. Reprod. Biol. 2018, 18, 5–11. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, M.; Wang, S.; Zhang, H.; Du, Z.; Li, Y.; Cao, Z.; Luan, P.; Leng, L.; Li, H. Genetic selection on abdominal fat content alters the reproductive performance of broilers. Animal 2018, 12, 1232–1241. [Google Scholar] [CrossRef]
- Zoth, N.; Weigt, C.; Laudenbach-Leschowski, U.; Diel, P. Physical activity and estrogen treatment reduce visceral body fat and serum levels of leptin in an additive manner in a diet induced animal model of obesity. J. Steroid Biochem. Mol. Biol. 2010, 122, 100–105. [Google Scholar] [CrossRef]
- Ceccarelli, I.; Bioletti, L.; Peparini, S.; Solomita, E.; Ricci, C.; Casini, I.; Miceli, E.; Aloisi, A.M. Estrogens and phytoestrogens in body functions. Neurosci. Biobehav. Rev. 2022, 132, 648–663. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Gu, Z.; Wang, H.; Liu, Y.; Wang, L.; Huang, L.; Huo, J.; Wu, Y. Effects of rutin supplementation on growth performance, slaughter performance, serum parameters, and meat quality of Nubian goats. Anim. Sci. J. 2023, 94, e13819. [Google Scholar] [CrossRef]
- Tan, X.; Sun, Z.; Chen, S.; Chen, S.; Huang, Z.; Zhou, C.; Zou, C.; Liu, Q.; Ye, H.; Lin, H. Effects of dietary dandelion extracts on growth performance, body composition, plasma biochemical parameters, immune responses and disease resistance of juvenile golden pompano Trachinotus ovatus. Fish Shellfish. Immunol. 2017, 66, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, X.; Yang, H.; Liang, G.; Gao, B.; Leng, X. Dietary quercetin improved the growth, antioxidation, and flesh quality of grass carp (Ctenopharyngodon idella). J. World Aquac. Soc. 2019, 50, 1182–1195. [Google Scholar] [CrossRef]
- Saifan, H.Y.; Shalaby, M.A.; Abo-EL-Sooud, K.; Tony, M.; Yassin, A.M. Effects of sodium butyrate and rosemary leaves on performance, biochemical parameters, immune status, and carcass traits of broiler chickens. bioRxiv 2024. [Google Scholar] [CrossRef]
- Al-Hijazeen, M.; Al-Rawashdeh, M. Preservative effects of rosemary extract (Rosmarinus officinalis L.) on quality and storage stability of chicken meat patties. Food Sci. Technol. 2019, 39, 27–34. [Google Scholar] [CrossRef]
- Lopez-Bote, C.J.; Gray, J.I.; Gomaa, E.A.; Flegal, C.J. Effect of dietary administration of oil extracts from rosemary and sage on lipid oxidation in broiler meat. Br. Poult. 1998, 39, 235–240. [Google Scholar] [CrossRef]
- Loetscher, Y.; Kreuzer, M.; Albiker, D.; Stephan, R.; Messikommer, R.E. Effect of replacing dietary vitamin E by sage on performance and meatiness of spent hens, and the oxidative stability of sausages produced from their meat. Br. Poult. Sci. 2014, 55, 576–584. [Google Scholar] [CrossRef]
- Sheng, G.; Peng, N.; Hu, C.; Zhong, L.; Zhong, M.; Zou, Y. The albumin-to-alkaline phosphatase ratio as an independent predictor of future non-alcoholic fatty liver disease in a 5-year longitudinal cohort study of a non-obese Chinese population. Lipids Health Dis. 2021, 20, 50. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, L.; Liu, Y.; Yu, X.; Qiao, X. Rutin Ameliorates Cadmium-Induced Necroptosis in the Chicken Liver via Inhibiting Oxidative Stress and MAPK/NF-κB Pathway. Biol. Trace Element Res. 2021, 200, 1799–1810. [Google Scholar] [CrossRef]
- Yu, J.; Hu, G.; Guo, X.; Cao, H.; Zhang, C. Quercetin Alleviates Inflammation and Energy Deficiency Induced by Lipopolysaccharide in Chicken Embryos. Animals 2023, 13, 2051. [Google Scholar] [CrossRef] [PubMed]
- Davaatseren, M.; Hur, H.J.; Yang, H.J.; Hwang, J.-T.; Park, J.H.; Kim, H.-J.; Kim, M.J.; Kwon, D.Y.; Sung, M.J. Taraxacum official (Dandelion) leaf extract alleviates high-fat diet-induced nonalcoholic fatty liver. Food Chem. Toxicol. 2013, 58, 30–36. [Google Scholar] [CrossRef]
- Noor, A.S.; Kadhim, A.H.; Ali, M.A. The effect of feeding different levels of dandelion leaf powder (Taraxacum officinale) in the diet on the productive and physiological performance of broiler chickens, strain Ross-308. IOP Conf. Ser. Earth Environ. Sci. 2021, 722, 012002. [Google Scholar] [CrossRef]
- Aabideen, Z.U.; Mumtaz, M.W.; Akhtar, M.T.; Mukhtar, H.; Raza, S.A.; Touqeer, T.; Saari, N. Anti-obesity attributes; UHPLC-QTOF-MS/MS-based metabolite profiling and molecular docking insights of Taraxacum officinale. Molecules 2020, 25, 4935. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, Y.; Zhang, J.; Hu, C.; Jiang, J.; Li, Y.; Peng, Z. ROS-induced lipid peroxidation modulates cell death outcome: Mechanisms behind apoptosis, autophagy, and ferroptosis. Arch. Toxicol. 2023, 97, 1439–1451. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, R.; Yan, X.; Fan, K. Superoxide dismutase nanozyme: An emerging star for anti-oxidation. J. Mater. Chem. B 2021, 9, 6939–6957. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, X.; Jia, W.; Zhang, C.; Boczek, T.; Harding, M.; Liu, Y.; Li, M.; Zhang, S.; Lei, S.; et al. Circulating glutathione peroxidase and superoxide dismutase levels in patients with epilepsy: A meta-analysis. Seizure 2021, 91, 278–286. [Google Scholar] [CrossRef]
- Chen, S.; Wang, X.; Cheng, Y.; Gao, H.; Chen, X. A Review of Classification, Biosynthesis, Biological Activities and Potential Applications of Flavonoids. Molecules 2023, 28, 4982. [Google Scholar] [CrossRef]
- L. Suraweera, T.; Rupasinghe, H.V.; Dellaire, G.; Xu, Z. Regulation of Nrf2/ARE pathway by dietary flavonoids: A friend or foe for cancer management? Antioxidants 2020, 9, 973. [Google Scholar] [CrossRef]
- Jin, H.-R.; Yu, J.; Choi, S.-J. Hydrothermal treatment enhances antioxidant activity and intestinal absorption of rutin in tartary buckwheat flour extracts. Foods 2019, 9, 8. [Google Scholar] [CrossRef]
- Banerjee, S.; Sarkar, R.; Mukherjee, A.; Miyoshi, S.-i.; Kitahara, K.; Halder, P.; Koley, H.; Chawla-Sarkar, M. Quercetin, a flavonoid, combats rotavirus infection by deactivating rotavirus-induced pro-survival NF-κB pathway. Front. Microbiol. 2022, 13, 951716. [Google Scholar] [CrossRef] [PubMed]
- Boyle, S.; Dobson, V.; Duthie, S.J.; Hinselwood, D.; Kyle, J.; Collins, A. Bioavailability and efficiency of rutin as an antioxidant: A human supplementation study. Eur. J. Clin. Nutr. 2000, 54, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Park, C.M.; Park, J.Y.; Noh, K.H.; Shin, J.H.; Song, Y.S. Taraxacum officinale Weber extracts inhibit LPS-induced oxidative stress and nitric oxide production via the NF-κB modulation in RAW 264.7 cells. J. Ethnopharmacol. 2011, 133, 834–842. [Google Scholar] [CrossRef] [PubMed]
| Group | C | T1 | T2 | T3 | T4 | |
|---|---|---|---|---|---|---|
| Items | ||||||
| Rutin (ng/mL) | 10 d | ND | ND | ND | ND | 26.17 ± 2.46 |
| 20 d | ND | ND | ND | ND | 21.43 ± 0.68 | |
| 30 d | ND | ND | ND | ND | 17.50 ± 2.85 | |
| Quercetin (ng/mL) | 10 d | ND | ND | ND | ND | 8.70 ± 2.25 |
| 20 d | ND | ND | ND | ND | 9.19 ± 1.87 | |
| 30 d | ND | ND | ND | ND | 7.83 ± 1.51 |
| Time | 1 h | 2 h | 4 h | 6 h | 7 h | 24 h | p- Value | |
|---|---|---|---|---|---|---|---|---|
| Items | ||||||||
| Rutin (ng/mL) | 3.82 ± 0.08 c | 2.80 ± 1.11 cd | 11.94 ± 1.96 a | 6.21 ± 1.69 b | 1.25 ± 0.60 c | ND | <0.01 | |
| Quercetin (ng/mL) | 5.15 ± 1.97 AB | 18.64 ± 7.34 A | 17.39 ± 3.67 A | 5.67 ± 2.54 AB | 5.60 ± 2.87 AB | ND | <0.01 | |
| Items | C | T1 | T2 | T3 | T4 | p-Value |
|---|---|---|---|---|---|---|
| Feed (mg/g) | 7.05 ± 0.12 D | 7.27 ± 0.12 D | 7.71 ± 0.13 C | 8.27 ± 0.07 B | 11.46 ± 0.27 A | <0.01 |
| layer hen thigh meat (mg/g) | 0.48 ± 0.12 D | 0.6 ± 0.19 D | 1.19 ± 0.23 C | 1.73 ± 0.23 B | 2.42 ± 0.18 A | 0.01 |
| layer hen breast meat (mg/g) | 0.46 ± 0.07 Cd | 0.51 ± 0.08 Cc | 0.63 ± 0.10 Cc | 0.94 ± 0.17 Bb | 1.25 ± 0.13 Aa | 0.01 |
| Items | Linear Regression Equation | R | R2 | p-Value |
|---|---|---|---|---|
| layer hen thigh meat | Y = −2.163 + 0.413X | 0.892 | 0.795 | <0.01 |
| layer hen breast meat | Y = −0.700 + 0.175X | 0.890 | 0.792 | <0.01 |
| Group | C | T1 | T2 | T3 | T4 | p-Value | |
|---|---|---|---|---|---|---|---|
| Items | |||||||
| Slaughtering rate | 79.15 ± 4.56 | 82.74 ± 2.99 | 80.62 ± 4.99 | 81.18 ± 4.48 | 81.52 ± 1.55 | 0.48 | |
| Half-clearance rate | 66.11 ± 2.56 | 66.05 ± 2.82 | 66.64 ± 3.58 | 66.30 ± 4.04 | 65.16 ± 2.53 | 0.91 | |
| Full bore rate | 51.93 ± 1.95 | 52.41 ± 2.45 | 53.54 ± 3.19 | 52.92 ± 3.08 | 52.42 ± 2.63 | 0.80 | |
| Pectoral muscle rate | 16.88 ± 1.73 | 15.60 ± 2.74 | 15.76 ± 1.51 | 16.32 ± 3.27 | 16.60 ± 3.79 | 0.87 | |
| Thigh muscle rate | 24.02 ± 2.25 | 23.70 ± 1.62 | 22.98 ± 3.44 | 24.57 ± 3.09 | 23.06 ± 3.03 | 0.76 | |
| Abdominal fat percentage | 5.49 ± 1.85 a | 4.42 ± 2.49 ab | 2.89 ± 1.39 bc | 1.92 ± 0.52 c | 2.99 ± 2.31 bc | <0.01 | |
| Thigh muscle coarse protein | 20.33 ± 0.39 | 19.53 ± 0.38 | 19.80 ± 0.36 | 19.70 ± 0.45 | 19.77 ± 0.55 | 0.09 | |
| Thigh muscle moisture | 64.90 ± 3.76 | 61.17 ± 0.9 | 62.93 ± 2.44 | 63.33 ± 2.29 | 63.73 ± 2.11 | 0.48 | |
| Thigh muscle crude fat | 8.43 ± 1.75 b | 13.23 ± 1.75 a | 12.33 ± 2.22 ab | 12.45 ± 2.33 ab | 12.73 ± 3.41 a | 0.20 | |
| Breast muscle crude protein | 18.53 ± 0.71 | 18.50 ± 0.35 | 18.10 ± 0.20 | 19.13 ± 1.16 | 18.57 ± 0.50 | 0.50 | |
| Breast muscle moisture | 53.77 ± 5.95 | 56.93 ± 2.78 | 54.30 ± 2.50 | 57.60 ± 9.35 | 56.37 ± 5.01 | 0.90 | |
| Breast muscle crude fat | 17.20 ± 2.72 | 17.47 ± 1.85 | 19.93 ± 0.70 | 15.57 ± 3.94 | 17.07 ± 2.21 | 0.38 | |
| Group | C | T1 | T2 | T3 | T4 | p-Value | |
|---|---|---|---|---|---|---|---|
| Items and Date | |||||||
| ALT (U/L) | 10 d | 13.28 ± 4.71 | 9.13 ± 2.96 | 12.93 ± 1.23 | 12.49 ± 1.05 | 10.99 ± 6.02 | 0.44 |
| 20 d | 14.08 ± 1.21 | 12.94 ± 1.63 | 12.33 ± 3.19 | 13.64 ± 3.67 | 13.85 ± 2.21 | 0.78 | |
| 30 d | 15.10 ± 2.12 a | 14.25 ± 2.23 a | 15.04 ± 2.57 a | 10.63 ± 1.64 b | 10.87 ± 3.75 b | 0.02 | |
| AST (U/L) | 10 d | 19.40 ± 5.50 | 19.38 ± 3.61 | 19.63 ± 4.59 | 19.56 ± 5.53 | 17.98 ± 2.08 | 0.98 |
| 20 d | 23.46 ± 2.39 a | 20.71 ± 4.79 a | 18.63 ± 5.34 ab | 18.21 ± 2.06 ab | 14.23 ± 3.79 b | 0.03 | |
| 30 d | 20.18 ± 2.84 a | 17.40 ± 2.60 a | 17.00 ± 3.68 a | 17.31 ± 4.24 a | 11.40 ± 5.01 b | 0.02 | |
| ALB (g/L) | 10 d | 19.13 ± 1.44 b | 23.41 ± 2.27 a | 23.94 ± 2.37 a | 25.35 ± 2.25 a | 23.66 ± 1.10 a | <0.01 |
| 20 d | 22.54 ± 1.69 b | 25.27 ± 1.89 a | 23.76 ± 1.50 ab | 22.78 ± 0.77 b | 23.33 ± 1.56 ab | 0.05 | |
| 30 d | 23.27 ± 2.72 | 23.92 ± 3.33 | 23.43 ± 2.46 | 22.72 ± 1.66 | 24.89 ± 2.97 | 0.67 | |
| TG (mmol/L) | 10 d | 5.07 ± 1.61 | 4.16 ± 1.41 | 4.10 ± 1.01 | 4.18 ± 0.77 | 3.66 ± 0.53 | 0.53 |
| 20 d | 5.71 ± 0.90 | 5.40 ± 0.66 | 4.32 ± 1.41 | 4.43 ± 0.77 | 3.99 ± 1.08 | 0.32 | |
| 30 d | 5.11 ± 0.77 a | 4.73 ± 0.57 a | 3.88 ± 0.05 ab | 5.04 ± 0.95 a | 3.39 ± 0.16 b | 0.03 | |
| HDL-C (mmol/L) | 10 d | 1.12 ± 0.69 | 1.03 ± 0.55 | 1.31 ± 0.46 | 1.58 ± 0.96 | 1.57 ± 0.75 | 0.63 |
| 20 d | 1.31 ± 0.50 | 1.35 ± 0.57 | 1.75 ± 1.09 | 1.81 ± 0.48 | 2.01 ± 0.29 | 0.50 | |
| 30 d | 0.97 ± 0.12 | 1.04 ± 0.46 | 1.17 ± 0.51 | 1.18 ± 0.26 | 1.11 ± 0.45 | 0.96 | |
| LDL-C (mmol/L) | 10 d | 1.37 ± 0.34 | 1.15 ± 0.31 | 1.27 ± 0.21 | 1.07 ± 0.38 | 0.94 ± 0.23 | 0.18 |
| 20 d | 1.55 ± 0.33 a | 1.38 ± 0.40 a | 1.36 ± 0.54 ab | 1.03 ± 0.25 ab | 0.83 ± 0.10 b | 0.50 | |
| 30 d | 1.57 ± 0.47 | 1.29 ± 0.18 | 1.36 ± 0.71 | 1.28 ± 0.33 | 1.19 ± 0.54 | 0.71 | |
| TC (mmol/L) | 10 d | 2.38 ± 0.56 | 2.24 ± 0.32 | 2.16 ± 0.22 | 2.33 ± 1.19 | 1.62 ± 0.27 | 0.26 |
| 20 d | 3.31 ± 0.87 a | 2.44 ± 0.36 b | 2.64 ± 0.53 ab | 2.34 ± 0.51 b | 2.16 ± 0.59 b | 0.03 | |
| 30 d | 2.27 ± 0.44 | 2.25 ± 0.44 | 1.99 ± 0.55 | 2.06 ± 0.30 | 1.83 ± 0.43 | 0.39 | |
| GLU (mmol/L) | 10 d | 13.14 ± 1.94 | 12.50 ± 2.52 | 11.42 ± 2.52 | 12.26 ± 1.36 | 10.78 ± 1.44 | 0.30 |
| 20 d | 12.73 ± 0.91 | 12.87 ± 1.31 | 12.26 ± 0.34 | 12.01 ± 1.36 | 11.79 ± 1.39 | 0.43 | |
| 30 d | 12.89 ± 1.03 a | 10.58 ± 1.62 c | 11.06 ± 1.02 bc | 12.39 ± 0.93 ab | 11.02 ± 1.03 bc | <0.01 |
| Group | C | T1 | T2 | T3 | T4 | p-Value | |
|---|---|---|---|---|---|---|---|
| Items | |||||||
| CAT (U/mL) | 10 d | 1.48 ± 0.83 | 1.51 ± 0.93 | 1.78 ± 0.51 | 1.78 ± 0.84 | 1.81 ± 0.56 | 0.91 |
| 20 d | 1.43 ± 0.77 | 2.13 ± 1.06 | 2.45 ± 0.65 | 2.30 ± 0.50 | 2.46 ± 0.88 | 0.21 | |
| 30 d | 1.19 ± 1.01 | 1.16 ± 0.57 | 1.19 ± 0.77 | 1.52 ± 0.33 | 2.20 ± 0.62 | 0.15 | |
| MDA (nmol/L) | 10 d | 11.43 ± 2.17 a | 10.12 ± 1.46 ab | 8.57 ± 3.56 b | 5.83 ± 1.53 c | 3.69 ± 1.39 c | <0.01 |
| 20 d | 11.71 ± 2.18 a | 10.83 ± 2.57 a | 10.00 ± 1.50 a | 8.10 ± 4.16 ab | 5.71 ± 3.06 b | 0.01 | |
| 30 d | 11.86 ± 3.48 | 8.93 ± 1.17 | 9.64 ± 1.48 | 10.00 ± 2.02 | 11.79 ± 2.85 | 0.15 | |
| T-AOC (mmol/L) | 10 d | 0.29 ± 0.15 | 0.44 ± 0.20 | 0.32 ± 0.17 | 0.33 ± 0.14 | 0.38 ± 0.08 | 0.70 |
| 20 d | 0.26 ± 0.03 b | 0.22 ± 0.10 b | 0.24 ± 0.13 b | 0.47 ± 0.13 a | 0.41 ± 0.12 ab | 0.03 | |
| 30 d | 0.23 ± 0.04 | 0.29 ± 0.13 | 0.34 ± 0.07 | 0.45 ± 0.26 | 0.39 ± 0.17 | 0.29 | |
| T-SOD (U/L) | 10 d | 174.11 ± 35.10 | 183.09 ± 26.25 | 170.92 ± 7.79 | 181.7 ± 12.88 | 209.05 ± 12.97 | 0.29 |
| 20 d | 172.71 ± 25.76 | 168.52 ± 54.35 | 184.09 ± 21.10 | 190.08 ± 17.97 | 185.49 ± 30.44 | 0.91 | |
| 30 d | 164.73 ± 9.41 c | 153.34 ± 6.34 c | 166.52 ± 6.91 c | 203.26 ± 15.87 b | 266.36 ± 10.55 a | <0.01 | |
| GSH-Px (U/mL) | 10 d | 2569.55 ± 21.89 c | 2860.9 ± 292.71 bc | 2985.07 ± 533.11 bc | 3792.24 ± 377.01 a | 3414.93 ± 240.33 ab | <0.01 |
| 20 d | 2388.06 ± 623.96 | 3094.93 ± 263.03 | 2712.84 ± 334.60 | 2789.25 ± 614.01 | 2779.7 ± 408.04 | 0.52 | |
| 30 d | 1977.31 ± 724.82 | 2459.7 ± 455.89 | 2880 ± 432.71 | 2870.45 ± 621.98 | 2870.45 ± 276.48 | 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Zhang, J.; Zhang, Y.; Liu, D.; You, C.; Zhang, W.; Ren, C.; Zhao, X.; Li, L.; Yu, X. Effects of Dandelion Flavonoid Extract on the Accumulation of Flavonoids in Layer Hen Meat, Slaughter Performance and Blood Antioxidant Indicators of Spent Laying Hens. Animals 2025, 15, 886. https://doi.org/10.3390/ani15060886
Wei Y, Zhang J, Zhang Y, Liu D, You C, Zhang W, Ren C, Zhao X, Li L, Yu X. Effects of Dandelion Flavonoid Extract on the Accumulation of Flavonoids in Layer Hen Meat, Slaughter Performance and Blood Antioxidant Indicators of Spent Laying Hens. Animals. 2025; 15(6):886. https://doi.org/10.3390/ani15060886
Chicago/Turabian StyleWei, Yuyu, Jingwen Zhang, Yiming Zhang, Dingkuo Liu, Chunxue You, Wenjuan Zhang, Chaoqi Ren, Xin Zhao, Liu’an Li, and Xiaoxue Yu. 2025. "Effects of Dandelion Flavonoid Extract on the Accumulation of Flavonoids in Layer Hen Meat, Slaughter Performance and Blood Antioxidant Indicators of Spent Laying Hens" Animals 15, no. 6: 886. https://doi.org/10.3390/ani15060886
APA StyleWei, Y., Zhang, J., Zhang, Y., Liu, D., You, C., Zhang, W., Ren, C., Zhao, X., Li, L., & Yu, X. (2025). Effects of Dandelion Flavonoid Extract on the Accumulation of Flavonoids in Layer Hen Meat, Slaughter Performance and Blood Antioxidant Indicators of Spent Laying Hens. Animals, 15(6), 886. https://doi.org/10.3390/ani15060886






