17α-Methyltestosterone Affected Growth, Gonadal Development, and Intestinal Microbial Analysis in the Giant Freshwater Prawn (Macrobrachium rosenbergii)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Dietary Preparation
2.2. Experimental Animals and Design
2.3. Analysis of Reproductive-Related Gene Expression Levels
2.4. Measurement of the Growth Traits
2.5. Sex Ratio Statistics
2.6. Histological Observations of Gonadal Development
2.7. Analysis of Intestinal Microbial Diversity
2.8. Statistical Analysis
3. Results
3.1. Effects of MT Concentration on the Sex Ratio of M. rosenbergii
3.2. Histological Observations of the Gonad
3.3. Effect of MT Concentration on the Growth Traits of M. rosenbergii
3.4. Effects of MT Concentration on Intestinal Microbial Diversity of M. rosenbergii
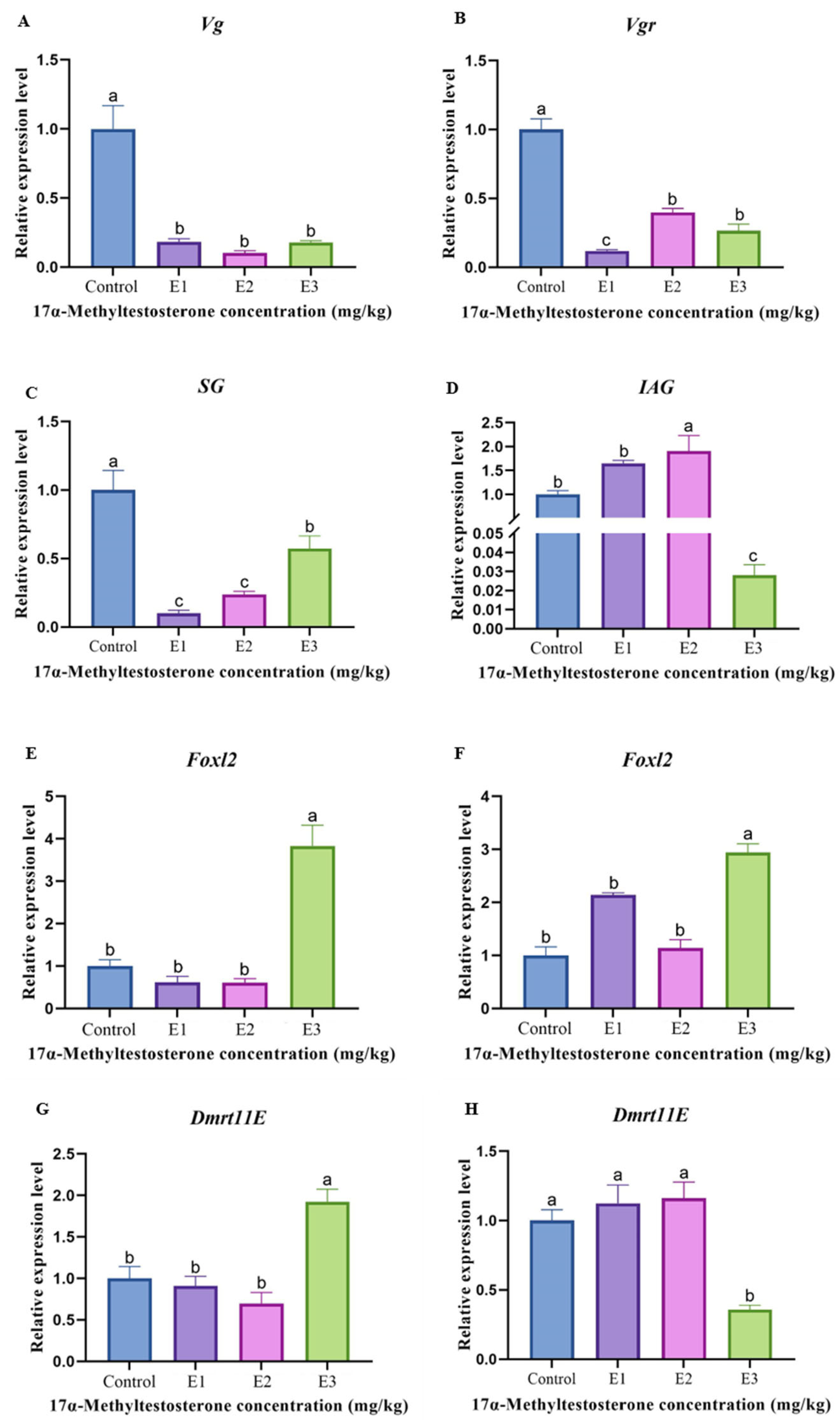
3.5. Gene Expression Levels Associated with Reproduction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, W.L. Molecular biology of steroid hormone synthesis. Endocr. Rev. 1988, 9, 295–318. [Google Scholar] [CrossRef] [PubMed]
- Wierman, M.E. Sex steroid effects at target tissues: Mechanisms of action. Adv. Physiol. Educ. 2007, 31, 26–33. [Google Scholar] [CrossRef]
- Whirledge, S.; Cidlowski, J.A. Steroid hormone action. In Yen and Jaffe’s Reproductive Endocrinology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 115–131. [Google Scholar] [CrossRef]
- Anderson, D.C. Sex-hormone-binding globulin. Clin. Endocrinol. 1974, 3, 69–96. [Google Scholar] [CrossRef]
- Beato, M.; Klug, J. Steroid hormone receptors: An update. Hum. Reprod. Update 2000, 6, 225–236. [Google Scholar] [CrossRef]
- Bosch, E.; Alviggi, C.; Lispi, M.; Conforti, A.; Hanyaloglu, A.C.; Chuderland, D.; Humaidan, P. Reduced FSH and LH action: Implications for medically assisted reproduction. Hum. Reprod. 2021, 36, 1469–1480. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, E.G.; Lee, G.; Jeong, M.G.; Kim, H.K.; Oh, J.H.; Hwang, E.S. Amodiaquine promotes testosterone production and de novo synthesis of cholesterol and triglycerides in Leydig cells. J. Lipid Res. 2021, 62, 100152. [Google Scholar] [CrossRef]
- Edelman, I.S. Mechanism of action of steroid hormones. J. Steroid Biochem. 1975, 6, 147–159. [Google Scholar] [CrossRef]
- Thibaut, R.; Porte, C. Effects of endocrine disrupters on sex steroid synthesis and metabolism pathways in fish. J. Steroid Biochem. Mol. Biol. 2004, 92, 485–494. [Google Scholar] [CrossRef]
- Drobnis, E.Z.; Nangia, A.K. Exogenous androgens and male reproduction. In Impacts of Medications on Male Fertility; Springer: Berlin/Heidelberg, Germany, 2017; pp. 25–28. [Google Scholar] [CrossRef]
- Beardmore, J.A.; Mair, G.C.; Lewis, R.I. Monosex male production in finfish as exemplified by tilapia: Applications, problems, and prospects. In Reproductive Biotechnology in Finfish Aquaculture; Elsevier: Amsterdam, The Netherlands, 2001; pp. 283–301. [Google Scholar] [CrossRef]
- Lange, I.G.; Hartel, A.; Meyer, H.H. Evolution of oestrogen functions in vertebrates. J. Steroid Biochem. Mol. Biol. 2002, 83, 219–226. [Google Scholar] [CrossRef]
- Park, C.B.; Aoki, J.Y.; Lee, J.S.; Nagae, M.; Lee, Y.D.; Sakakura, Y.; Soyano, K. The effects of 17β-estradiol on various reproductive parameters in the hermaphrodite fish Kryptolebias marmoratus. Aquat. Toxicol. 2010, 96, 273–279. [Google Scholar] [CrossRef]
- Abo-Al-Ela, H.G. Hormones and fish monosex farming: A spotlight on immunity. Fish Shellfish Immunol. 2018, 72, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Pace, F.; Watnick, P.I. The interplay of sex steroids, the immune response, and the intestinal microbiota. Trends Microbiol. 2021, 29, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ke, X. Effect of induction on sex reversal of Monopterus albus using heterologous hormone. Southwest China J. Agric. Sci. 1992, 01, 74–78. (In Chinese) [Google Scholar] [CrossRef]
- Martinez-Bengochea, A.; Doretto, L.; Rosa, I.F.; Oliveira, M.A.; Silva, C.; Silva, D.M.Z.A.; Nóbrega, R.H. Effects of 17β-estradiol on early gonadal development and expression of genes implicated in sexual differentiation of a South American teleost, Astyanax altiparanae. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2020, 248, 110467. [Google Scholar] [CrossRef]
- Zhu, Q.; Han, C.; Liu, S.; Ouyang, H.; Liu, D.; Zhang, Z.; Zhang, Y. Development and gene expression analysis of gonad during 17α-methyltestosterone-induced sex reversal in mandarin fish (Siniperca chuatsi). Aquac. Rep. 2022, 23, 101049. [Google Scholar] [CrossRef]
- Moreno-Indias, I.; Sánchez-Alcoholado, L.; Sánchez-Garrido, M.Á.; Martín-Núñez, G.M.; Pérez-Jiménez, F.; Tena-Sempere, M.; Queipo-Ortuño, M.I. Neonatal androgen exposure causes persistent gut microbiota dysbiosis related to metabolic disease in adult female rats. Endocrinology 2016, 157, 4888–4898. [Google Scholar] [CrossRef]
- Asad, F.; Naz, S.; Ali, T.; Gul, Y.; Jamal, R.; Shaheen, Z.; Bano, S. Effect of natural and synthetic androgen hormone on sex reversal of Nile Tilapia (Oreochromis niloticus). Braz. J. Biol. 2023, 84, e272413. [Google Scholar] [CrossRef]
- Jin, S.B.; Yue, D.; Fu, H.; Jiang, S.; Xiong, Y.; Qiao, H.; Wu, Y. Effects of dietary supplementation with 17β-estradiol and 17α-methyltestosterone on growth performance and gonadal development of the juvenile oriental river prawn (Macrobrachium nipponense). Aquac. Rep. 2022, 23, 101042. [Google Scholar] [CrossRef]
- Cai, P.; Yuan, H.; Gao, Z.; Daka, P.; Qiao, H.; Zhang, W.; Fu, H. Sex reversal induced by dietary supplementation with 17α-methyltestosterone during the critical period of sex differentiation in oriental river prawn (Macrobrachium nipponense). Animals 2023, 13, 1369. [Google Scholar] [CrossRef]
- Bureau of Fisheries, The Ministry of Agriculture of the People’s Republic of China. Fisheries economic statistics. In China Fishery Yearbook; Beijing China Agricultural Press: Beijing, China, 2024; p. 24. (In Chinese) [Google Scholar]
- Sagi, A.; Ra’anan, Z.; Cohen, D.; Wax, Y. Production of Macrobrachium rosenbergii in monosex populations: Yield characteristics under intensive monoculture conditions in cages. Aquaculture 1986, 51, 265–275. [Google Scholar] [CrossRef]
- Yu, Y.Q. The Molecular Characterization and Functional Analysis of Sexual Development Related Genes Sxl and Dmrt in the Prawn, Macrobrachium rosenbergii. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2013. (In Chinese). [Google Scholar]
- Sagi, A.; Cohen, D.; Milner, Y. Effect of androgenic gland ablation on morphotypic differentiation and sexual characteristics of male freshwater prawns, Macrobrachium rosenbergii. Gen. Comp. Endocrinol. 1990, 77, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Ventura, T.; Sagi, A. The insulin-like androgenic gland hormone in crustaceans: From a single gene silencing to a wide array of sexual manipulation-based biotechnologies. Biotechnol. Adv. 2012, 30, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Levy, T.; Rosen, O.; Eilam, B.; Azulay, D.; Aflalo, E.D.; Manor, R.; Sagi, A. A single injection of hypertrophied androgenic gland cells produces all-female aquaculture. Mar. Biotechnol. 2016, 18, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Antiporda, J.L. Preliminary Studies on the Effect of Methyltestosterone on Macrobrachium rosenbergii Juveniles; Network of Aquaculture in Asia Pacific: Bangkok, Thailand, 1986. [Google Scholar]
- Baghel, D.S.; Lakra, W.S.; Satyanarayana Rao, G.P. Altered sex ratio in giant fresh water prawn, Macrobrachium rosenbergii (de Man) using hormone bioencapsulated live Artemia feed. Aquac. Res. 2004, 35, 943–947. [Google Scholar] [CrossRef]
- Ohs, C.L.; D’Abramo, L.R.; Kelly, A.M. Effect of dietary administration of 17a-methyltestosterone on the sex ratio of postlarval freshwater prawn, Macrobrachium rosenbergii, during the nursery stage of culture. J. World Aquac. Soc. 2006, 37, 328–333. [Google Scholar] [CrossRef]
- Shen, S.Q.; Li, J.W.; Xu, H.J.; Yang, J.S.; Ma, W.M.; Qian, G.Y. Sexual characteristic development and sex identification of juvenile prawns, Macrobrachium rosenbergii. Aquac. Res. 2020, 51, 3718–3728. [Google Scholar] [CrossRef]
- Yamamoto, T.O. 3 Sex differentiation. Fish Physiol. 1969, 3, 117–175. [Google Scholar] [CrossRef]
- Lou, Y. Progress on research of sex control in fish. J. Shanghai Fish. Univ. 1992, 1, 168–173. (In Chinese) [Google Scholar]
- Fang, Y.; Li, Z. Comparison of 17α-methyltestosterone on spermatogenesis in grey mullet of fresh water and seawater. J. Oceanogr. Taiwan Strait 1992, 11, 301–304. (In Chinese) [Google Scholar]
- Blázquez, M.; Piferrer, F.; Zanuy, S.; Carrillo, M.; Donaldson, E.M. Development of sex control techniques for European sea bass (Dicentrarchus labrax L.) aquaculture: Effects of dietary 17 α-methyltestosterone prior to sex differentiation. Aquaculture 1995, 135, 329–342. [Google Scholar] [CrossRef]
- Kitano, T.; Takamune, K.; Nagahama, Y.; Abe, S.I. Aromatase inhibitor and 17α-methyltestosterone cause sex-reversal from genetical females to phenotypic males and suppression of P450 aromatase gene expression in Japanese flounder (Paralichthys olivaceus). Mol. Reprod. Dev. Inc. Gamete Res. 2000, 56, 1–5. [Google Scholar] [CrossRef]
- Guerrero, R.D., III. Culture of Male Tilapia mossambica Produced Through Artificial Sex Reversal; Advances in Aquaculture; Fishing News Books Ltd: Farnham, UK, 1979; pp. 166–168. [Google Scholar]
- Macintosh, D.J.; Varghese, T.J.; Rao Satyanarayana, G.P. Hormonal sex reversal of wild-spawned tilapia in India. J. Fish Biol. 1985, 26, 87–94. [Google Scholar] [CrossRef]
- Zheng, S.M.; Chen, Z.B.; He, R. The sex reversal of methyltestosterone against Guppies and Mariposities. Sichuan J. Zool. 1997, 16, 183–185. (In Chinese) [Google Scholar]
- Rinchard, J.; Dabrowski, K.; Garcia–Abiado, M.A.; Ottobre, J. Uptake and depletion of plasma 17α-methyltestosterone during induction of masculinization in muskellunge, Esox masquinongy: Effect on plasma steroids and sex reversal. Steroids 1999, 64, 518–525. [Google Scholar] [CrossRef]
- Zerulla, M.; Länge, R.; Steger-Hartmann, T.; Panter, G.; Hutchinson, T.; Dietrich, D.R. Morphological sex reversal upon short-term exposure to endocrine modulators in juvenile fathead minnow (Pimephales promelas). Toxicol. Lett. 2002, 131, 51–63. [Google Scholar] [CrossRef]
- Örn, S.; Holbech, H.; Madsen, T.H.; Norrgren, L.; Petersen, G.I. Gonad development and vitellogenin production in zebrafish (Danio rerio) exposed to ethinylestradiol and methyltestosterone. Aquat. Toxicol. 2003, 65, 397–411. [Google Scholar] [CrossRef]
- LONE, K.P.; Ridha, M.T. Sex reversal and growth of Oreochromis spilurus (Günther) in brackish and sea water by feeding 17α-methyltestosterone. Aquac. Res. 1993, 24, 593–602. [Google Scholar] [CrossRef]
- Pawlowski, S.; Sauer, A.; Shears, J.A.; Tyler, C.R.; Braunbeck, T. Androgenic and estrogenic effects of the synthetic androgen 17α-methyltestosterone on sexual development and reproductive performance in the fathead minnow (Pimephales promelas) determined using the gonadal recrudescence assay. Aquat. Toxicol. 2004, 68, 277–291. [Google Scholar] [CrossRef]
- Lyu, Q.; Hu, J.; Yang, X.; Liu, X.; Chen, Y.; Liao, L.; Zhao, H. Expression profiles of dmrts and foxls during gonadal development and sex reversal induced by 17α-methyltestosterone in the orange-spotted grouper. Gen. Comp. Endocrinol. 2019, 274, 26–36. [Google Scholar] [CrossRef]
- Ankley, G.T.; Jensen, K.M.; Kahl, M.D.; Korte, J.J.; Makynen, E.A. Description and evaluation of a short-term reproduction test with the fathead minnow (Pimephales promelas). Environ. Toxicol. Chem. Int. J. 2001, 20, 1276–1290. [Google Scholar] [CrossRef]
- Li, M.; Zhang, F.; Ding, J.; Zuo, R.; Chang, Y. Effects of lipid sources on the growth performance, gonad development, fatty acid profile and transcription of related genes in juvenile sea urchin (Strongylocentrotus intermedius). Aquac. Nutr. 2021, 27, 28–38. [Google Scholar] [CrossRef]
- Neuman, H.; Debelius, J.W.; Knight, R.; Koren, O. Microbial endocrinology: The interplay between the microbiota and the endocrine system. FEMS Microbiol. Rev. 2015, 39, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Org, E.; Mehrabian, M.; Parks, B.W.; Shipkova, P.; Liu, X.; Drake, T.A.; Lusis, A.J. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes 2016, 7, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Tan, X.; Huang, C.; Zhao, H.; Lan, W. Sources, pollution characteristics, and ecological risk assessment of steroids in Beihai Bay, Guangxi. Water 2022, 14, 1399. [Google Scholar] [CrossRef]
- Wang, C.; Li, R.; Li, S.; Yi, M.; Li, J.; Sun, Y.; Ni, J. Dysbiosis of gut microbiota by actual long-term exposure to textile wastewater treatment plant effluents in adult zebrafish (Danio rerio) using aquatic microcosm systems. J. Water Process Eng. 2023, 54, 104047. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, Y.; Li, H.; Qiao, F.; Wu, J.; Du, Z.Y.; Zhang, M. Influence of endogenous and exogenous estrogenic endocrine on intestinal microbiota in zebrafish. PLoS ONE 2016, 11, e0163895. [Google Scholar] [CrossRef]
- Gao, S.; Pan, L.; Huang, F.; Song, M.; Tian, C.; Zhang, M. Metagenomic insights into the structure and function of intestinal microbiota of the farmed Pacific white shrimp (Litopenaeus vannamei). Aquaculture 2019, 499, 109–118. [Google Scholar] [CrossRef]
- Semova, I.; Carten, J.D.; Stombaugh, J.; Mackey, L.C.; Knight, R.; Farber, S.A.; Rawls, J.F. Microbiota regulate intestinal absorption and metabolism of fatty acids in the zebrafish. Cell Host Microbe 2012, 12, 277–288. [Google Scholar] [CrossRef]
- Li, T.; Xia, F.; You, J.; Zhang, J.; Zhang, P.; Zhang, Z.; Zhang, X.; Song, H.; Cai, H.; Shi, H. Effects of the intestinal microbial community structure on the growth of Litopenaeus vannamei. J. Zhejiang Ocean. Univ. Nat. Sci. 2022, 41, 499–507. (In Chinese) [Google Scholar]
- Huang, K.; Cheng, Y.; Huo, Z.; Qing, Y. Advances in the Control of Aquatic Invertebrates’ Gonadal Development. Open J. Fish. Res. 2024, 11, 167–176. [Google Scholar] [CrossRef]
- Otani, A.; Nakajima, T.; Okumura, T.; Fujii, S.; Tomooka, Y. Sex reversal and analyses of possible involvement of sex steroids in scallop gonadal development in newly established organ-culture systems. Zool. Sci. 2017, 34, 86–92. [Google Scholar] [CrossRef]


| Primer Name | Sequence (5′→3′) | Sources | Product Size (bp) | Amplification Efficiency (%) | R2 |
|---|---|---|---|---|---|
| β-actin-F | CAGGGAAAAGATGACCCAGA | AY651918.2 | 171 | 98.5 | 0.997 |
| β-actin-R | GGAAGTGCATACCCCTCGTA | ||||
| Vg-F | GGACGCTGATCGTAACCC | AB056458.1 | 192 | 98.7 | 0.996 |
| Vg-R | TACCTCTAGCATCAAACT | ||||
| Vgr-F | TACCTTAGCATCAAACT | GU454802.1 | 145 | 96.8 | 0.987 |
| Vgr-R | GAGAAGGCGGTAAGTCTGGTT | ||||
| Foxl2-F | AGTCCCGACAGAAAGCTTCA | MZ647492.1 | 191 | 97.7 | 0.995 |
| Foxl2-R | TGCCCAAAGATCCTCCGATT | ||||
| IAG-F | GGACAGCGTGAGGAGAAGTC | FJ409645.1 | 185 | 97.5 | 0.991 |
| IAG-R | ACTAAAGCAGCGGGAAGACA | ||||
| Dmrt11E-F | ACCACCAGTAGCACCACAATACAACAG | KC801044.1 | 189 | 96.7 | 0.991 |
| Dmrt11E-R | CAAGAATCTGAAGGAAGTTCGTGAGTG | ||||
| SG-F | CCACCCATTCCTGGTAAGCATCA | EF647641.1 | 104 | 99.2 | 0.998 |
| SG-R | GAGTGTCCATTCGGTAACTCGTAG |
| Index | Group | ||||
|---|---|---|---|---|---|
| Control | E1 | E2 | E3 | ||
| all | IMW (mg) | 9.33 ± 0.78 | 9.30 ± 0. 97 | 9.37 ± 0.71 | 9.40 ± 0.60 |
| FMW (g) | 9.47 ± 1.20 b | 8.27 ± 0.66 c | 9.98 ± 0.78 ab | 11.07 ± 0.93 a | |
| WGR (%) | 101,421.97 ± 12,840.77 b | 88,528.08 ± 7025.70 c | 106,481.61 ± 8277.65 ab | 118,086.47 ± 9955.57 a | |
| SGR (%/d) | 4.48 ± 0.05 b | 4.49 ± 0.05 b | 4.66 ± 0.05 ab | 4.72 ± 0.06 a | |
| male | FMW (g) | 13.55 ± 0.72 a | 9.90 ± 0.61 b | 9.94 ± 0.50 b | 10.17 ± 1.13 b |
| WGR (%) | 177,965.93 ± 18,392.46 a | 105,955.73 ± 6516.02 b | 105,990.53 ± 5360.25 b | 108,503.15 ± 10,113.37 b | |
| GR (%/d) | 4.95 ± 0.07 a | 4.64 ± 0.04 b | 4.64 ± 0.03 b | 4.67 ± 0.07 b | |
| female | MW (g) | 6.76 ± 1.33 b | 7.19 ± 0.90 b | 10.01 ± 1.27 ab | 11.59 ± 1.33 a |
| WGR (%) | 72,286.51 ± 14,271.60 b | 76,909.65 ± 9664.70 b | 106,809.00 ± 13,602.91 ab | 123,676.73 ± 14,240.77 a | |
| SGR (%/d) | 4.23 ± 0.14 b | 4.38 ± 0.07 ab | 4.65 ± 0.08 a | 4.77 ± 0.09 a | |
| 17α-Methyltestosterone Treatment Time (Days) | Group (mg/kg) | |||
|---|---|---|---|---|
| Control | E1 | E2 | E3 | |
| 60 | 1.94 ± 0.17 b | 1.78 ± 0.20 b | 2.55 ± 0.16 a | 2.05 ± 0.20 b |
| 150 | 1.70 ± 0.55 b | 2.66 ± 0.29 a | 1.90 ± 0.21 b | 0.93 ± 0.06 c |
| Index | Group | |||
|---|---|---|---|---|
| Control | E1 | E2 | E3 | |
| Sobs | 469.50 ± 29.50 b | 615.00 ± 38.18 a | 700.33 ± 25.83 a | 796.00 ± 122.53 a |
| Shannon | 3.65 ± 0.04 c | 4.65 ± 0.05 a | 4.24 ± 0.18 b | 4.82 ± 0.06 a |
| Simpson | 0.85 ± 0.00 b | 0.91 ± 0.01 a | 0.89 ± 0.03 a | 0.90 ± 0.00 a |
| Chao1 | 526.51 ± 23.41 c | 630.95 ± 36.64 b | 755.98 ± 25.52 b | 952.00 ± 19.28 a |
| Coverage (%) | 99.86 ± 0.00 | 99.83 ± 0.01 | 99.82 ± 0.00 | 99.84 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liufu, B.; Su, Q.; Hong, K.; Wei, J.; Wang, Y.; Han, Z.; Yu, L. 17α-Methyltestosterone Affected Growth, Gonadal Development, and Intestinal Microbial Analysis in the Giant Freshwater Prawn (Macrobrachium rosenbergii). Animals 2025, 15, 870. https://doi.org/10.3390/ani15060870
Liufu B, Su Q, Hong K, Wei J, Wang Y, Han Z, Yu L. 17α-Methyltestosterone Affected Growth, Gonadal Development, and Intestinal Microbial Analysis in the Giant Freshwater Prawn (Macrobrachium rosenbergii). Animals. 2025; 15(6):870. https://doi.org/10.3390/ani15060870
Chicago/Turabian StyleLiufu, Bai, Qiyao Su, Kunhao Hong, Jie Wei, Yakun Wang, Zhiqiang Han, and Lingyun Yu. 2025. "17α-Methyltestosterone Affected Growth, Gonadal Development, and Intestinal Microbial Analysis in the Giant Freshwater Prawn (Macrobrachium rosenbergii)" Animals 15, no. 6: 870. https://doi.org/10.3390/ani15060870
APA StyleLiufu, B., Su, Q., Hong, K., Wei, J., Wang, Y., Han, Z., & Yu, L. (2025). 17α-Methyltestosterone Affected Growth, Gonadal Development, and Intestinal Microbial Analysis in the Giant Freshwater Prawn (Macrobrachium rosenbergii). Animals, 15(6), 870. https://doi.org/10.3390/ani15060870






