Indirect Enzyme-Linked Immunosorbent Assay Based on Immunoglobulin Y Antibodies for the Evaluation of Humoral Immunity Against Flavobacterium oreochromis in Colossoma macropomum: A Preliminary Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Hen Immunization
2.2. Bacterial Inoculum and Immunization of Fish Against Flavobacterium oreochromis
2.3. Standardization of the Indirect Enzyme-Linked Immunosorbent Assay (iELISA)
2.4. Statistical Analysis
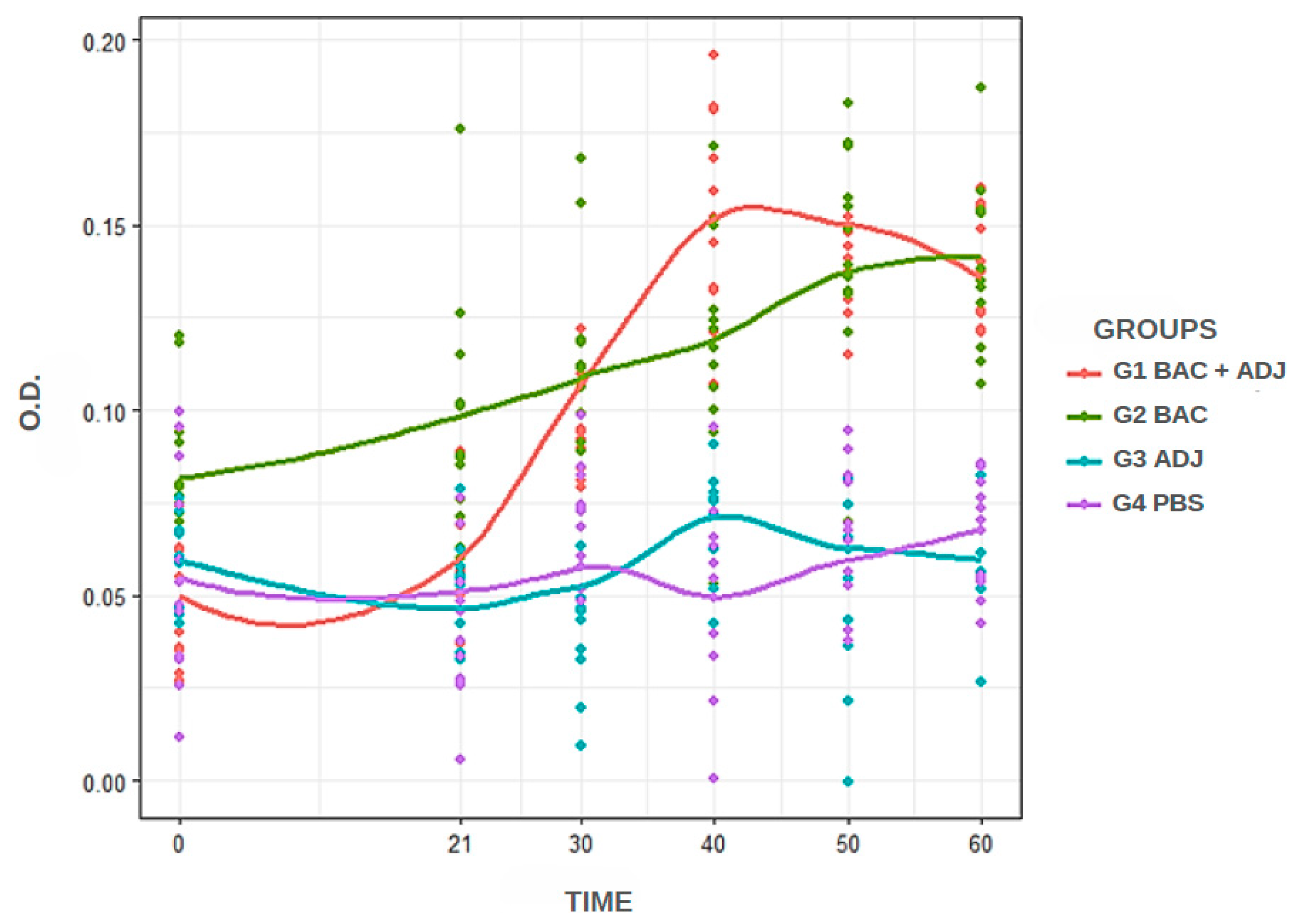
3. Results
3.1. Immunized Animals
3.2. Standardized iELISA and Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valladão, G.M.R.; Gallani, S.U.; Pilarski, F. South American Fish for Continental Aquaculture. Rev. Aquac. 2018, 10, 351–369. [Google Scholar] [CrossRef]
- IBGE. Produção Da Pecuária Municipal, 50th ed.; Instituto Brasileiro de Geografia e Estatística: Rio de Janeiro, Brasil, 2024. [Google Scholar]
- PeixeBR. Anuário Brasileiro Da Piscicultura PeixeBR 2025; PeixeBR: São Paulo, Brazil, 2025; p. 67. [Google Scholar]
- Gallani, S.U.; Valladão, G.M.R.; Assane, I.M.; de Alves, L.O.; Kotzent, S.; Hashimoto, D.T.; Pilarski, F. Motile Aeromonas Septicemia in Tambaqui Colossoma macropomum: Pathogenicity, Lethality and New Insights for Control and Disinfection in Aquaculture. Microb. Pathog. 2020, 149, 104512. [Google Scholar] [CrossRef] [PubMed]
- Mielke, T.D.; Francisco, C.J.; Dorella, F.A.; Figueiredo, H.C.P.; Tavares, G.C.; Gallani, S.U. The Strategic Use of Water Additives for Tambaqui Colossoma macropomum Transport: New Insights of Bacteriosis and Productivity Approach. Aquaculture 2022, 558, 738406. [Google Scholar] [CrossRef]
- Reis, F.Y.; Rocha, V.P.; Janampa-Sarmiento, P.C.; Costa, H.L.; Egger, R.C.; Passos, N.C.; de Assis, C.H.; Carneiro, S.P.; Santos, Á.F.; Silva, B.A.; et al. Edwardsiella tarda in Tambaqui (Colossoma macropomum): A Pathogenicity, Antimicrobial Susceptibility, and Genetic Analysis of Brazilian Isolates. Animals 2023, 13, 2910. [Google Scholar] [CrossRef]
- Hilsdorf, A.W.S.; Hallerman, E.; Valladão, G.M.R.; Zaminhan-Hassemer, M.; Hashimoto, D.T.; Dairiki, J.K.; Takahashi, L.S.; Albergaria, F.C.; de Gomes, M.E.S.; Venturieri, R.L.L.; et al. The Farming and Husbandry of Colossoma macropomum: From Amazonian Waters to Sustainable Production. Rev. Aquac. 2022, 14, 993–1027. [Google Scholar] [CrossRef]
- Barony, G.; Tavares, G.; Assis, G.; Luz, R.; Figueiredo, H.; Leal, C. New Hosts and Genetic Diversity of Flavobacterium columnare Isolated from Brazilian Native Species and Nile Tilapia. Dis. Aquat. Organ. 2015, 117, 1–11. [Google Scholar] [CrossRef]
- Pilarski, F.; Rossini, A.J.; Ceccarelli, P.S. Isolation and Characterization of Flavobacterium columnare (Bernardet et al. 2002) from Four Tropical Fish Species in Brazil. Braz. J. Biol. 2008, 68, 409–414. [Google Scholar] [CrossRef]
- Mohammed, H.; Arias, C. Epidemiology of Columnaris Disease Affecting Fishes within the Same Watershed. Dis. Aquat. Organ. 2014, 109, 201–211. [Google Scholar] [CrossRef]
- Shoemaker, C.A.; Olivares-Fuster, O.; Arias, C.R.; Klesius, P.H. Flavobacterium columnare Genomovar Influences Mortality in Channel Catfish (Ictalurus punctatus). Vet. Microbiol. 2008, 127, 353–359. [Google Scholar] [CrossRef]
- Bunnoy, A.; Thangsunan, P.; Chokmangmeepisarn, P.; Yata, T.; Klongklaew, N.; Pirarat, N.; Kitiyodom, S.; Srisapoome, P.; Rodkhum, C. Mucoadhesive Cationic Lipid-Based Flavobacterium oreochromis Nanoencapsulation Enhanced the Efficacy of Mucoadhesive Immersion Vaccination against Columnaris Disease and Strengthened Immunity in Asian Sea Bass (Lates calcarifer). Fish Shellfish. Immunol. 2022, 127, 633–646. [Google Scholar] [CrossRef]
- Ma, J.; Bruce, T.J.; Jones, E.M.; Cain, K.D. A Review of Fish Vaccine Development Strategies: Conventional Methods and Modern Biotechnological Approaches. Microorganisms 2019, 7, 569. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S.; Putalun, W.; Vimolmangkang, S.; Phoolcharoen, W.; Shoyama, Y.; Tanaka, H.; Morimoto, S. Enzyme-Linked Immunosorbent Assay for the Quantitative/Qualitative Analysis of Plant Secondary Metabolites. J. Nat. Med. 2018, 72, 32–42. [Google Scholar] [CrossRef]
- Aydin, S. A Short History, Principles, and Types of ELISA, and Our Laboratory Experience with Peptide/Protein Analyses Using ELISA. Peptides 2015, 72, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Barati, B.; Ebrahimi, F.; Nazarian, S. Production of Chicken Egg Yolk Antibody (IgY) against Recombinant Cholera Toxin B Subunit and Evaluation of Its Prophylaxis Potency in Mice. Iran. J. Immunol. 2018, 15, 47–58. [Google Scholar] [PubMed]
- Lanzarini, N.M.; Bentes, G.A.; de Volotão, E.M.; Pinto, M.A. Use of Chicken Immunoglobulin Y in General Virology. J. Immunoass. Immunochem. 2018, 39, 235–248. [Google Scholar] [CrossRef]
- Amro, W.A.; Al-Qaisi, W.; Al-Razem, F. Production and Purification of IgY Antibodies from Chicken Egg Yolk. J. Genet. Eng. Biotechnol. 2018, 16, 99–103. [Google Scholar] [CrossRef]
- Carlander, D.; Stalberg, J.; Larsson, A. Chicken Antibodies. Upsala J. Med. Sci. 2010, 104, 179–189. [Google Scholar] [CrossRef]
- Zhang, Q.; He, D.; Xu, L.; Ge, S.; Wang, J.; Zhang, X. Generation and Evaluation of Anti-Mouse IgG IgY as Secondary Antibody. Prep. Biochem. Biotechnol. 2020, 50, 788–793. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, L.; Qin, Z. A Review on the Application of Chicken Immunoglobulin Y in Aquaculture. Rev. Aquac. 2024, 16, 536–551. [Google Scholar] [CrossRef]
- Fernandes, D.C.; Eto, S.F.; Claudiano, G.S.; Marcusso, P.F.; Ramos-Espinoza, F.C.; Moraes, J.R.E. Production and Use of Immunoglobulin Y in the Diagnosis of Haemorrhagic Septicemia Caused by Aeromonas hydrophila in Piaractus mesopotamicus. Aquac. Res. 2022, 53, 2930–2936. [Google Scholar] [CrossRef]
- de Dias, C.A.C.V.; de Góes Filho, J.D.; Oliveira, R.R.C.O.; de Sousa, R.L.; Duncan, W.L.P.; Ono, E.; Mariúba, L.A.; Lameiras, J.L.V.; dos Santos, M.C. Caracterização Parcial Das Imunoglobulinas de Plesiotrygon iwamae (Chondrichtyes—Potamotrygonidae) e de Colossoma macropomum (Osteichtyes Characidae) Isoladas Com Ácido Caprílico Caprílico. Sci. Amaz. 2015, 2, 1–9. [Google Scholar]
- Pereira, E.C.; Botinelly, T.F.; Nogueira, L.F.F.; Kotzent, S.; Pilarski, F.; Figueiredo, H.C.P.; Valladão, G.M.R.; Tavares, G.C.; Gallani, S.U. Identification, Virulence and Genetic Diversity of Flavobacterium oreochromis of Tambaqui (Colossoma macropomum) in Different Brazilian States. In Proceedings of the I Integrative International Congress on Animal and Environmental Health, Nilton Lins University, Manaus, Brazil, 25–28 June 2024; pp. 1–2. [Google Scholar]
- Miles, A.A.; Misra, S.S.; Irwin, J.O. The Estimation of the Bactericidal Power of the Blood. Epidemiol. Infect. 1938, 38, 732–749. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.A.A.; Leal, C.A.G.; Schuenker, N.D.; Leite, R.C.; Figueiredo, H.C.P. Characterization of Weissella ceti Infections in Brazilian Rainbow Trout, Oncorhynchus mykiss (Walbaum), Farms and Development of an Oil-Adjuvanted Vaccine. J. Fish Dis. 2015, 38, 295–302. [Google Scholar] [CrossRef]
- Wangkaghart, E.; Deville, S.; Wang, B.; Srisapoome, P.; Wang, T.; Secombes, C.J. Immune Response and Protective Efficacy of Two New Adjuvants, MontanideTM ISA 763B VG and MontanideTM GEL02, Administered with a Streptococcus agalactiae Ghost Vaccine in Nile Tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2021, 116, 19–29. [Google Scholar] [CrossRef]
- Crowther, J.R. (Ed.) Basic Immunology BT. In ELISA: Theory and Practice; Humana Press: Totowa, NJ, USA, 1995; pp. 1–34. ISBN 978-1-59259-529-7. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; Scientific Research: Wuhan, China, 2024. [Google Scholar]
- Elgueta, R.; De Vries, V.C.; Noelle, R.J. The Immortality of Humoral Immunity. Immunol. Rev. 2010, 236, 139–150. [Google Scholar] [CrossRef]
- El-Adawy, M.M.; Attia, M.M.; Elgendy, M.Y.; Abdelsalam, M.; Fadel, A. Development of Silver Nano-Based Indirect ELISA and Dot-ELISA Methods for Serological Diagnosis of a Bacterial Fish Pathogen Aeromonas veronii. J. Microbiol. Methods 2023, 211, 106782. [Google Scholar] [CrossRef]
- Adams, A.; Thompson, K.D. Biotechnology Offers Revolution to Fish Health Management. Trends Biotechnol. 2006, 24, 201–205. [Google Scholar] [CrossRef]
- Lin, A. V Indirect ELISA BT. In ELISA: Methods and Protocols; Hnasko, R., Ed.; Springer New York: New York, NY, USA, 2015; pp. 51–59. ISBN 978-1-4939-2742-5. [Google Scholar]
- Adams, A.; Thompson, K.D. Development of Diagnostics for Aquaculture: Challenges and Opportunities. Aquac. Res. 2011, 42, 93–102. [Google Scholar] [CrossRef]
- Yang, F.; Xu, L.; Dias, A.C.P.; Zhang, X. A Sensitive Sandwich ELISA Using a Modified Biotin-Streptavidin Amplified System for Histamine Detection in Fish, Prawn and Crab. Food Chem. 2021, 350, 129196. [Google Scholar] [CrossRef]
- Empey Campora, C.; Hokama, Y.; Yabusaki, K.; Isobe, M. Development of an Enzyme-Linked Immunosorbent Assay for the Detection of Ciguatoxin in Fish Tissue Using Chicken Immunoglobulin Y. J. Clin. Lab. Anal. 2008, 22, 239–245. [Google Scholar] [CrossRef]
- Oliveri Conti, G.; Copat, C.; Wang, Z.; D’Agati, P.; Cristaldi, A.; Ferrante, M. Determination of Illegal Antimicrobials in Aquaculture Feed and Fish: An ELISA Study. Food Control 2015, 50, 937–941. [Google Scholar] [CrossRef]
- Gye, H.J.; Nishizawa, T. Reducing Background Optical Density in Enzyme-Linked Immunosorbent Assay for Detecting Nervous Necrosis Virus (NNV)-Specific IgM by Immobilizing Fish Sera. Aquaculture 2018, 485, 93–100. [Google Scholar] [CrossRef]
- Wang, T.; Jin, S.; Lv, R.; Meng, Y.; Li, G.; Han, Y.; Zhang, Q. Development of an Indirect ELISA for Detection of the Adaptive Immune Response of Black Carp (Mylopharyngodon piceus). J. Immunol. Methods 2023, 521, 113550. [Google Scholar] [CrossRef]
- Oliver, L.P.; Bruce, T.J.; Ma, J.; Jones, E.M.; Cain, K.D. Development of a Monoclonal Antibody Specific to Burbot (Lota lota) IgM and Optimization of an ELISA to Measure Anti-Aeromonas sp. Antibody Titers Following Pathogen Challenge. Fish Shellfish Immunol. 2023, 137, 108775. [Google Scholar] [CrossRef]
- Shoemaker, C.A.; Shelby, R.A.; Klesius, P.H. Development of an Indirect ELISA to Detect Humoral Response to Flavobacterium columnare Infection of Channel Catfish, Ictalurus punctatus. J. Appl. Aquac. 2003, 14, 43–52. [Google Scholar] [CrossRef]
- Leal, C.A.G.; Carvalho-Castro, G.A.; Sacchetin, P.S.C.; Lopes, C.O.; Moraes, A.M.; Figueiredo, H.C.P. Oral and Parenteral Vaccines against Flavobacterium columnare: Evaluation of Humoral Immune Response by ELISA and in Vivo Efficiency in Nile Tilapia (Oreochromis niloticus). Aquac. Int. 2010, 18, 657–666. [Google Scholar] [CrossRef]
- Farias, T.H.V.; Silva, K.R.; Mariguela, V.C.; Montassier, H.J.; Pilarski, F. Development of an Indirect ELISA Assay to Evaluation of the Adaptive Immune Response of Pacu (Piaractus mesopotamicus). An. Acad. Bras. Cienc. 2018, 90, 3327–3335. [Google Scholar] [CrossRef]
- Glória, J.C.; Chaves, Y.O.; de Figueiredo, A.M.; de Souza, C.C.; da Silva, E.R.D.; Batista, J.C.L.; Nogueira, P.A.; Mariúba, L.A.M. Standardization of a Cytometric Bead Assay Based on Egg-Yolk Antibodies. JoVE 2023, 195, e65123. [Google Scholar] [CrossRef]
- Karachaliou, C.E.; Vassilakopoulou, V.; Livaniou, E. IgY Technology: Methods for Developing and Evaluating Avian Immunoglobulins for the in Vitro Detection of Biomolecules. World J. Methodol. 2021, 11, 243–262. [Google Scholar] [CrossRef]
- Xu, L.; Xu, Y.; He, L.; Zhang, M.; Wang, L.; Li, Z.; Li, X. Immunomodulatory Effects of Chicken Egg Yolk Antibodies (IgY) against Experimental Shewanella marisflavi AP629 Infections in Sea Cucumbers (Apostichopus japonicus). Fish Shellfish Immunol. 2019, 84, 108–119. [Google Scholar] [CrossRef]
- Sheng, L.; He, Z.; Chen, J.; Liu, Y.; Ma, M.; Cai, Z. The Impact of N-Glycosylation on Conformation and Stability of Immunoglobulin Y from Egg Yolk. Int. J. Biol. Macromol. 2017, 96, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Zhen, Y.; Li, S.; Xu, Y. Chicken Egg Yolk Antibodies (IgY) as Non-Antibiotic Production Enhancers for Use in Swine Production: A Review. J. Anim. Sci. Biotechnol. 2015, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.; Meenatchisundaram, S.; Parameswari, G.; Subbraj, T.; Selvakumaran, R.; Ramalingam, S. Chicken Egg Yolk Antibodies (IgY) as an Alternative to Mammalian Antibodies. Indian J. Sci. Technol. 2010, 3, 468–474. [Google Scholar] [CrossRef]
- Baloch, A.R.; Zhang, X.-Y.; Schade, R. IgY Technology in Aquaculture—A Review. Rev. Aquac. 2015, 7, 153–160. [Google Scholar] [CrossRef]
- Xu, L.; Che, J.; Xu, Y.; Chen, Y.; Li, Y.; Murtaza, B.; Wang, L.; Zhang, M.; Li, X. Oral Administration of Microencapsulated Egg Yolk Immunoglobulin (IgY) in Turbot (Scophthalmus maximus) to Combat against Edwardsiella tarda 2CDM001 Infections. Fish Shellfish Immunol. 2020, 106, 609–620. [Google Scholar] [CrossRef]
- Sun, B.-Y.; Kou, H.-Y.; Jian, P.-Y.; Kong, L.-J.; Fang, J.; Meng, P.-K.; Wu, K.; Yang, C.-G.; Yang, G.; Song, X.-H. Protective Effects of Egg Yolk Immunoglobulins (IgY) against CyHV-2 Infection in Gibel Carp (Carassius Gibelio). Aquaculture 2023, 569, 739371. [Google Scholar] [CrossRef]
- Jin, L.; Li, X.; Zou, D.; Li, S.; Song, W.; Xu, Y. Protection of Crucian Carp (Carassius auratus Gibelio) against Septicaemia Caused by Aeromonas hydrophila Using Specific Egg Yolk Immunoglobulins. Aquac. Res. 2013, 44, 928–936. [Google Scholar] [CrossRef]
- Zhang, M.; Geng, H.; Tariq Javed, M.; Xu, L.; Li, X.; Wang, L.; Li, S.; Xu, Y. Passive Protection of Japanese Pufferfish (Takifugu rubripes) against Vibrio harveyi Infection Using Chicken Egg Yolk Immunoglobulins (IgY). Aquaculture 2021, 532, 736009. [Google Scholar] [CrossRef]
- Tini, M.; Jewell, U.R.; Camenisch, G.; Chilov, D.; Gassmann, M. Generation and Application of Chicken Egg-Yolk Antibodies. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2002, 131, 569–574. [Google Scholar] [CrossRef]
- Fernandes, D.C.; Eto, S.F.; Funnicelli, M.I.G.; Fernandes, C.C.; Charlie-Silva, I.; Belo, M.A.A.; Pizauro, J.M. Immunoglobulin Y in the Diagnosis of Aeromonas hydrophila Infection in Nile Tilapia (Oreochromis niloticus). Aquaculture 2019, 500, 576–585. [Google Scholar] [CrossRef]
- Eto, S.F.; Fernandes, D.C.; Moraes, A.C.; Prado, E.J.R.; Baldassi, A.C.; Manrique, W.G.; Silva, I.C.; Medeiros, A.S.R.; Belo, M.A.A.; Balbuena, T.S.; et al. Validation of IgY for the Diagnosis of Streptococcus agalactiae-Caused Endocarditis and Bacterial Meningitis in Nile Tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2018, 76, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Kieliszewski, M.; Lamport, D.T.A. Cross-Reactivities of Polyclonal Antibodies against Extensin Precursors Determined via Elisa Techniques. Phytochemistry 1986, 25, 673–677. [Google Scholar] [CrossRef]
- Martins, N.N.N.; Azevedo, B.A.M.; Miranda, S.M.D.; Diniz, D.C.; Calixto, M.L.S.; Fagundes, E.M.; Vitelli-Avelar, D.M. Experiência Da Utilização Do Anticorpo Monoclonal Jovi-1 Em Laboratório de Citometria de Fluxo. Hematol. Transfus. Cell Ther. 2022, 44, S570–S571. [Google Scholar] [CrossRef]
- Suomalainen, L.-R.; Tiirola, M.A.; Valtonen, E.T. Influence of Rearing Conditions on Flavobacterium columnare Infection of Rainbow Trout, Oncorhynchus mykiss (Walbaum). J. Fish Dis. 2005, 28, 271–277. [Google Scholar] [CrossRef]
- Pilarski, F.; Ishikawa, M.M.; de Pádua, S.B.; Sakabe, R. Columnariose: Etiologia, Sinais Clínicos e Envio de Amostras Para Análise Laboratorial; Embrapa Agropecuária Oeste: Dourados, Brazil, 2011. [Google Scholar]
- Strømsheim, A.; Eide, D.M.; Fjalestad, K.T.; Larsen, H.J.S.; Røed, K.H. Genetic Variation in the Humoral Immune Response in Atlantic Salmon (Salmo salar) against Aeromonas salmonicida A-Layer. Vet. Immunol. Immunopathol. 1994, 41, 341–352. [Google Scholar] [CrossRef]
- Thuvander, A.N.N.; Hongslo, T.; Jansson, E.V.A.; Sundquist, B.O. Duration of Protective Immunity and Antibody Titres Measured by ELISA after Vaccination of Rainbow Trout, Salmo gairdneri Richardson, against Vibriosis. J. Fish Dis. 1987, 10, 479–486. [Google Scholar] [CrossRef]
- Queiróz, G.A.; Silva, T.M.; Leal, C.A. Duration of Protection and Humoral Immune Response in Nile Tilapia (Oreochromis niloticus L.) Vaccinated against Streptococcus agalactiae. Animals 2024, 14, 2440. [Google Scholar] [CrossRef]
- de Soveral, L.F.; de Almeida, P.A.; Kreutz, Y.; Ribeiro, V.A.; Frandoloso, R.; Kreutz, L.C. Modulation of Expression of Proinflammatory Genes and Humoral Immune Response Following Immunization or Infection with Aeromonas hydrophila in Silver Catfish (Rhamdia quelen). Fish Shellfish Immunol. Rep. 2022, 3, 100053. [Google Scholar] [CrossRef]
- Tattiyapong, P.; Dechavichitlead, W.; Waltzek, T.B.; Surachetpong, W. Tilapia Develop Protective Immunity Including a Humoral Response Following Exposure to Tilapia Lake Virus. Fish Shellfish Immunol. 2020, 106, 666–674. [Google Scholar] [CrossRef]
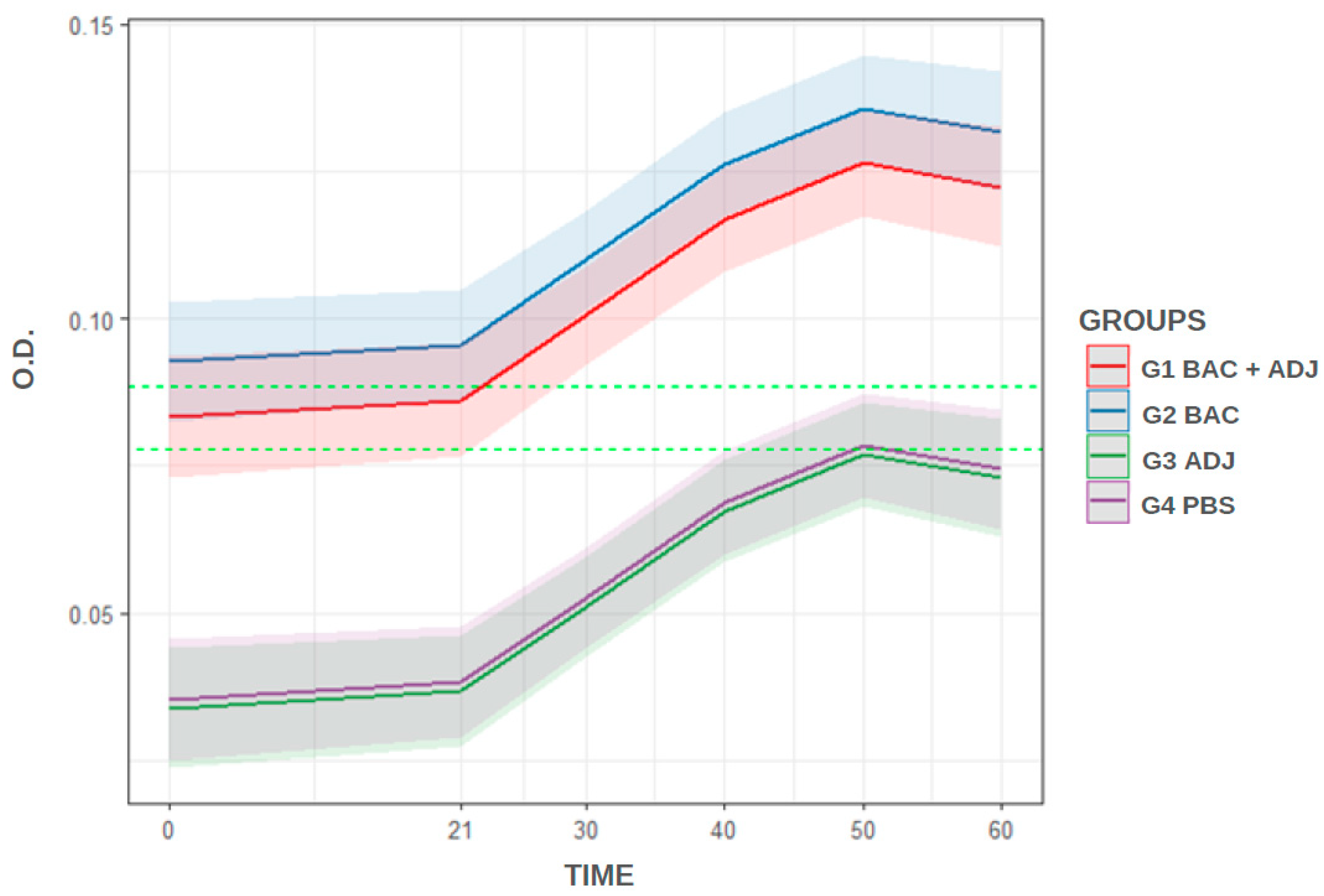
| Predictors | Estimates | Confidence Interval | p-Value |
|---|---|---|---|
| G1—Bacterin + Adjuvant | Ref. | Ref. | a |
| G2—Bacterin | 0.01 | −0.00–0.02 | 0.091 a |
| G3—Adjuvant | −0.05 | −0.06–−0.04 | <0.001 b |
| G4—Control | −0.05 | −0.06–−0.04 | <0.001 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corado, M.V.L.; Pereira, E.C.; Botinelly, T.F.; Glória, J.C.; Sousa, R.L.d.; Corado, A.d.L.G.; Balieiro, A.A.d.S.; Mariúba, L.A.M.; Gallani, S.U.; Tavares, G.C. Indirect Enzyme-Linked Immunosorbent Assay Based on Immunoglobulin Y Antibodies for the Evaluation of Humoral Immunity Against Flavobacterium oreochromis in Colossoma macropomum: A Preliminary Study. Animals 2025, 15, 869. https://doi.org/10.3390/ani15060869
Corado MVL, Pereira EC, Botinelly TF, Glória JC, Sousa RLd, Corado AdLG, Balieiro AAdS, Mariúba LAM, Gallani SU, Tavares GC. Indirect Enzyme-Linked Immunosorbent Assay Based on Immunoglobulin Y Antibodies for the Evaluation of Humoral Immunity Against Flavobacterium oreochromis in Colossoma macropomum: A Preliminary Study. Animals. 2025; 15(6):869. https://doi.org/10.3390/ani15060869
Chicago/Turabian StyleCorado, Maria Vitória Lobo, Elcimara Cardoso Pereira, Taísa Freitas Botinelly, Juliane Corrêa Glória, Rafael Luckwu de Sousa, André de Lima Guerra Corado, Antônio Alcirley da Silva Balieiro, Luís André Morais Mariúba, Silvia Umeda Gallani, and Guilherme Campos Tavares. 2025. "Indirect Enzyme-Linked Immunosorbent Assay Based on Immunoglobulin Y Antibodies for the Evaluation of Humoral Immunity Against Flavobacterium oreochromis in Colossoma macropomum: A Preliminary Study" Animals 15, no. 6: 869. https://doi.org/10.3390/ani15060869
APA StyleCorado, M. V. L., Pereira, E. C., Botinelly, T. F., Glória, J. C., Sousa, R. L. d., Corado, A. d. L. G., Balieiro, A. A. d. S., Mariúba, L. A. M., Gallani, S. U., & Tavares, G. C. (2025). Indirect Enzyme-Linked Immunosorbent Assay Based on Immunoglobulin Y Antibodies for the Evaluation of Humoral Immunity Against Flavobacterium oreochromis in Colossoma macropomum: A Preliminary Study. Animals, 15(6), 869. https://doi.org/10.3390/ani15060869





