Growth Performance and Gut Health of Cold-Stressed Broilers in Response to Supplementation with a Combination of Sodium Butyrate and Vitamin D3
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds, Diet, and Experimental Design
2.2. Growth Performance
2.3. Sample Collection
2.4. Histological Analyses
2.5. Antioxidant-Related Biochemical Kit Assay
2.6. Enzyme-Linked Immunosorbent Assay
2.7. Real-Time Quantitative PCR Analysis
2.8. 16S rRNA Gene Sequencing
2.9. Statistical Analysis
3. Results
3.1. Growth Performance and Organ Index
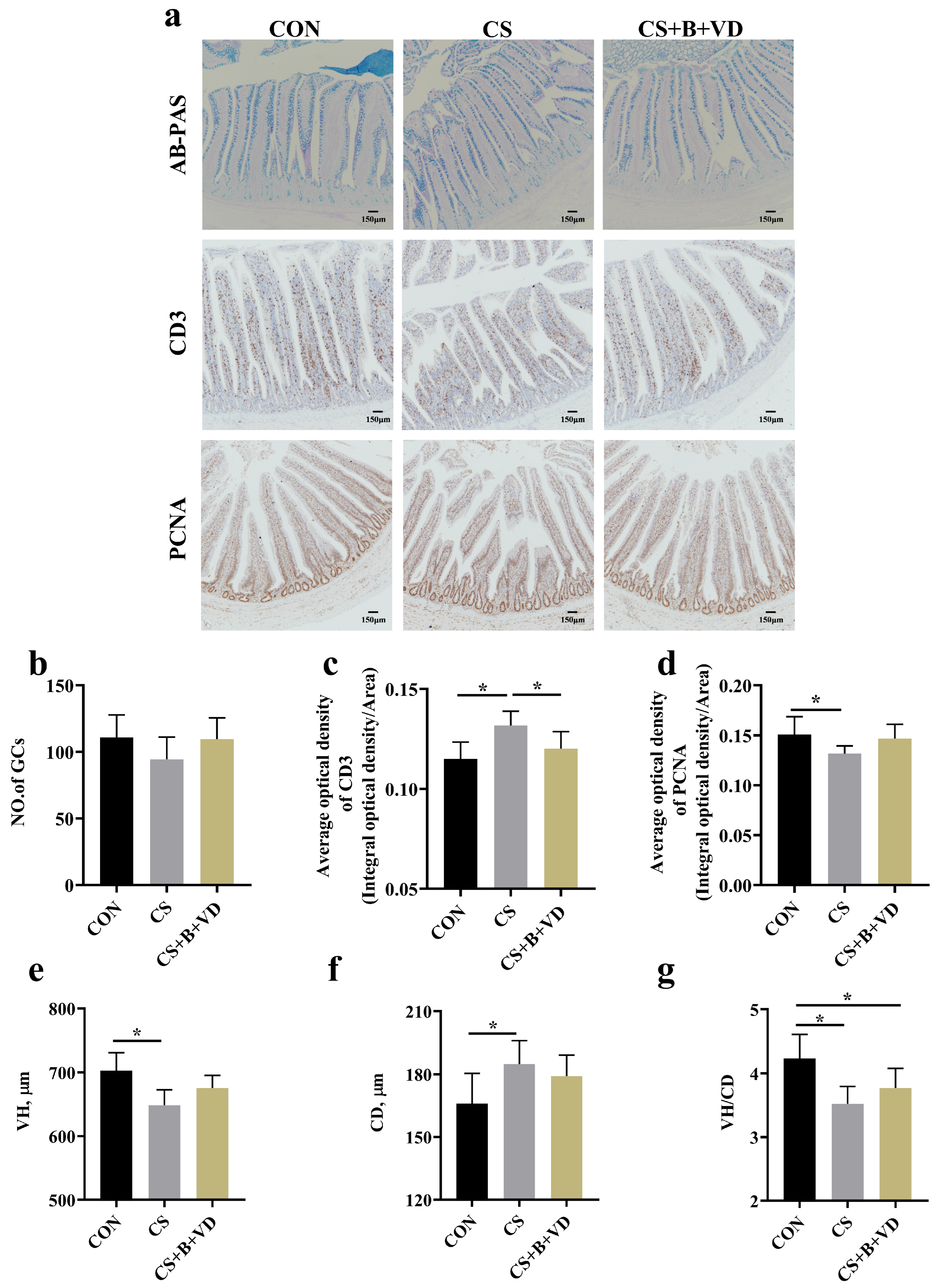
3.2. Intestinal Morphology and Immuno-Histochemical Analyses
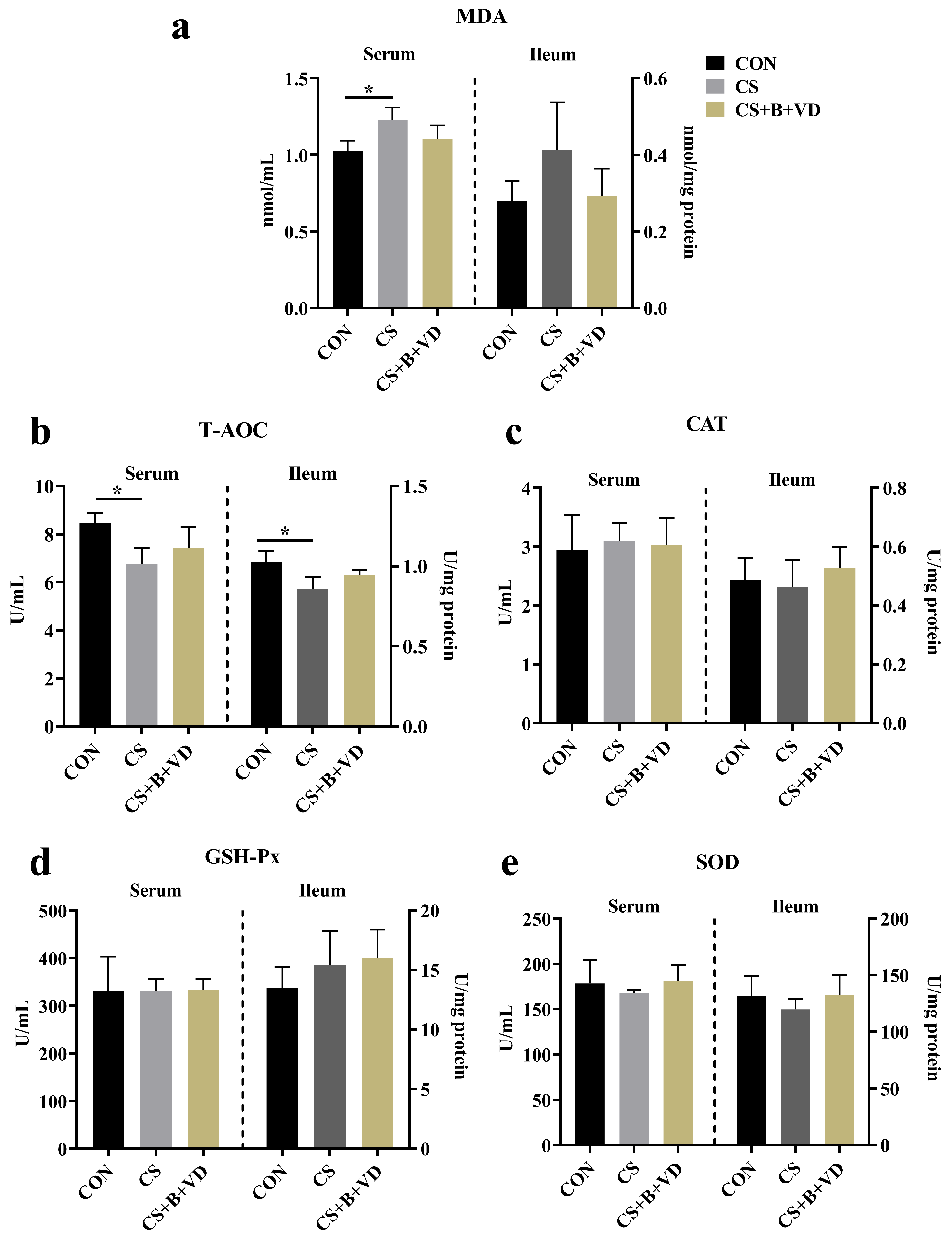
3.3. Antioxidant Parameters
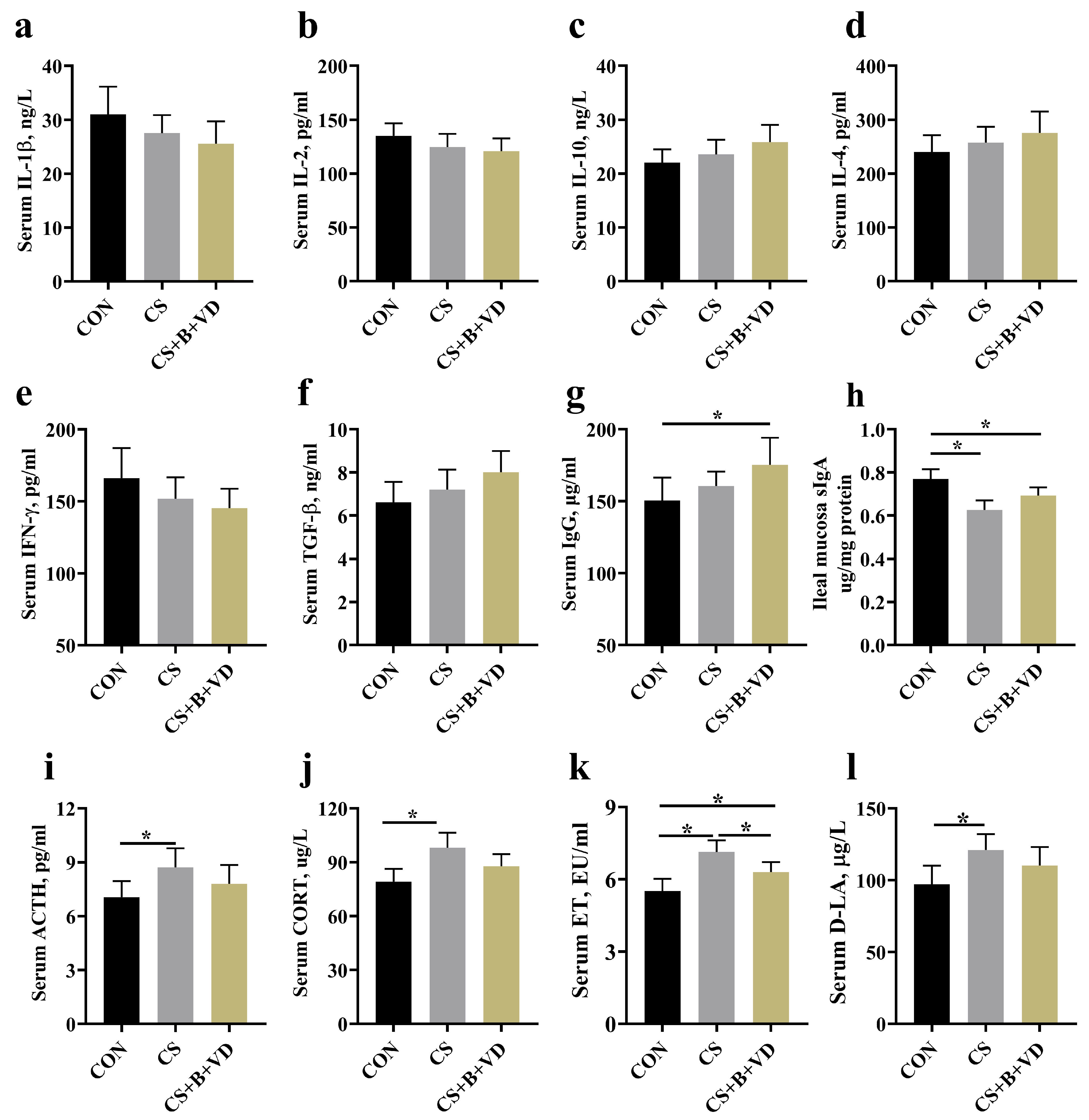
3.4. Immunity, Stress-Related Hormones, and Gut Barrier Biomarkers
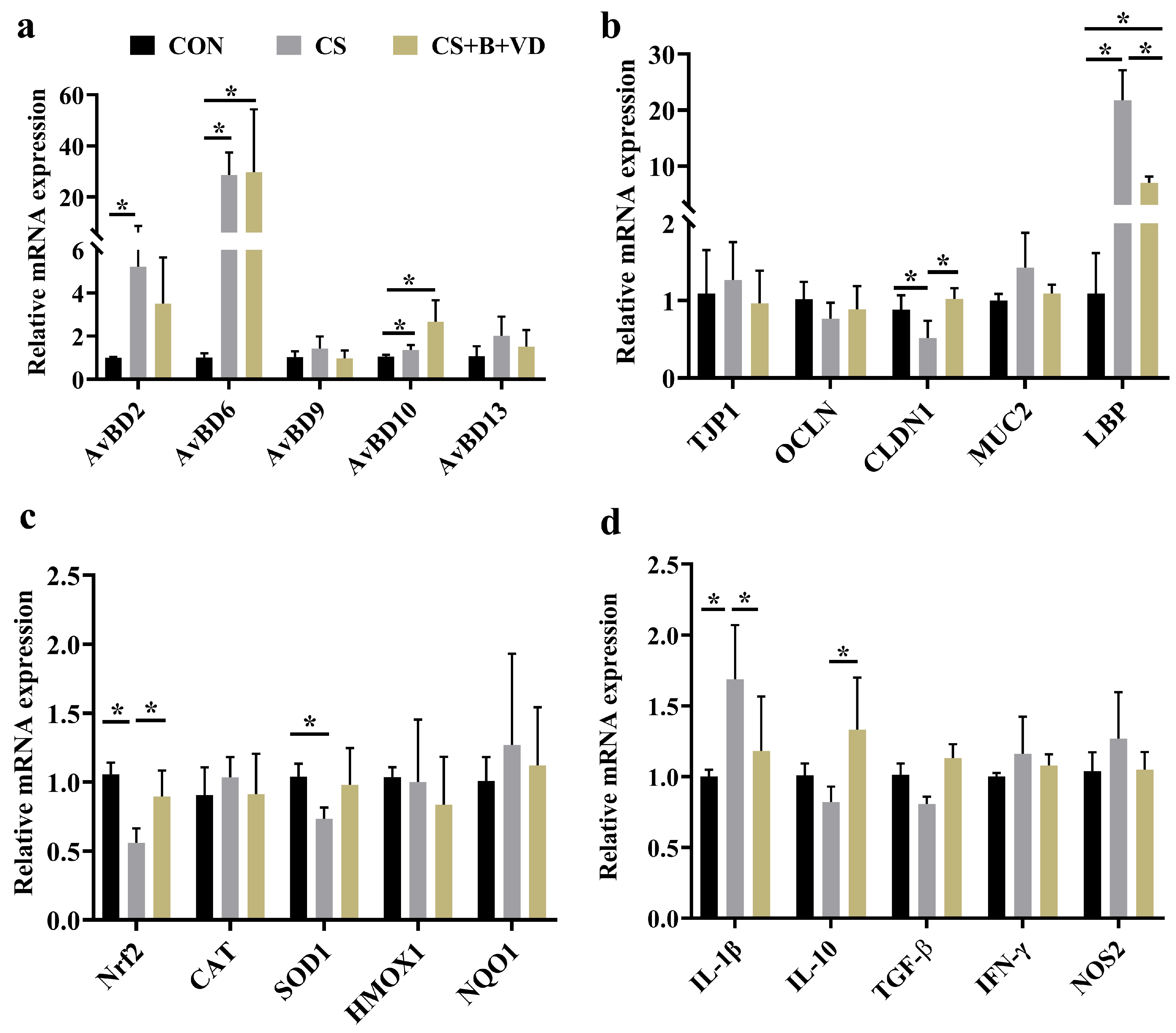
3.5. Ileal Gene Expression
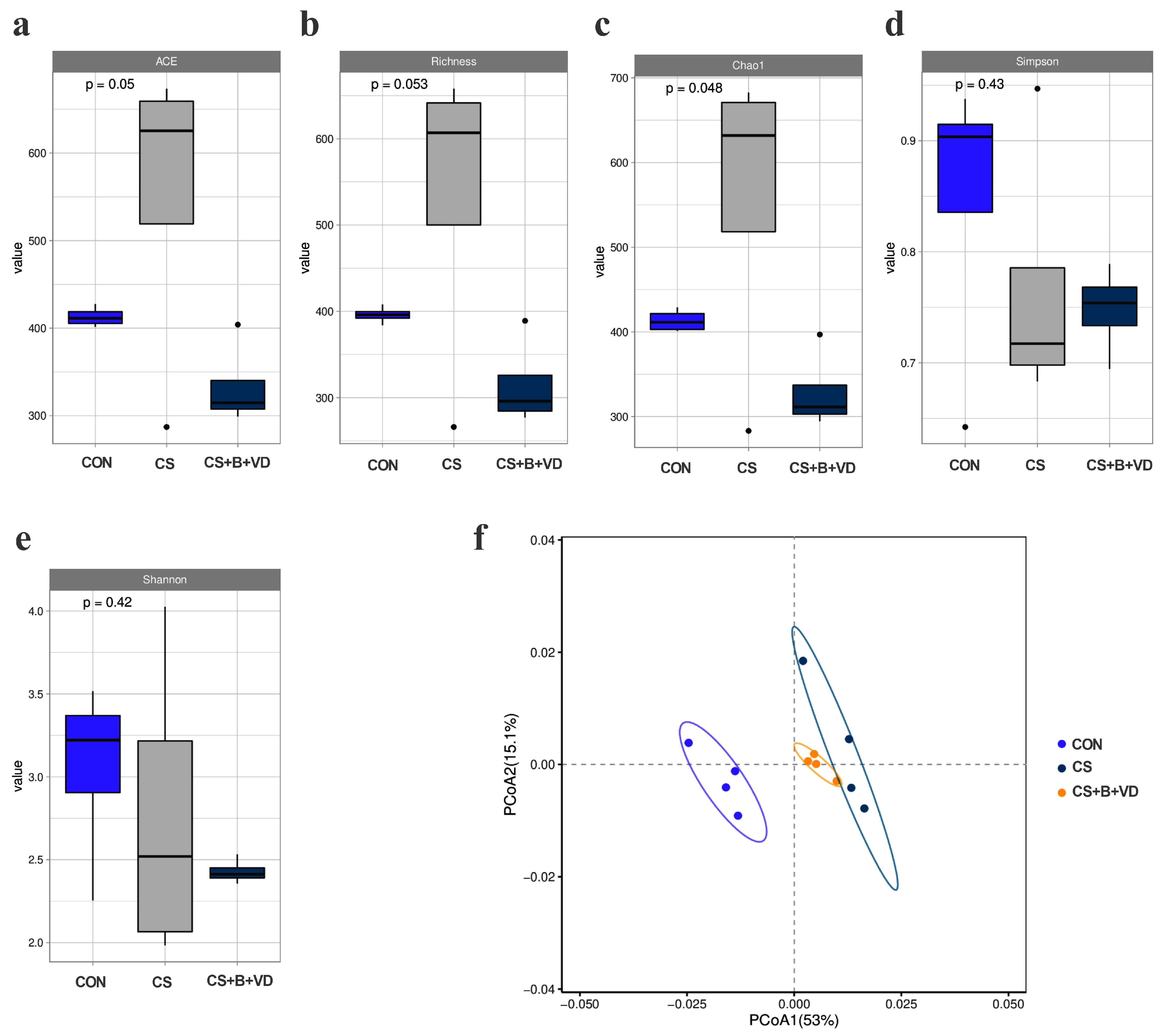
3.6. Ileal Microbial Profile
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leishman, E.M.; Ellis, J.; van Staaveren, N.; Barbut, S.; Vanderhout, R.J.; Osborne, V.R.; Wood, B.J.; Harlander-Matauschek, A.; Baes, C.F. Meta-analysis to predict the effects of temperature stress on meat quality of poultry. Poult. Sci. 2021, 100, 101471. [Google Scholar] [CrossRef]
- Moreira, L.M.; Sousa, L.S.; Guamán, C.A.G.; Vieira, M.C.; Santini, M.B.; Cardoso, A.R.; Leme, F.d.O.P.; Lara, L.J.C.; Araújo, I.C.S. Effects of cold stress on physiologic metabolism in the initial phase and performance of broiler rearing. J. Therm. Biol. 2023, 119, 103773. [Google Scholar] [CrossRef]
- Zhou, H.; Kong, L.; Zhu, L.; Hu, X.; Busye, J.; Song, Z. Effects of cold stress on growth performance, serum biochemistry, intestinal barrier molecules, and adenosine monophosphate-activated protein kinase in broilers. Animal 2021, 15, 100138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.W.; Lv, Z.H.; Li, J.L.; Li, S.; Xu, S.W.; Wang, X.L. Effects of cold stress on nitric oxide in duodenum of chicks. Poult. Sci. 2011, 90, 1555–1561. [Google Scholar] [CrossRef]
- Huff, G.R.; Huff, W.E.; Rath, N.C.; Solis de Los Santos, F.; Farnell, M.B.; Donoghue, A.M. Influence of Hen Age on the Response of Turkey Poults to Cold Stress, Escherichia coli Challenge, and Treatment with a Yeast Extract Antibiotic Alternative. Poult. Sci. 2007, 86, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Tsiouris, V.; Georgopoulou, I.; Batzios, C.; Pappaioannou, N.; Ducatelle, R.; Fortomaris, P. The effect of cold stress on the pathogenesis of necrotic enteritis in broiler chicks. Avian Pathol. 2015, 44, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Huff, G.R.; Huff, W.E.; Rath, N.C.; El-Gohary, F.A.; Zhou, Z.Y.; Shini, S. Efficacy of a novel prebiotic and a commercial probiotic in reducing mortality and production losses due to cold stress and Escherichia coli challenge of broiler chicks. Poult. Sci. 2015, 94, 918–926. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Yao, R.; Hu, Y.; Liu, P.; Lian, S.; Lv, H.; Xu, B.; Li, S. Dietary supplementary glutamine and L-carnitine enhanced the anti-cold stress of Arbor Acres broilers. Arch. Anim. Breed. 2021, 64, 231–243. [Google Scholar] [CrossRef]
- Perry, F.; Perry, F.; Lahaye, L.; Lahaye, L.; Santin, E.; Santin, E.; Johnson, C.; Johnson, C.; Korver, D.; Korver, D.; et al. Protected biofactors and antioxidants reduce the negative consequences of virus and cold challenge while enhancing performance by modulating immunometabolism through cytoskeletal and immune signaling in the jejunum. Poult. Sci. 2022, 101, 102172. [Google Scholar] [CrossRef]
- Arsenault, R.J.; Kogut, M.H. Immunometabolism and the Kinome Peptide Array: A New Perspective and Tool for the Study of Gut Health. Front. Vet. Sci. 2015, 2, 44. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, C.; Jiang, Q.; Yin, Y. Butyrate in Energy Metabolism: There Is Still More to Learn. Trends Endocrinol. Metab. 2021, 32, 159–169. [Google Scholar] [CrossRef] [PubMed]
- El-Saadony, M.T.; Umar, M.; Hassan, F.-U.; Alagawany, M.; Arif, M.; Taha, A.E.; Elnesr, S.S.; El-Tarabily, K.A.; El-Hack, M.E.A. Applications of butyric acid in poultry production: The dynamics of gut health, performance, nutrient utilization, egg quality, and osteoporosis. Anim. Health Res. Rev. 2022, 23, 136–146. [Google Scholar] [CrossRef]
- Abdellatif, I.A.; El-Gendy, S.A.A.; Abumandour, M.M.; Noreldin, A.; Bassuoni, N.F. Analyzing the morphology and avian β-defensins genes (AvβD) expression in the small intestine of Cobb500 broiler chicks fed with sodium butyrate. BMC Veter Res. 2024, 20, 434. [Google Scholar] [CrossRef]
- Wan, F.; Deng, F.; Chen, L.; Zhong, R.; Wang, M.; Yi, B.; Liu, L.; Zhao, H.; Zhang, H. Long-term chemically protected sodium butyrate supplementation in broilers as an antibiotic alternative to dynamically modulate gut microbiota. Poult. Sci. 2022, 101, 102221. [Google Scholar] [CrossRef]
- Xiao, X.; Cui, T.; Qin, S.; Wang, T.; Liu, J.; Sa, L.; Wu, Y.; Zhong, Y.; Yang, C. Beneficial effects of Lactobacillus plantarum on growth performance, immune status, antioxidant function and intestinal microbiota in broilers. Poult. Sci. 2024, 103, 104280. [Google Scholar] [CrossRef] [PubMed]
- Seidavi, A.; Tavakoli, M.; Asroosh, F.; Scanes, C.G.; El-Hack, M.E.A.; Naiel, M.A.E.; Taha, A.E.; Aleya, L.; El-Tarabily, K.A.; Swelum, A.A. Antioxidant and antimicrobial activities of phytonutrients as antibiotic substitutes in poultry feed. Environ. Sci. Pollut. Res. 2021, 29, 5006–5031. [Google Scholar] [CrossRef]
- Xiao, C.; Zhang, L.; Zhang, B.; Kong, L.; Pan, X.; Goossens, T.; Song, Z. Dietary sodium butyrate improves female broiler breeder performance and offspring immune function by enhancing maternal intestinal barrier and microbiota. Poult. Sci. 2023, 102, 102658. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Sun, Y.; Dai, Z.; Liu, J.; Xiao, S.; Liu, Y.; Wang, X.; Yang, S.; Zhang, R.; Yang, C.; et al. Microencapsulated Sodium Butyrate Alleviates Immune Injury and Intestinal Problems Caused by Clostridium Perfringens through Gut Microbiota. Animals 2023, 13, 3784. [Google Scholar] [CrossRef]
- Wang, B.; Liu, J.; Lei, R.; Xue, B.; Li, Y.; Tian, X.; Zhang, K.; Luo, B. Cold exposure, gut microbiota, and hypertension: A mechanistic study. Sci. Total. Environ. 2022, 833, 155199. [Google Scholar] [CrossRef]
- Cho, T.-Z.A.; Sadiq, M.B.; Srichana, P.; Anal, A.K. Vitamin D3 enhanced intestinal phosphate cotransporter genes in young and growing broilers. Poult. Sci. 2020, 99, 2041–2047. [Google Scholar] [CrossRef]
- Zhang, W.; Geng, Y.; Yang, K.; Hu, Y.; Xue, M.; Cui, X.; Zhang, L.; Wang, S.; Li, T.; Luo, X.; et al. 1,25-dihydroxyvitamin D3 enhances the expression of phosphorus transporters via vitamin D receptor in ligated duodenal loops of Arbor Acres male broilers. Poult. Sci. 2024, 103, 104503. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, L.; Peng, Y.; Luo, J.; Chen, T.; Xi, Q.; Zhang, Y.; Sun, J. The effects of optimal dietary vitamin d(3) on growth and carcass performance, tibia traits, meat quality, and intestinal morphology of chinese yellow-feathered broiler chickens. Animals 2024, 14, 920. [Google Scholar] [CrossRef]
- Sharma, M.K.; Lee, J.; Shi, H.; Ko, H.; Goo, D.; Paneru, D.; Holladay, S.D.; Gogal, R.M., Jr.; Kim, W.K. Effect of dietary inclusion of 25-hydroxyvitamin d₃ and vitamin e on performance, gut health, oxidative status, and immune response in laying hens infected with coccidiosis. Poult. Sci. 2024, 103, 104033. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, L.; Li, S.; Zhang, G.; Ouyang, L.; Robinson, K.; Tang, Y.; Zhu, Q.; Li, D.; Hu, Y.; et al. 1,25-Dihydroxyvitamin-D3 Induces Avian β-Defensin Gene Expression in Chickens. PLoS ONE 2016, 11, e0154546. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, Y.; Liu, K.; Fan, R.; Li, Q.; Zhou, Z. Dietary sodium butyrate and/or vitamin D3 supplementation alters growth performance, meat quality, chemical composition, and oxidative stability in broilers. Food Chem. 2022, 390, 133138. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhao, X.; Guo, Y.; Li, Z.; Zhou, Z. Coated sodium butyrate and vitamin d(3) supplementation improve gut health through influencing intestinal immunity, barrier, and microflora in early-stage broilers. J. Sci. Food Agric. 2024, 104, 4058–4069. [Google Scholar] [CrossRef]
- National Research Council (NRC). Nutrient Requirements of Poultry; National Academy Press: Washington, DC, USA, 1994. [Google Scholar]
- Gong, R.; Xing, L.; Yin, J.; Ding, Y.; Liu, X.; Bao, J.; Li, J. Appropriate cold stimulation changes energy distribution to improve stress resistance in broilers. J. Anim. Sci. 2023, 101, skad185. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, S.; Zhao, N.; Xing, L.; Li, T.; Liu, X.; Bao, J.; Li, J. Effect of mild intermittent cold stimulation on thymus immune function in broilers. Poult. Sci. 2022, 101, 102073. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ni, Y.; Guo, F.; Fu, W.; Grossmann, R.; Zhao, R. Effect of corticosterone on growth and welfare of broiler chickens showing long or short tonic immobility. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 164, 537–543. [Google Scholar] [CrossRef]
- Wu, J.; Li, G.; Guo, H.; Huang, B.; Li, G.; Dai, S. Acute cold stress induces intestinal injury via CIRP–TLR4–IRE1 signaling pathway in pre-starter broilers. Mol. Biol. Rep. 2023, 50, 6299–6304. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The Microbiome and Butyrate Regulate Energy Metabolism and Autophagy in the Mammalian Colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Dixit, P.; Jain, D.K.; Rajpoot, J.S. Differential effect of oxidative stress on intestinal apparent permeability of drugs transported by paracellular and transcellular route. Eur. J. Drug Metab. Pharmacokinet. 2012, 37, 203–209. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Li, R.; Geng, Z.Y. Cold stress initiates the nrf2/ugt1a1/l-fabp signaling pathway in chickens. Poult. Sci. 2015, 94, 2597–2603. [Google Scholar] [CrossRef]
- Wang, H.-B.; Wang, P.-Y.; Wang, X.; Wan, Y.-L.; Liu, Y.-C. Butyrate Enhances Intestinal Epithelial Barrier Function via Up-Regulation of Tight Junction Protein Claudin-1 Transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef]
- Stephens, M.; von der Weid, P.Y. Lipopolysaccharides modulate intestinal epithelial permeability and inflammation in a species-specific manner. Gut Microbes 2020, 11, 421–432. [Google Scholar] [CrossRef]
- Trickett, A.; Kwan, Y.L. T cell stimulation and expansion using anti-cd3/cd28 beads. J. Immunol. Methods 2003, 275, 251–255. [Google Scholar] [CrossRef]
- Shaw, D.M.; Merien, F.; Braakhuis, A.; Dulson, D. T-cells and their cytokine production: The anti-inflammatory and immunosuppressive effects of strenuous exercise. Cytokine 2018, 104, 136–142. [Google Scholar] [CrossRef]
- Zou, X.; Ji, J.; Qu, H.; Wang, J.; Shu, D.; Wang, Y.; Liu, T.; Li, Y.; Luo, C. Effects of sodium butyrate on intestinal health and gut microbiota composition during intestinal inflammation progression in broilers. Poult. Sci. 2019, 98, 4449–4456. [Google Scholar] [CrossRef]
- Fatemi, S.A.; Elliott, K.E.C.; Macklin, K.S.; Bello, A.; Peebles, E.D. Effects of the In Ovo Injection of Vitamin D3 and 25-Hydroxyvitamin D3 in Ross 708 Broilers Subsequently Challenged with Coccidiosis: II Immunological and Inflammatory Responses and Small Intestine Histomorphology. Animals 2022, 12, 1027. [Google Scholar] [CrossRef] [PubMed]
- Isobe, J.; Maeda, S.; Obata, Y.; Iizuka, K.; Nakamura, Y.; Fujimura, Y.; Kimizuka, T.; Hattori, K.; Kim, Y.-G.; Morita, T.; et al. Commensal-bacteria-derived butyrate promotes the T-cell-independent IgA response in the colon. Int. Immunol. 2019, 32, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, X.; Zou, Y.; He, Y.; Liang, C.; Li, J.; Li, P.; Zhang, J. Cold stress induces colitis-like phenotypes in mice by altering gut microbiota and metabolites. Front. Microbiol. 2023, 14, 1134246. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nan, X.; Zhao, Y.; Jiang, L.; Wang, H.; Zhang, F.; Hua, D.; Liu, J.; Yao, J.; Yang, L.; et al. Dietary Supplementation of Inulin Ameliorates Subclinical Mastitis via Regulation of Rumen Microbial Community and Metabolites in Dairy Cows. Microbiol. Spectr. 2021, 9, e0010521. [Google Scholar] [CrossRef]
- Glendinning, L.; Watson, K.A.; Watson, M. Development of the duodenal, ileal, jejunal and caecal microbiota in chickens. Anim. Microbiome 2019, 1, 17. [Google Scholar] [CrossRef]
- Yang, W.-Y.; Lee, Y.; Lu, H.; Chou, C.-H.; Wang, C. Analysis of gut microbiota and the effect of lauric acid against necrotic enteritis in Clostridium perfringens and Eimeria side-by-side challenge model. PLoS ONE 2019, 14, e0205784. [Google Scholar] [CrossRef]
- Bai, X.; Shi, Y.; Tang, L.; Chen, L.; Fan, H.; Wang, H.; Wang, J.; Jia, X.; Chen, S.; Lai, S. Heat Stress Affects Faecal Microbial and Metabolic Alterations of Rabbits. Front. Microbiol. 2022, 12, 817615. [Google Scholar] [CrossRef]
- Niu, J.; Liu, X.; Xu, J.; Li, F.; Wang, J.; Zhang, X.; Yang, X.; Wang, L.; Ma, S.; Li, D.; et al. Effects of Silage Diet on Meat Quality through Shaping Gut Microbiota in Finishing Pigs. Microbiol. Spectr. 2023, 11, e0241622. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, Y.; Yang, X.; Lv, Z.; Li, P.; Zhang, M.; Wei, F.; Jin, X.; Hu, Y.; Guo, Y.; et al. Mining chicken ileal microbiota for immunomodulatory microorganisms. ISME J. 2023, 17, 758–774. [Google Scholar] [CrossRef]

| Items 1 | CON | CS | CS+B+VD | SEM 2 | p-Value |
|---|---|---|---|---|---|
| BW (g) | |||||
| 18 d | 516.00 | 525.06 | 526.77 | 2.52 | 0.101 |
| 21 d | 650.17 a | 625.03 b | 645.90 a | 4.39 | 0.032 |
| ADG (g/d) | |||||
| 1–18 d | 26.27 | 26.77 | 26.87 | 0.14 | 0.101 |
| 18–21 d | 44.45 a | 33.06 b | 39.45 ab | 1.67 | 0.010 |
| 1–21d | 28.87 a | 27.67 b | 28.66 a | 0.21 | 0.032 |
| ADFI (g/d) | |||||
| 1–18 d | 30.93 | 31.33 | 30.29 | 0.25 | 0.111 |
| 18–21 d | 68.88 | 65.00 | 68.42 | 0.91 | 0.165 |
| 1–21d | 36.36 | 36.14 | 35.74 | 0.30 | 0.714 |
| FCR (g/g) | |||||
| 1–18 d | 1.18 | 1.17 | 1.13 | 0.01 | 0.064 |
| 18–21 d | 1.58 b | 1.99 a | 1.75 ab | 0.07 | 0.044 |
| 1–21d | 1.26 | 1.31 | 1.25 | 0.01 | 0.121 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Wang, Y.; Zhao, X.; Yu, Y.; Guo, Y.; Li, Z.; Zhou, Z. Growth Performance and Gut Health of Cold-Stressed Broilers in Response to Supplementation with a Combination of Sodium Butyrate and Vitamin D3. Animals 2025, 15, 861. https://doi.org/10.3390/ani15060861
Gao H, Wang Y, Zhao X, Yu Y, Guo Y, Li Z, Zhou Z. Growth Performance and Gut Health of Cold-Stressed Broilers in Response to Supplementation with a Combination of Sodium Butyrate and Vitamin D3. Animals. 2025; 15(6):861. https://doi.org/10.3390/ani15060861
Chicago/Turabian StyleGao, Hang, Yi Wang, Xingkai Zhao, Yaling Yu, Yizhe Guo, Zhendong Li, and Zhenlei Zhou. 2025. "Growth Performance and Gut Health of Cold-Stressed Broilers in Response to Supplementation with a Combination of Sodium Butyrate and Vitamin D3" Animals 15, no. 6: 861. https://doi.org/10.3390/ani15060861
APA StyleGao, H., Wang, Y., Zhao, X., Yu, Y., Guo, Y., Li, Z., & Zhou, Z. (2025). Growth Performance and Gut Health of Cold-Stressed Broilers in Response to Supplementation with a Combination of Sodium Butyrate and Vitamin D3. Animals, 15(6), 861. https://doi.org/10.3390/ani15060861






