In Vitro Evaluation of Three Pisum sativum L. Varieties to Partially Replace Soybean and Corn Meal in Dairy Cow Diet
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Chemical Analysis
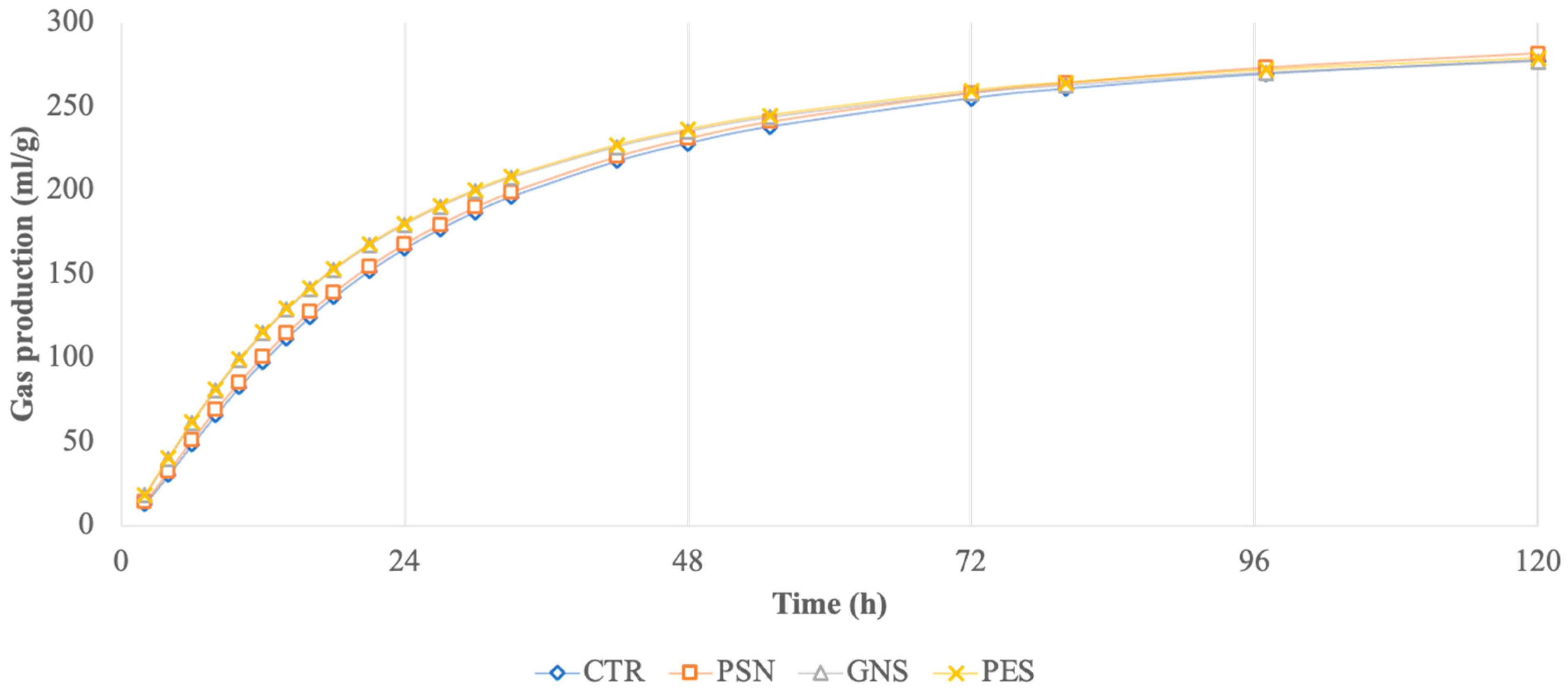
2.3. In Vitro Gas Production Technique
2.4. In Vitro End Products Fermentation
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ace | Acetate; |
| AOAC | Association of Official Analytical Chemists; |
| BCFA | Branched-chain fatty acids; |
| But | Butyrate; |
| CP | Crude protein; |
| CPD | Crude protein degradability; |
| CTR | Control diet; |
| DM | Dry matter; |
| EE | Ether extract; |
| GNS | Ganster; |
| Iso-But | Iso-butyrate; |
| Iso-Val | Iso-valerate; |
| MSE | Mean square error; |
| NDF | Neutral detergent fiber; |
| NS | Not significant; |
| NSCs | Non-structural carbohydrates; |
| OM | Organic matter; |
| OMCV | Cumulative volume of gas related to incubated organic matter; |
| OMD | Organic matter disappearance; |
| PES | Peps; |
| PNS | Poseidon; |
| Prop | Propionate; |
| RDS | Rumen-degradable starch; |
| Rmax | Maximum fermentation rate; |
| SBM | Soybean meal; |
| Tmax | Time to reach maximum fermentation rate; |
| UFL | Milk forage unit; |
| Val | Valerate; |
| VFA | Volatile fatty acid. |
References
- Reveglia, P.; Blanco, M.; Cobos, M.J.; Labuschagne, M.; Joy, M.; Rubiales, D. Metabolic profiling of pea (Pisum sativum) cultivars in changing environments: Implications for nutritional quality in animal feed. Food Chem. 2025, 462, 140972. [Google Scholar] [CrossRef] [PubMed]
- Vastolo, A.; Matera, R.; Passarelli, G.; Calabrò, S.; Santinello, M.; Neglia, G.; Cutrignelli, M.I. Pisum sativum L. as an alternative protein and starch source in Italian Mediterranean buffaloes’ feeding plan: In vitro evaluation of different varieties. Ital. Anim. Sci. 2025, 24, 631–641. [Google Scholar] [CrossRef]
- Buza, M.H.; Holden, L.A.; White, R.A.; Ishler, V.A. Evaluating the effect of ration composition on income over feed cost and milk yield. J. Dairy Sci. 2014, 97, 3073–3080. [Google Scholar] [CrossRef] [PubMed]
- Joy, M.; Rufino-Moya, P.; Lobón, S.; Blanco, M. The effect of the inclusion of pea in lamb fattening concentrate on in vitro and in situ rumen fermentation. J. Sci. Food Agric. 2021, 101, 3041–3048. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.; Kuhnitzsch, C.; Michel, S.; Thierbach, A.; Bochnia, M.; Greef, J.M.; Martens, S.D.; Steinhöfel, O.; Zeyner, A. Effect of toasting grain silages from field peas (Pisum sativum) and field beans (Vicia faba) on in vitro gas production methane production and post-ruminal crude protein content. Anim. Nutr. 2020, 6, 342–352. [Google Scholar] [CrossRef]
- Pulido, R.G.; Beltran, I.E.; Aleixo, J.A.; Morales, Á.G.; Gutierrez, M.; Ponce, M.; Melendez, P. Effect of Replacing Corn Grain and Soybean Meal with Field Peas at Different Levels on Feed Intake, Milk Production, and Metabolism in Dairy Cows under a Restrictive Grazing. Animals 2024, 14, 2830. [Google Scholar] [CrossRef]
- Sauvant, D.; Nozière, P. INRA Feeding System for Ruminants 2018; Wageningen Academic Publishers: Wageningen, The Netherlands, 2018. [Google Scholar]
- AOAC. Official Methods of Analysis, 22nd ed.; Association of Official Analytical Chemists: Rockville, MD, USA, 2023. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber neutral detergent fiber and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Groot, J.C.; Williams, B.A.; Oostdam, A.J.; Boer, H.; Tamminga, S. The use of cumulative gas and volatile fatty acid production to predict in vitro fermentation kinetics of Italian ryegrass leaf cell walls and contents at various time intervals. Br. J. Nutr. 1998, 79, 519–525. [Google Scholar] [CrossRef]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J. Simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed. Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- EC Council. Regulation 882/2004 on Official controls performed to ensure verification of compliance with feed and food law, animal health and animal welfare rules. Off. J. Eur. Union 2004, L191, 1–52. [Google Scholar]
- Groot, J.C.J.; Cone, J.W.; Williams, B.A.; Debersaques, F.M.A.; Lantinga, E.A. Multiphasic analysis of gas production kinet- ics for in vitro fermentation of ruminant feedstuff. J. Feed. Sci. Technol. 1996, 64, 77–89. [Google Scholar] [CrossRef]
- Bauer, E.; Williams, B.A.; Voigt, C.; Mosenthin, R.; Verstegen, M.W.A. Microbial activities of faeces from unweaned and adult pigs, in relation to selected fermentable carbohydrates. J. Anim. Sci. 2001, 73, 313–322. [Google Scholar] [CrossRef]
- Searle, P.L. The Berthelot or indophenol reaction and its use in the analytical chemistry of nitrogen. A Review. Analyst 1984, 109, 549–568. [Google Scholar] [CrossRef]
- Zagorakis, K.; Liamadis, D.; Milis, C.; Dotas, V.; Dotas, D. Nutrient digestibility and in situ degradability of alternatives to soybean meal protein sources for sheep. Small Rumin. Res. 2015, 124, 38–44. [Google Scholar] [CrossRef]
- Lobon, S.; Alvarez-Rodriguez, J.; Joy, M. Nutritional evaluation of pea starch as an alternative to corn in ruminant diets. J. Anim. Sci. 2022, 100, 345–356. [Google Scholar] [CrossRef]
- Solanas, E.; Castrillo, C.; Fondevila, M. The influence of different legume seeds on rumen degradability and intestinal digestibility in cattle. J. Anim. Physiol. Anim. Nutr. 2004, 88, 449–456. [Google Scholar]
- Vaga, M.; Hetta, M.; Huhtanen, P. Effects of heat treatment on protein feeds evaluated in vitro by the method of estimating utilisable crude protein at the duodenum. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1259–1272. [Google Scholar] [CrossRef]
- Yáñez-Ruiz, D.R.; Martín-García, A.I.; Weisbjerg, M.R.; Hvelplund, T.; Molina-Alcaide, E. A comparison of different legume seeds as protein supplement to optimise the use of low-quality forages by ruminants. Arch. Anim. Nutr. 2009, 63, 39–55. [Google Scholar] [CrossRef]
- Huntington, G.B.; Harmon, D.L.; Richards, C.J. Sites, rates, and limits of starch digestion and glucose metabolism in growing cattle. J. Anim. Sci. 2006, 84, E14–E24. [Google Scholar] [CrossRef]
- Vastolo, A.; Matera, R.; Serrapica, F.; Cutrignelli, M.I.; Neglia, G.; Kiatti, D.; Calabrò, S. Improvement of Rumen Fermentation Efficiency Using Different Energy Sources: In Vitro Comparison Between Buffalo and Cow. Fermentation 2022, 8, 351. [Google Scholar] [CrossRef]
- Ortega, M.E.; Mendoza, G. Starch digestion and glucose metabolism in the ruminant: A review. Interciencia 2003, 28, 380–386. [Google Scholar]
- Jin, C.X.; Su, P.; Wang, Z.; Liang, X.; Lei, H.; Bai, H.; Yao, J. Effects of rumen degradable starch on growth performance, carcass, rumen fermentation, and ruminal VFA absorption in growing goats. Anim. Feed. Sci. Technol. 2023, 299, 115618. [Google Scholar] [CrossRef]
- Shen, S.; Hou, H.; Ding, C.; Bing, D.J.; Lu, Z.X. Protein content correlates with starch morphology composition and physicochemical properties in field peas. Can. J. Plant Sci. 2016, 96, 404–412. [Google Scholar] [CrossRef]
- Han, X.; Lei, X.; Yang, X.; Shen, J.; Zheng, L.; Jin, C.; Cao, Y.; Yao, J. A Metagenomic Insight Into the Hindgut Microbiota and Their Metabolites for Dairy Goats Fed Different Rumen Degradable Starch. Front. Microbiol. 2021, 12, 651631. [Google Scholar] [CrossRef]
- Pereira, A.B.D.; Whitehouse, N.L.; Aragon, K.M.; Schwab, C.S.; Reis, S.F.; Brito, A.F. Production and nitrogen utilization in lactating dairy cows fed ground field peas with or without ruminally protected lysine and methionine. J. Dairy Sci. 2017, 100, 6239–6255. [Google Scholar] [CrossRef]
- Judd, L.M.; Kohn, R.A. Test of conditions that affect in vitro production of volatile fatty acids and gases. J. Anim. Sci. 2018, 96, 694–704. [Google Scholar] [CrossRef]
- Swierk, S.; Przybyło, M.; Flaga, J.; Szczepanik, K.; Garus-Pietak, A.; Biernat, W.; Molik, E.; Wojtysiak, D.; Miltko, R.; Górka, P. Effect of increased intake of concentrates and sodium butyrate supplementation on ruminal epithelium structure and function in growing rams. Animal 2023, 17, 100898. [Google Scholar] [CrossRef]

| CTR | PSP | PSG | PSE | |
|---|---|---|---|---|
| Maize silage | 24.68 | 24.59 | 33.82 | 33.81 |
| Alfalfa hay | 54.64 | 54.45 | 44.94 | 44.93 |
| Soybean meal | 10.34 | 5.15 | 7.09 | 7.09 |
| Corn meal | 10.34 | 5.15 | 6.93 | 6.92 |
| Pea grain var. Poseidon | 0 | 10.66 | 0 | 0 |
| Pea grain var. Ganster | 0 | 0 | 7.22 | 0 |
| Pea grain var. Peps | 0 | 0 | 0 | 7.25 |
| Varieties | DM | CP | EE | NDF | Starch | Ash | NSCs |
|---|---|---|---|---|---|---|---|
| % | % DM | % DM | % DM | % DM | % DM | % DM | |
| Ganster | 89.7 | 27.1 | 1.33 | 10.8 | 45.3 | 2.87 | 57.9 |
| Peps | 90.0 | 27.3 | 1.21 | 12.1 | 44.6 | 2.82 | 56.8 |
| Poseidon | 91.4 | 26.5 | 1.12 | 14.7 | 45.2 | 2.46 | 55.2 |
| Diets | DM | CP | EE | NDF | Ash | UFL | NSCs |
|---|---|---|---|---|---|---|---|
| CTR | 89.7 | 13.6 | 2.04 | 42.9 | 7.15 | 0.83 | 34.3 |
| PSP | 89.7 | 13.4 | 1.46 | 42.5 | 7.18 | 0.86 | 35.5 |
| PSG | 89.7 | 13.3 | 1.46 | 42.6 | 8.22 | 0.85 | 34.4 |
| PSE | 90.3 | 13.4 | 1.49 | 42.7 | 7.28 | 0.86 | 35.1 |
| Diet | OMD | CPD | OMCV | Yield | Tmax | Rmax |
|---|---|---|---|---|---|---|
| % | % | mL/g | mL/g | h | mL/h | |
| CTR | 72.4 | 81.6 | 261 | 362 | 4.69 | 9.13 |
| PSN | 72.0 | 83.8 | 265 | 359 | 3.97 | 9.62 |
| GNS | 72.1 | 85.1 | 262 | 356 | 3.15 | 11.1 |
| PES | 73.2 | 84.3 | 263 | 366 | 3.31 | 11.1 |
| Dunnet test | ||||||
| CTR vs. | ||||||
| PSN | NS | NS | NS | NS | * | NS |
| GNS | NS | * | NS | NS | * | *** |
| PES | NS | * | NS | NS | * | *** |
| Tukey test | ||||||
| NS | NS | NS | NS | * | ** | |
| MSE | 1.33 | 2.54 | 3.68 | 12.3 | 0.44 | 0.32 |
| Diet | pH | N-NH3 | VFA | BCFA | Ace | Prop | Iso-But | But | Iso-Val | Val |
|---|---|---|---|---|---|---|---|---|---|---|
| mmol/L | mmol/giOM | mmol/giOM | ||||||||
| CTR | 6.30 | 8.71 | 76.8 | 8.86 | 41.7 | 16.3 | 1.88 | 10.3 | 3.76 | 2.88 |
| PSN | 6.33 | 7.64 | 77.0 | 8.34 | 41.4 | 17.0 | 1.66 | 10.8 | 3.61 | 2.57 |
| GNS | 6.34 | 6.39 | 72.3 | 8.48 | 37.5 | 15.6 | 1.84 | 11.8 | 3.28 | 2.62 |
| PES | 6.36 | 6.77 | 83.2 | 7.98 | 45.4 | 17.9 | 1.81 | 11.7 | 3.62 | 2.75 |
| Dunnet test | ||||||||||
| CTR vs. | ||||||||||
| PSN | NS | * | NS | NS | NS | ** | NS | NS | NS | NS |
| GNS | NS | * | * | NS | * | * | NS | * | NS | NS |
| PES | *** | * | *** | NS | *** | *** | NS | * | NS | NS |
| Tukey test | ||||||||||
| NS | * | *** | NS | *** | *** | NS | NS | NS | NS | |
| MSE | 0.17 | 0.29 | 1.19 | 0.78 | 0.67 | 0.15 | 0.06 | 0.56 | 0.47 | 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrara, M.; D’Anza, E.; Montefusco, T.; Iommelli, P.; Piccirillo, B.; Ruggiero, A.; Vastolo, A. In Vitro Evaluation of Three Pisum sativum L. Varieties to Partially Replace Soybean and Corn Meal in Dairy Cow Diet. Animals 2025, 15, 855. https://doi.org/10.3390/ani15060855
Ferrara M, D’Anza E, Montefusco T, Iommelli P, Piccirillo B, Ruggiero A, Vastolo A. In Vitro Evaluation of Three Pisum sativum L. Varieties to Partially Replace Soybean and Corn Meal in Dairy Cow Diet. Animals. 2025; 15(6):855. https://doi.org/10.3390/ani15060855
Chicago/Turabian StyleFerrara, Maria, Emanuele D’Anza, Teresa Montefusco, Piera Iommelli, Barbara Piccirillo, Alessio Ruggiero, and Alessandro Vastolo. 2025. "In Vitro Evaluation of Three Pisum sativum L. Varieties to Partially Replace Soybean and Corn Meal in Dairy Cow Diet" Animals 15, no. 6: 855. https://doi.org/10.3390/ani15060855
APA StyleFerrara, M., D’Anza, E., Montefusco, T., Iommelli, P., Piccirillo, B., Ruggiero, A., & Vastolo, A. (2025). In Vitro Evaluation of Three Pisum sativum L. Varieties to Partially Replace Soybean and Corn Meal in Dairy Cow Diet. Animals, 15(6), 855. https://doi.org/10.3390/ani15060855







