Effects of Feed Restriction on Growth Performance, Nutrient Utilisation, Biochemical Parameters, and the Caecum Microbiota and Metabolites in Rabbits
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Growth Performance and Digestibility of Feed Nutrients
2.3. Plasma Parameters
2.4. Caecal Microbiota Analysis
2.5. Caecal Metabolomic Analysis
2.6. Statistical Analysis
3. Results
3.1. Dry Matter Intake, Growth Performance, and Apparent Faecal Digestibility
3.2. Nitrogen Utilisation
3.3. Energy Utilisation
3.4. Plasma Biochemical, Lipid Metabolism, and Antioxidant Activity
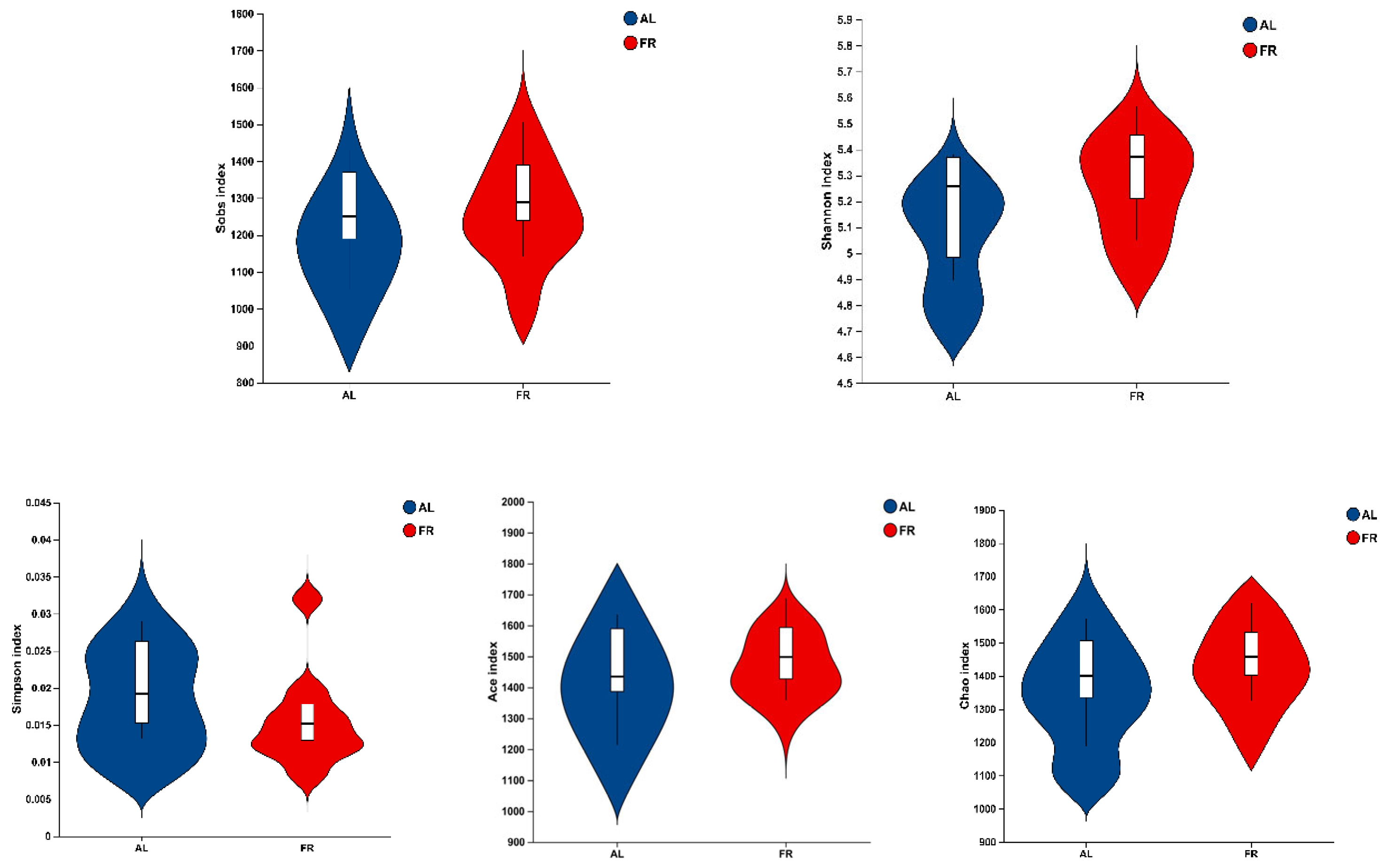
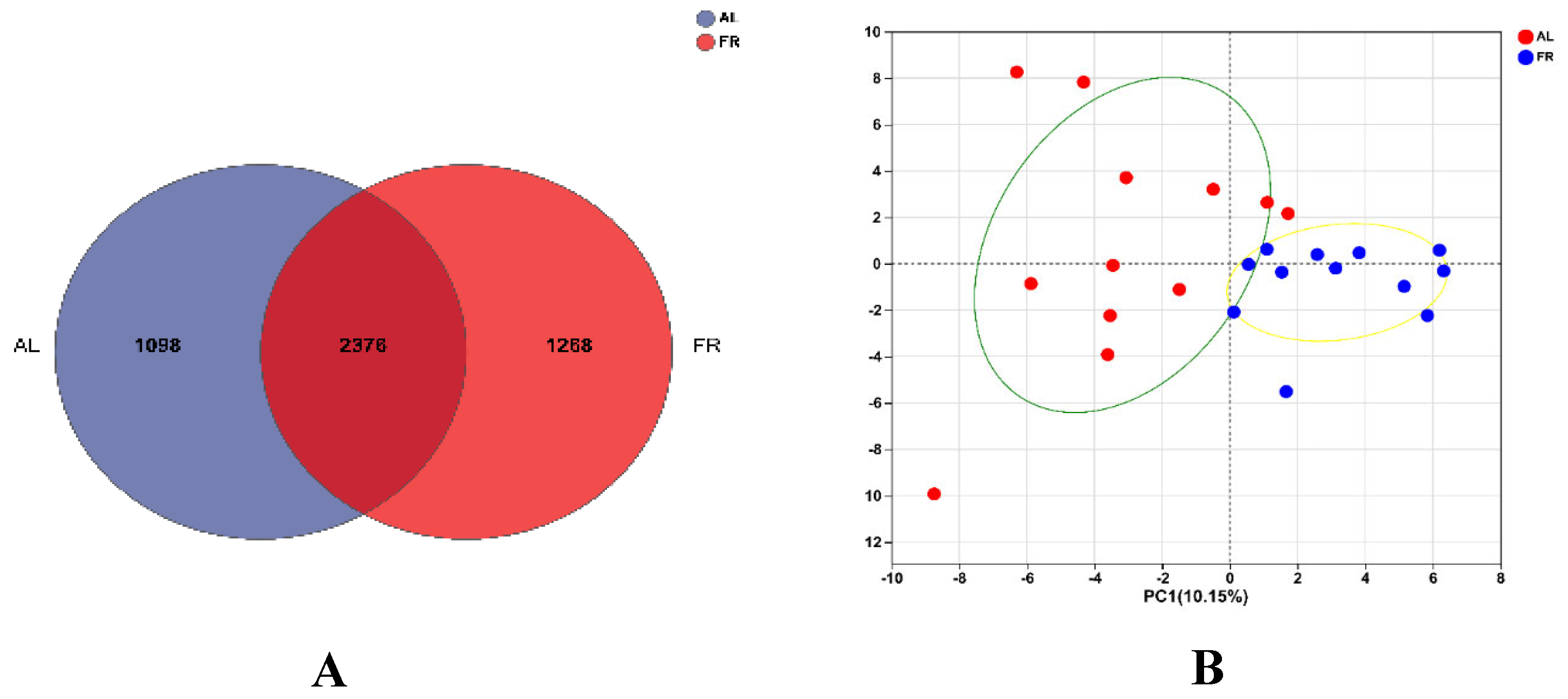
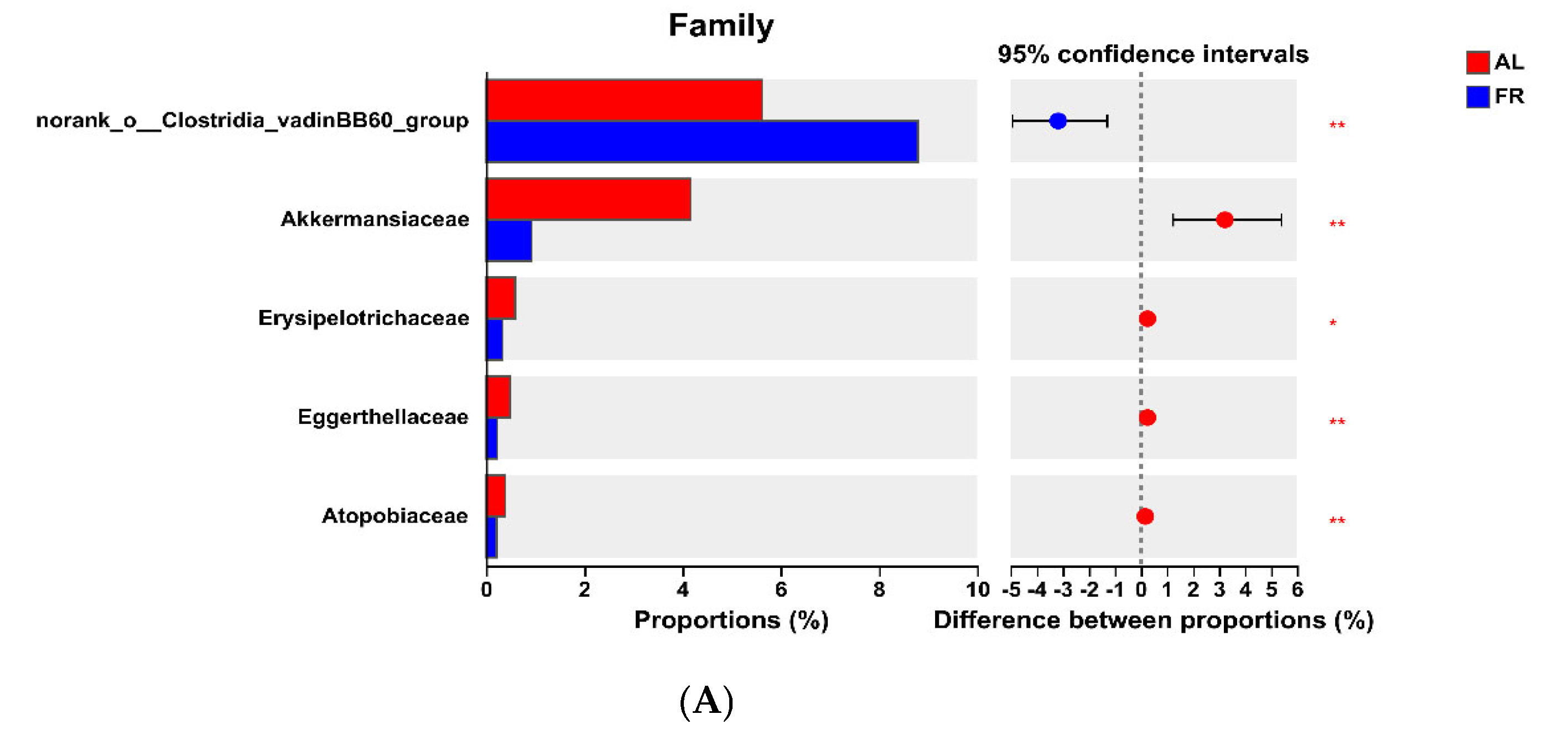
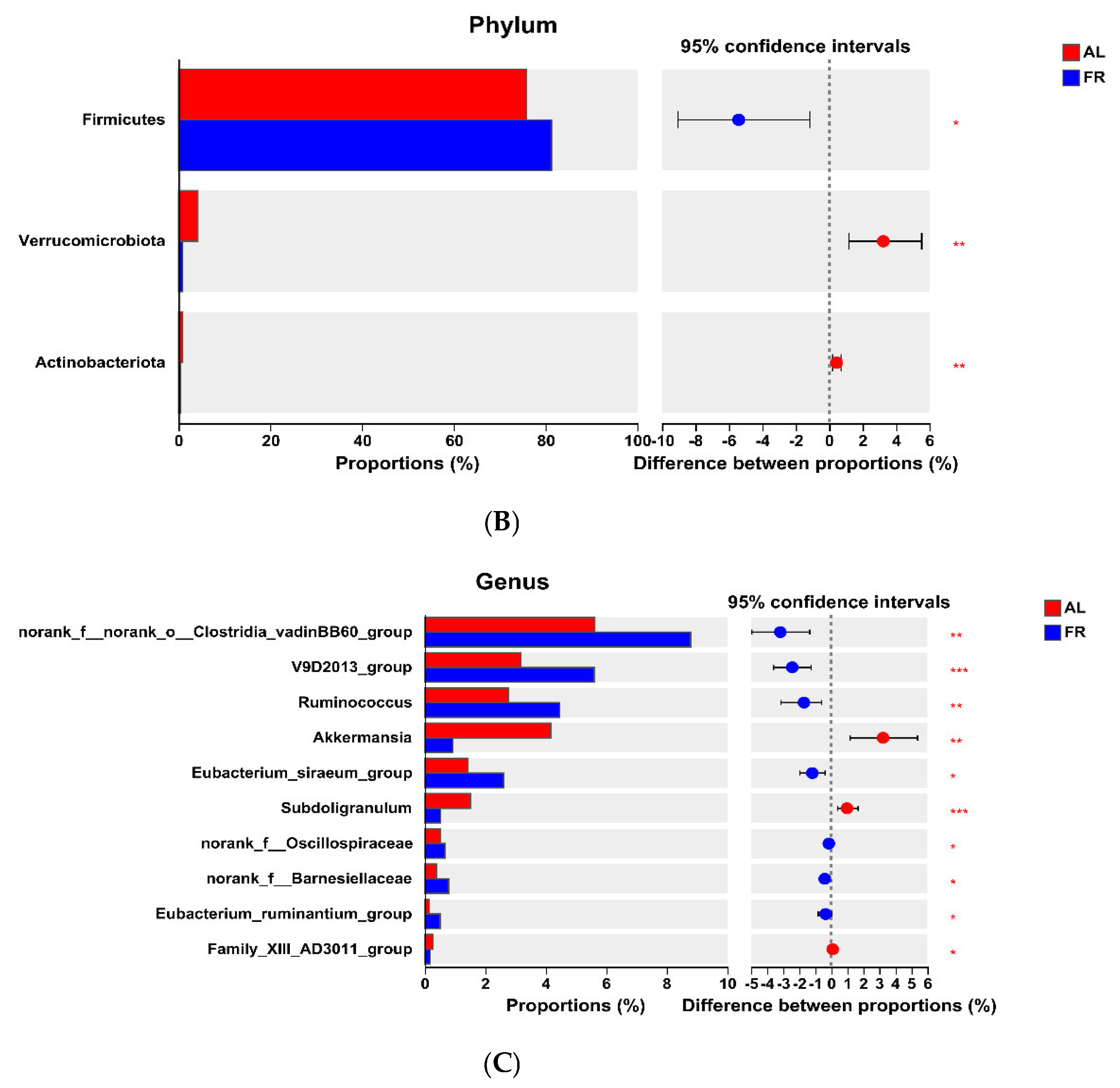
3.5. Caecal Microbiota
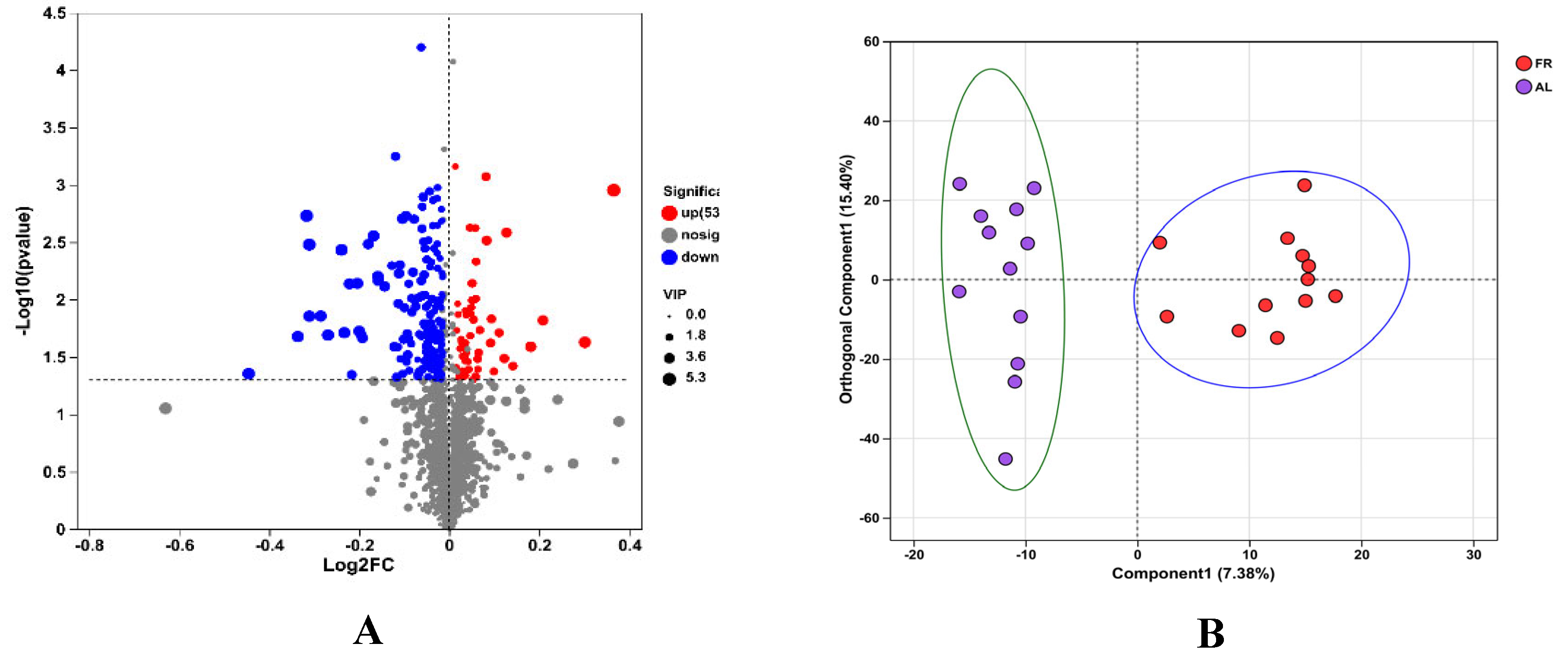
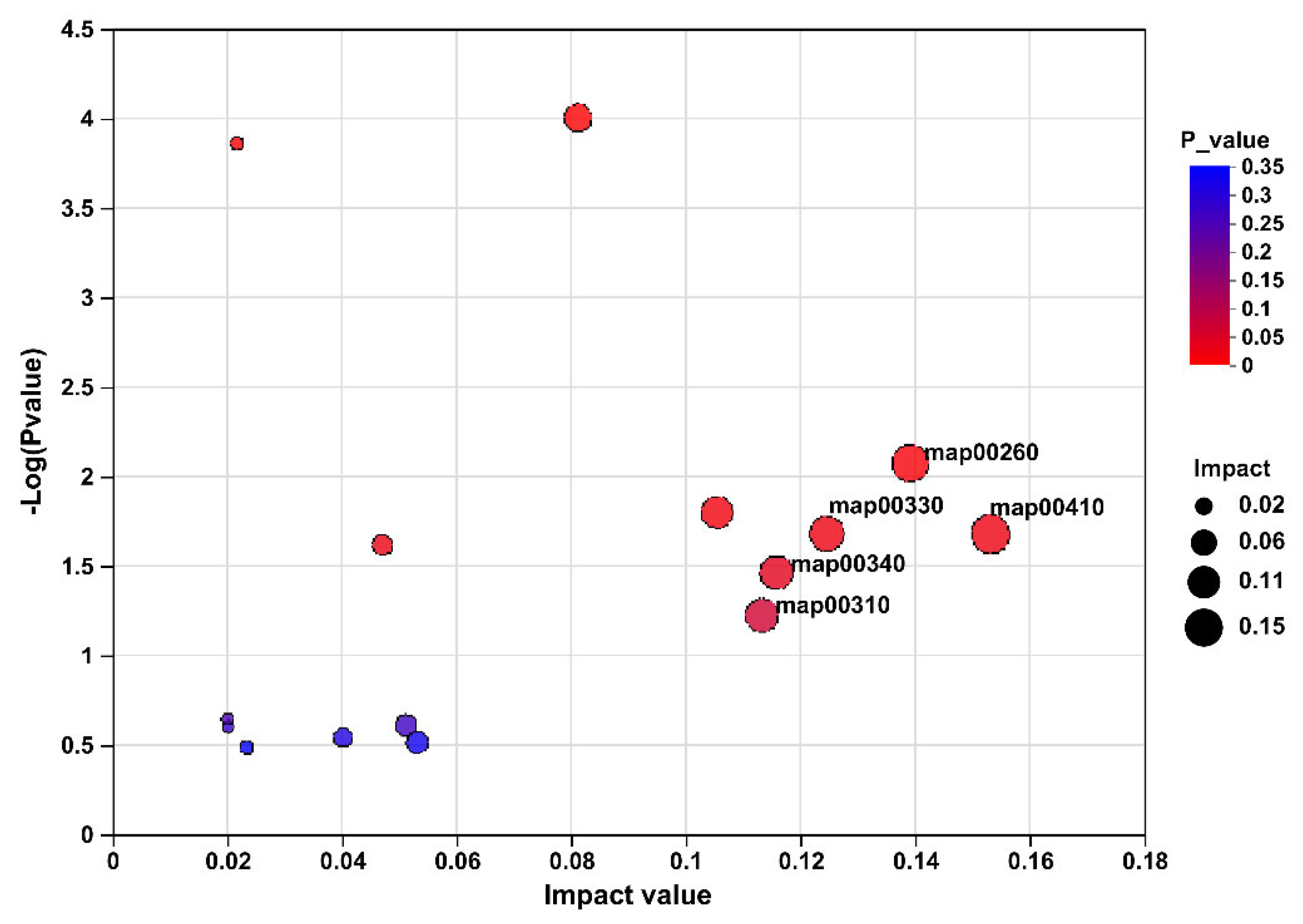
3.6. Caecal Metabolome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Harten, S.; Cardoso, L.A. Feed restriction and genetic selection on the expression and activity of metabolism regulatory enzymes in rabbits. Animal 2010, 4, 1873–1883. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, C.; Combes, S.; Briens, C.; Duperray, J.; Rebours, G.; Salaun, J.M.; Travel, A.; Weissman, D.; Gidenne, T.; Oswald, I.P. Quantitative feed restriction rather than caloric restriction modulates the immune response of growing rabbits. J. Nutr. 2015, 145, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Halai, S.F. The Effects of Dietary Free Radical Inhibitors and Feed Restriction on Age Associated Biochemical Parameter and Lifespan of AKR Male Mice. Ph.D. Thesis, University of Tennessee, Knoxville, TN, USA, 1972. [Google Scholar]
- Zhang, L.; Zhang, T.; Sun, J.; Huang, Y.; Liu, T.; Ye, Z.; Hu, J.; Zhang, G.; Chen, H.; Ye, Z.; et al. Calorie restriction ameliorates hyperglycemia, modulates the disordered gut microbiota, and mitigates metabolic endotoxemia and inflammation in type 2 diabetic rats. J. Endocrinol. Investig. 2023, 46, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Gidenne, T.; Combes, S.; Feugier, A.; Jehl, N.; Arveux, P.; Boisot, P.; Briens, C.; Corrent, E.; Fortune, H.; Montessuy, S.; et al. Feed restriction strategy in the growing rabbit. 2. Impact on digestive health, growth and carcass characteristics. Animal 2009, 3, 509–515. [Google Scholar] [CrossRef]
- Martignon, M.; Burel, C.; Cauquil, L.; Combes, S.; Gidenne, T. Impact of feed restriction and fragmented feed distribution on performance, intake behaviour and digestion of the growing rabbit. Animal 2021, 15, 100270. [Google Scholar] [CrossRef]
- Ebeid, T.A.; Tůmová, E.; Al-Homidan, I.H.; Ketta, M.; Chodová, D. Recent advances in the role of feed restriction in poultry productivity: Part I—performance, gut development, microbiota and immune response. World Poultry Sci. J. 2022, 78, 971–988. [Google Scholar] [CrossRef]
- Xu, C.; Liu, W.B.; Remø, S.C.; Wang, B.K.; Shi, H.J.; Zhang, L.; Liu, J.D.; Li, X.F. Feeding restriction alleviates high carbohydrate diet-induced oxidative stress and inflammation of Megalobrama amblycephala by activating the AMPK-SIRT1 pathway. Fish Shellfish Immunol. 2019, 92, 637–648. [Google Scholar] [CrossRef]
- Tmová, E.; Volek, Z.; Chodová, D.; Skivanová, V.; Němeek, T.; Ketta, M. Effect of quantitative feed restriction on the performance, organ development and cecal activity of growing nutrias (Myocastor coypus). Anim. Feed Sci. Technol. 2021, 280, 115077. [Google Scholar] [CrossRef]
- Hou, L.; Wang, L.; Qiu, Y.; Xiong, Y.; Xiao, H.; Yi, H.; Wen, X.; Lin, Z.; Wang, Z.; Yang, X.; et al. Effects of protein restriction and subsequent realimentation on body composition, gut microbiota and metabolite profiles in weaned piglets. Animals 2021, 11, 686. [Google Scholar] [CrossRef]
- Fondevila, G.; Archs, J.L.; Cámara, L.; de Juan, A.F.; Mateos, G.G. The length of the feed restriction period affects eating behavior, growth performance, and the development of the proximal part of the gastrointestinal tract of young broilers. Poult. Sci. 2020, 99, 1010–1018. [Google Scholar] [CrossRef]
- Artdita, C.A.; Zhuang, Y.R.; Liu, T.Y.; Cheng, C.Y.; Hsiao, F.S.; Lin, Y.Y. The effect of feeding restriction on the microbiota and metabolome response in late-phase laying hens. Animals 2021, 11, 3043. [Google Scholar] [CrossRef] [PubMed]
- Makovicky, P.; Tumova, E.; Volek, Z.; Makovicky, P.; Vodicka, P. Histological aspects of the small intestine under variable feed restriction: The effects of short and intense restriction on a growing rabbit model. Exp. Ther. Med. 2014, 8, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Tůmová, E.; Volek, Z.; Chodová, D.; Härtlová, H.; Makovický, P.; Svobodová, J.; Ebeid, T.A.; Uhlířová, L. The effect of 1-week feed restriction on performance, digestibility of nutrients and digestive system development in the growing rabbit. Animal 2016, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Xu, H.; Xie, Z.; Wang, L.; Sun, Y.; Yang, H.; Hu, D.; Mao, Y. Time-restricted feeding reduces the detrimental effects of a high-fat diet, possibly by modulating the circadian rhythm of hepatic lipid metabolism and gut microbiota. Front. Nutr. 2020, 7, 596285. [Google Scholar] [CrossRef]
- Schmidt, N.S.; Lorentz, A. Dietary restrictions modulate the gut microbiota: Implications for health and disease. Nutr. Res. 2021, 89, 10–22. [Google Scholar] [CrossRef]
- Snipes, R.L. Anatomy of the rabbit cecum. Anat. Embryol. 1978, 155, 57–80. [Google Scholar] [CrossRef]
- Abdel-Wareth, A.A.; Kehraus, S.; Ali, A.H.; Ismail, Z.S.; Südekum, K.H. Effects of temporary intensive feed restriction on performance, nutrient digestibility and carcass criteria of growing male Californian rabbits. Arch. Anim. Nutr. 2015, 69, 69–78. [Google Scholar] [CrossRef]
- Gidenne, T.; Combes, S.; Fortun-Lamothe, L. Feed intake limitation strategies for the growing rabbit: Effect on feeding behaviour, welfare, performance, digestive physiology and health: A review. Animal 2012, 6, 1407–1419. [Google Scholar] [CrossRef]
- Dalmau, A.; Abdel-Khalek, A.M.; Ramon, J.; Piles, M.; Sanchez, J.P.; Velarde, A.; Rafel, O. Comparison of behaviour, performance and mortality in restricted and ad libitum-fed growing rabbits. Animal 2015, 9, 1172–1180. [Google Scholar] [CrossRef]
- Gidenne, T.; Feugier, A. Feed restriction strategy in the growing rabbit. 1. Impact on digestion, rate of passage and microbial activity. Animal 2009, 3, 501–508. [Google Scholar] [CrossRef]
- Crespo, R.; Alfonso, C.; del Barrio, A.S.; Garcia-Ruiz, A.I.; Marco, M.; Nicodemus, N. Effect of feed restriction on performance, carcass yield and nitrogen and energy balance in growing rabbits. Livest. Sci. 2020, 241, 104278. [Google Scholar] [CrossRef]
- Lu, Q.; Qin, J.X.; Xie, S.L.; Chen, R.; Xu, Y.Q.; Wang, X.; Zhou, D.; Tian, X.Z. Effects of feed restriction on the slaughter performance, antioxidant activity, and meat quality of rabbits. Food Sci. Anim. Prod. 2024, 2, 9240080. [Google Scholar] [CrossRef]
- Chinese Standard NY/T 4049-2021; Nutrient Requirement of Meat Rabbit. The Standard Press of PR China: Beijing, China, 2021. (In Chinese)
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analtical Chemists: Arlington, MA, USA, 2005. [Google Scholar]
- Van Soest, P.V.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Ebeid, T.A.; Al-Homidan, I.H.; Saleh, A.A.; Barakat, H.A. Physiological and immunological aspects of feed restriction and its beneficial impacts in fattening rabbits’ productivity-an updated review. Trop. Anim. Health Prod. 2024, 6, 33. [Google Scholar] [CrossRef]
- Birolo, M.; Trocino, A.; Tazzoli, M.; Xiccato, G. Effect of feed restriction and feeding plans on performance, slaughter traits and body composition of growing rabbits. World Rabbit Sci. 2017, 25, 113–122. [Google Scholar] [CrossRef]
- Le Floc’h, N.; Knudsen, C.; Gidenne, T.; Montagne, L.; Merlot, E.; Zemb, O. Impact of feed restriction on health, digestion and faecal microbiota of growing pigs housed in good or poor hygiene conditions. Animal 2014, 8, 1632–1642. [Google Scholar] [CrossRef]
- Zhuang, J.; Zhou, T.; Bai, S.; Zhao, B.; Wu, X.; Chen, Y. Effects of restricted feeding on growth performance, intestinal immunity, and skeletal muscle development in New Zealand rabbits. Animals 2022, 12, 160. [Google Scholar] [CrossRef]
- Combes, S.; Massip, K.; Martin, O.; Furbeyre, H.; Cauquil, L.; Pascal, G.; Bouchez, O.; Le Floc’h, N.; Zemb, O.; Oswald, I.P.; et al. Impact of feed restriction and housing hygiene conditions on specific and inflammatory immune response, the cecal bacterial community and the survival of young rabbits. Animal 2017, 11, 854–863. [Google Scholar] [CrossRef]
- Drouilhet, L.; Achard, C.S.; Zemb, O.; Molette, C.; Gidenne, T.; Larzul, C.; Ruesche, J.; Tircazes, A.; Segura, M.; Bouchez, T.; et al. Direct and correlated responses to selection in two lines of rabbits selected for feed efficiency under ad libitum and restricted feeding: I. Production traits and gut microbiota characteristics. J. Anim. Sci. 2016, 94, 38–48. [Google Scholar] [CrossRef]
- Romero, C.; Cuesta, S.; Astillero, J.R.; Nicodemus, N.; Blas, C.D. Effect of early feed restriction on performance and health status in growing rabbits slaughtered at 2 kg live-weight. World Rabbit Sci. 2010, 18, 211–218. [Google Scholar] [CrossRef]
- Dong, L.; Li, Y.; Zhang, Y.; Zhang, Y.; Ren, J.; Zheng, J.; Diao, J.; Ni, H.; Yin, Y.; Sun, R.; et al. Effects of organic zinc on production performance, meat quality, apparent nutrient digestibility and gut microbiota of broilers fed low-protein diets. Sci. Rep. 2023, 13, 10803. [Google Scholar] [CrossRef] [PubMed]
- Gidenne, T.; Fortun-Lamothe, L.; Bannelier, C.; Molette, C.; Gilbert, H.; Chemit, M.L.; Segura, M.; Benitez, F.; Richard, F.; Garreau, H.; et al. Direct and correlated responses to selection in two lines of rabbits selected for feed efficiency under ad libitum and restricted feeding: III. digestion and excretion of nitrogen and minerals. J. Anim. Sci. 2017, 95, 1301–1312. [Google Scholar] [PubMed]
- Rebollar, P.G.; Pereda, N.; Schwarz, B.F.; Millán, P.; Lorenzo, P.L.; Nicodemus, N. Effect of feed restriction or feeding high-fibre diet during the rearing period on body composition, serum parameters and productive performance of rabbit does. Anim. Feed Sci. Technol. 2021, 163, 67–76. [Google Scholar] [CrossRef]
- Richards, M.P.; Poch, S.M.; Coon, C.N.; Rosebrough, R.W.; Ashwell, C.M.; Mcmurtry, J.P. Feed restriction significantly alters lipogenic gene expression in broiler breeder chickens. J. Nutr. 2003, 133, 707. [Google Scholar] [CrossRef]
- Aouichat, S.; Chayah, M.; Bouguerra-Aouichat, S.; Agil, A. Time-restricted feeding improves body weight gain, lipid profiles, and atherogenic indices in cafeteria-diet-fed rats: Role of browning of inguinal white adipose tissue. Nutrients 2020, 12, 2185. [Google Scholar] [CrossRef]
- Liu, J.; Wei, Y.; Di, H.; Zhong, Y.; Hua, W.; Wang, H. Calorie restriction improves serum lipid metabolism, colon metabolites and microbiota in pigs. Anim. Nutr. 2024, 1, e11. [Google Scholar] [CrossRef]
- Woodie, L.N.; Luo, Y.; Wayne, M.J.; Graff, E.C.; Ahmed, B.; O’Neill, A.M.; Greene, M.W. Restricted feeding for 9 h in the active period partially abrogates the detrimental metabolic effects of a Western diet with liquid sugar consumption in mice. Metabolism 2018, 82, 1–13. [Google Scholar] [CrossRef]
- Chen, W.; Guo, Y.M.; Huang, Y.Q.; Shi, Y.H.; Zhang, C.X.; Wang, J.W. Effect of energy restriction on growth, slaughter performance, serum biochemical parameters and Lpin2/WDTC1/mRNA expression of broilers in the later phase. J. Poult. Sci. 2012, 49, 12–19. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. The definition and measurement of antioxidants in biological systems. Free Radic. Biol. Med. 1995, 18, 125–126. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Hangalapura, B.N.; Nieuwland, M.G.; De Vries Reilingh, G.; Buyse, J.; Van Den Brand, H.; Kemp, B.; Parmentier, H.K. Severe feed restriction enhances innate immunity but suppresses cellular immunity in chicken lines divergently selected for antibody responses. Poult. Sci. 2005, 84, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Julian, D.; Leeuwenburgh, C. Linkage between insulin and the free radical theory of aging. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R20–R21. [Google Scholar] [CrossRef] [PubMed]
- Cotozzolo, E.; Cremonesi, P.; Curone, G.; Menchetti, L.; Riva, F.; Biscarini, F.; Marongiu, M.L.; Castrica, M.; Castiglioni, B.; Miraglia, D.; et al. Characterization of bacterial microbiota composition along the gastrointestinal tract in rabbits. Animals 2020, 11, 31. [Google Scholar] [CrossRef]
- Ye, J.; Jiang, S.; Cheng, Z.; Ding, F.; Fan, Q.; Lin, X.; Wang, Y.; Gou, Z. Feed restriction improves lipid metabolism by changing the structure of the cecal microbial community and enhances the meat quality and flavor of bearded chickens. Animals 2022, 12, 970. [Google Scholar] [CrossRef]
- Liu, B.; Cui, Y.; Ali, Q.; Zhu, X.; Li, D.; Ma, S.; Wang, Z.; Wang, C.; Shi, Y. Gut microbiota modulate rabbit meat quality in response to dietary fiber. Front. Nutr. 2022, 9, 849429. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef]
- Fan, P.; Liu, P.; Song, P.; Chen, X.; Ma, X. Moderate dietary protein restriction alters the composition of gut microbiota and improves ileal barrier function in adult pig model. Sci. Rep. 2017, 7, 43412. [Google Scholar] [CrossRef]
- Fujiki, Y.; Tanaka, T.; Yakabe, K.; Seki, N.; Akiyama, M.; Uchida, K.; Kim, Y.G. Hydrogen gas and the gut microbiota are potential biomarkers for the development of experimental colitis in mice. Gut Microbiome 2023, 5, e3. [Google Scholar] [CrossRef]
- Tian, S.; Chu, Q.; Ma, S.; Ma, H.; Song, H. Dietary fiber and its potential role in obesity: A focus on modulating the gut microbiota. J. Agric. Food Chem. 2023, 71, 14853–14869. [Google Scholar] [CrossRef]
- Yadav, S.; Jha, R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019, 10, 2. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Sale, C.; Saunders, B.; Harris, R.C. Effect of beta-alanine supplementation on muscle carnosine concentrations and exercise performance. Amino Acids 2010, 39, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Caruso, J.; Charles, J.; Unruh, K.; Giebel, R.; Learmonth, L.; Potter, W. Ergogenic effects of β-alanine and carnosine: Proposed future research to quantify their efficacy. Nutrients 2012, 4, 585–601. [Google Scholar] [CrossRef]
- Smith-Ryan, A.E.; Fukuda, D.H.; Stout, J.R.; Kendall, K.L. The influence of β-alanine supplementation on markers of exercise-induced oxidative stress. Appl. Physiol. Nutr. Metab. 2014, 39, 38–46. [Google Scholar] [CrossRef]
- Derave, W.; Everaert, I.; Beeckman, S.; Baguet, A. Muscle carnosine metabolism and beta-alanine supplementation in relation to exercise and training. Sports Med. 2010, 40, 247–263. [Google Scholar] [CrossRef]
- Smith, A.E.; Stout, J.R.; Kendall, K.L.; Fukuda, D.H.; Cramer, J.T. Exercise-induced oxidative stress: The effects of β-alanine supplementation in women. Amino Acids 2012, 43, 77–90. [Google Scholar] [CrossRef]
- Wu, H.C.; Shiau, C.Y.; Chen, H.M.; Chiou, T.K. Antioxidant activities of carnosine, anserine, some free amino acids and their combination. J. Food Drug Anal. 2003, 11, 148–153. [Google Scholar] [CrossRef]
- Mannion, A.F.; Jakeman, P.M.; Dunnett, M.; Harris, R.C.; Willan, P.L. Carnosine and anserine concentrations in the quadriceps femoris muscle of healthy humans. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 64, 47–50. [Google Scholar] [CrossRef]
- Intarapichet, K.O.; Maikhunthod, B. Genotype and gender differences in carnosine extracts and antioxidant activities of chicken breast and thigh meats. Meat Sci. 2005, 71, 634–642. [Google Scholar] [CrossRef]
| Ingredient, g/kg | Content |
|---|---|
| Corn | 237 |
| Soybean meal | 67 |
| Rapeseed meal | 32 |
| Cottonseed meal | 31 |
| Wheat bran | 158 |
| Alfalfa | 425 |
| CaHPO4 | 4.0 |
| NaCl | 3.5 |
| Stone powder | 2.5 |
| Compound premix | 40 |
| Chemical Composition | Content |
|---|---|
| Dry matter, g/kg | 894 |
| Crude protein, g/kg of DM | 163 |
| Gross energy, MJ/kg of DM | 15.2 |
| Neutral detergent fibre, g/kg of DM | 348 |
| Acid detergent fibre, g/kg of DM | 214 |
| Ash, g/kg of DM | 97 |
| Item | AL | FR | SEM | p-Value |
|---|---|---|---|---|
| Dry matter intake, g/d | 117.25 a | 93.39 b | 0.802 | <0.001 |
| Initial weight, g | 1866.58 | 1877.67 | 53.384 | 0.885 |
| Final weight, g | 2693.53 | 2681.86 | 51.211 | 0.873 |
| Average daily weight gain, g | 19.69 | 19.15 | 1.794 | 0.833 |
| Feed conversion ratio | 6.18 | 5.67 | 0.522 | 0.499 |
| Apparent faecal digestibility, % | ||||
| Dry matter | 35.67 | 34.66 | 1.321 | 0.617 |
| Neutral detergent fibre | 29.64 | 29.37 | 1.471 | 0.904 |
| Acid detergent fibre | 18.57 b | 20.88 a | 0.514 | 0.034 |
| Ash | 25.23 | 25.02 | 0.631 | 0.826 |
| Item | AL | FR | SEM | p-Value |
|---|---|---|---|---|
| Nitrogen intake, g/d | 3.07 a | 2.44 b | 0.016 | <0.001 |
| Nitrogen excretion in faeces, g/d | 0.74 a | 0.49 b | 0.013 | 0.002 |
| Nitrogen excretion in urine, g/d | 0.05 a | 0.02 b | 0.002 | 0.001 |
| Total nitrogen excretion, g/d | 0.79 a | 0.51 b | 0.015 | 0.002 |
| Nitrogen digestibility, % | 75.95 b | 79.92 a | 0.433 | 0.003 |
| Nitrogen retention, % | 74.45 b | 78.96 a | 0.934 | 0.027 |
| Item | AL | FR | SEM | p-Value |
|---|---|---|---|---|
| Gross energy intake, kJ/d | 1784.55 a | 1421.45 b | 9.236 | <0.001 |
| Gross energy excretion in faeces, kJ/d | 918.11 a | 614.10 b | 4.880 | <0.001 |
| Gross energy excretion in urine, kJ/d | 23.35 a | 9.88 b | 0.502 | <0.001 |
| Total gross energy excretion, kJ/d | 941.46 a | 623.98 b | 5.139 | <0.001 |
| Gross energy digestibility, % | 48.55 b | 56.80 a | 0.304 | <0.001 |
| Gross energy retention, % | 47.24 b | 56.10 a | 0.311 | <0.001 |
| Item | AL | FR | SEM | p-Value |
|---|---|---|---|---|
| Biochemical parameters | ||||
| Glucose, mmol/L | 5.71 | 5.19 | 0.190 | 0.069 |
| Total protein, g/L | 3.56 | 3.49 | 0.050 | 0.320 |
| Albumin, g/L | 62.54 | 62.01 | 3.081 | 0.904 |
| Lipid metabolism | ||||
| Triglyceride, mmol/L | 0.77 a | 0.57 b | 0.063 | 0.042 |
| Creatinine, μmol/L | 118.87 a | 97.53 b | 5.161 | 0.008 |
| Total cholesterol, mmol/L | 2.86 | 2.86 | 0.402 | 0.995 |
| Low-density lipoprotein cholesterol, mmol/L | 0.75 | 0.57 | 0.073 | 0.091 |
| High-density lipoprotein cholesterol, mmol/L | 1.30 a | 1.02 b | 0.081 | 0.022 |
| Antioxidant activity | ||||
| Total antioxidant capacity, U/mL | 0.76 b | 1.15 a | 0.129 | 0.048 |
| Superoxide dismutase, U/mL | 106.56 b | 136.66 a | 0.532 | <0.001 |
| G lutathione peroxidase, U/mL | 23.68 | 23.08 | 1.455 | 0.776 |
| Catalase, U/mL | 2.82 | 2.16 | 0.407 | 0.281 |
| 2,2-diphenyl-1-picrylhydrazyl scavenging activity, % | 81.42 | 71.70 | 4.107 | 0.108 |
| Malondialdehyde, nmol/mL | 0.89 a | 0.70 b | 0.016 | 0.017 |
| Hydroxyl free radical, U/mL | 1341 a | 1150 b | 28.450 | <0.001 |
| Superoxide anion, U/L | 334.06 | 333.67 | 4.264 | 0.949 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Q.; Qin, J.; Xie, S.; Chen, R.; Wang, X.; Xu, Y.; Ban, Y.; Gao, C.; Li, P.; Zhou, D.; et al. Effects of Feed Restriction on Growth Performance, Nutrient Utilisation, Biochemical Parameters, and the Caecum Microbiota and Metabolites in Rabbits. Animals 2025, 15, 842. https://doi.org/10.3390/ani15060842
Lu Q, Qin J, Xie S, Chen R, Wang X, Xu Y, Ban Y, Gao C, Li P, Zhou D, et al. Effects of Feed Restriction on Growth Performance, Nutrient Utilisation, Biochemical Parameters, and the Caecum Microbiota and Metabolites in Rabbits. Animals. 2025; 15(6):842. https://doi.org/10.3390/ani15060842
Chicago/Turabian StyleLu, Qi, Jixiao Qin, Shuanglong Xie, Rui Chen, Xu Wang, Yiqing Xu, Yiming Ban, Chengcheng Gao, Peiyao Li, Di Zhou, and et al. 2025. "Effects of Feed Restriction on Growth Performance, Nutrient Utilisation, Biochemical Parameters, and the Caecum Microbiota and Metabolites in Rabbits" Animals 15, no. 6: 842. https://doi.org/10.3390/ani15060842
APA StyleLu, Q., Qin, J., Xie, S., Chen, R., Wang, X., Xu, Y., Ban, Y., Gao, C., Li, P., Zhou, D., & Tian, X. (2025). Effects of Feed Restriction on Growth Performance, Nutrient Utilisation, Biochemical Parameters, and the Caecum Microbiota and Metabolites in Rabbits. Animals, 15(6), 842. https://doi.org/10.3390/ani15060842






