Come out of Your Shell—A Comparative Pilot Study for Teaching the Central Plastrotomy in Chelonians Using a 3D-Printed Simulator and a Virtual 3D Simulation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Privacy
2.2. Developed Resources
2.2.1. The 3D-Printed Simulator
2.2.2. Virtual 3D Simulation
2.3. Study Design
2.3.1. Study with Students from Semester 5 to 8
2.3.2. Evaluation by Experienced Veterinarians
2.4. Data Acquisition
2.4.1. Questionnaires
2.4.2. Objective Structured Clinical Examination (OSCE)
2.5. Statistical Analyses
2.5.1. Study with Students from Semester 5 to 8
2.5.2. Evaluation Analysis
3. Results
3.1. Study with Students in Semester 5 to 8
3.1.1. Objective Structured Clinical Examination (OSCE)
3.1.2. Correlation of Shell Thickness and OSCE Results
3.1.3. Self-Assessment of Skills
3.1.4. Correlation of Self-Assessment of Skills and OSCE Results
3.2. Evaluation
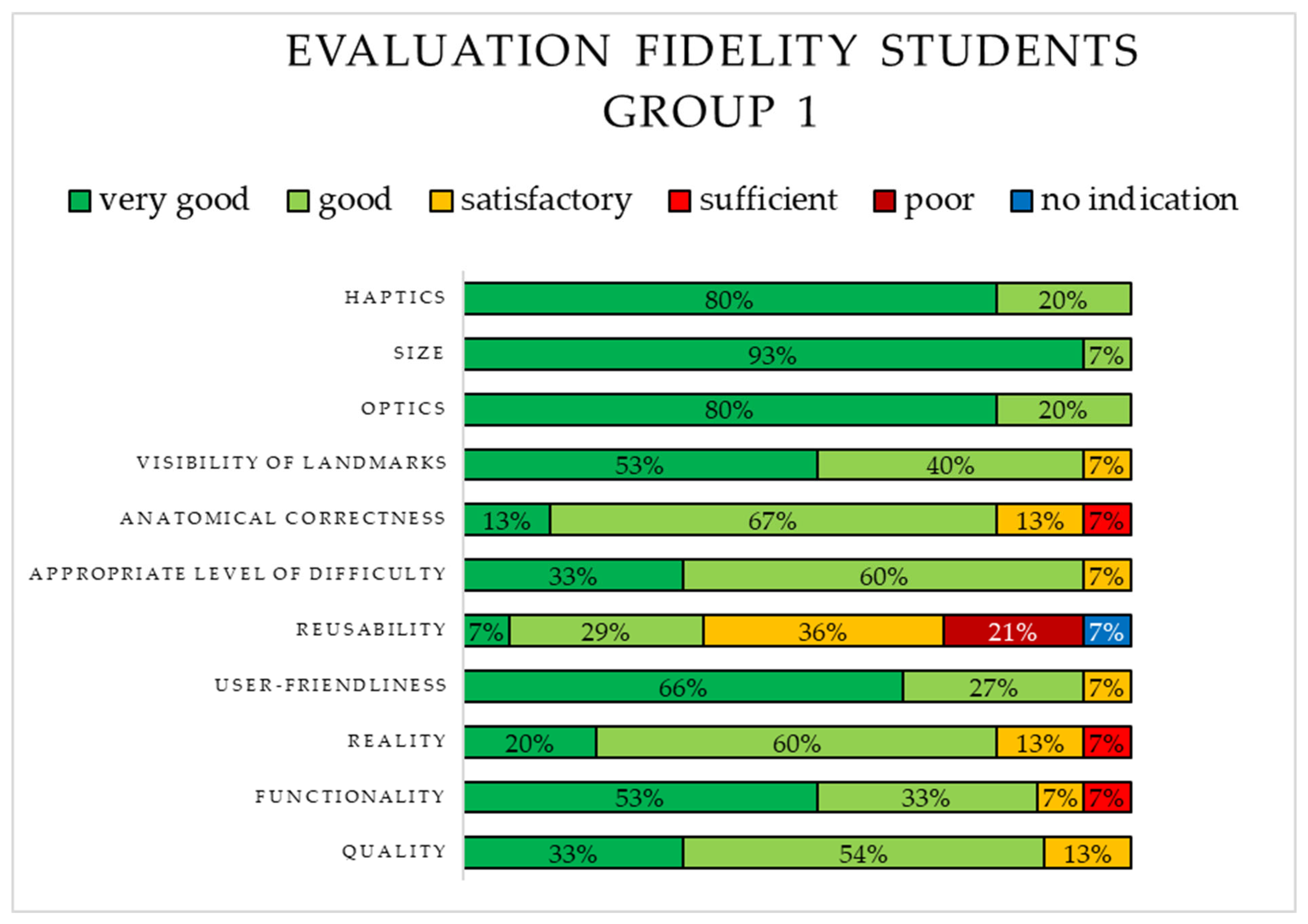
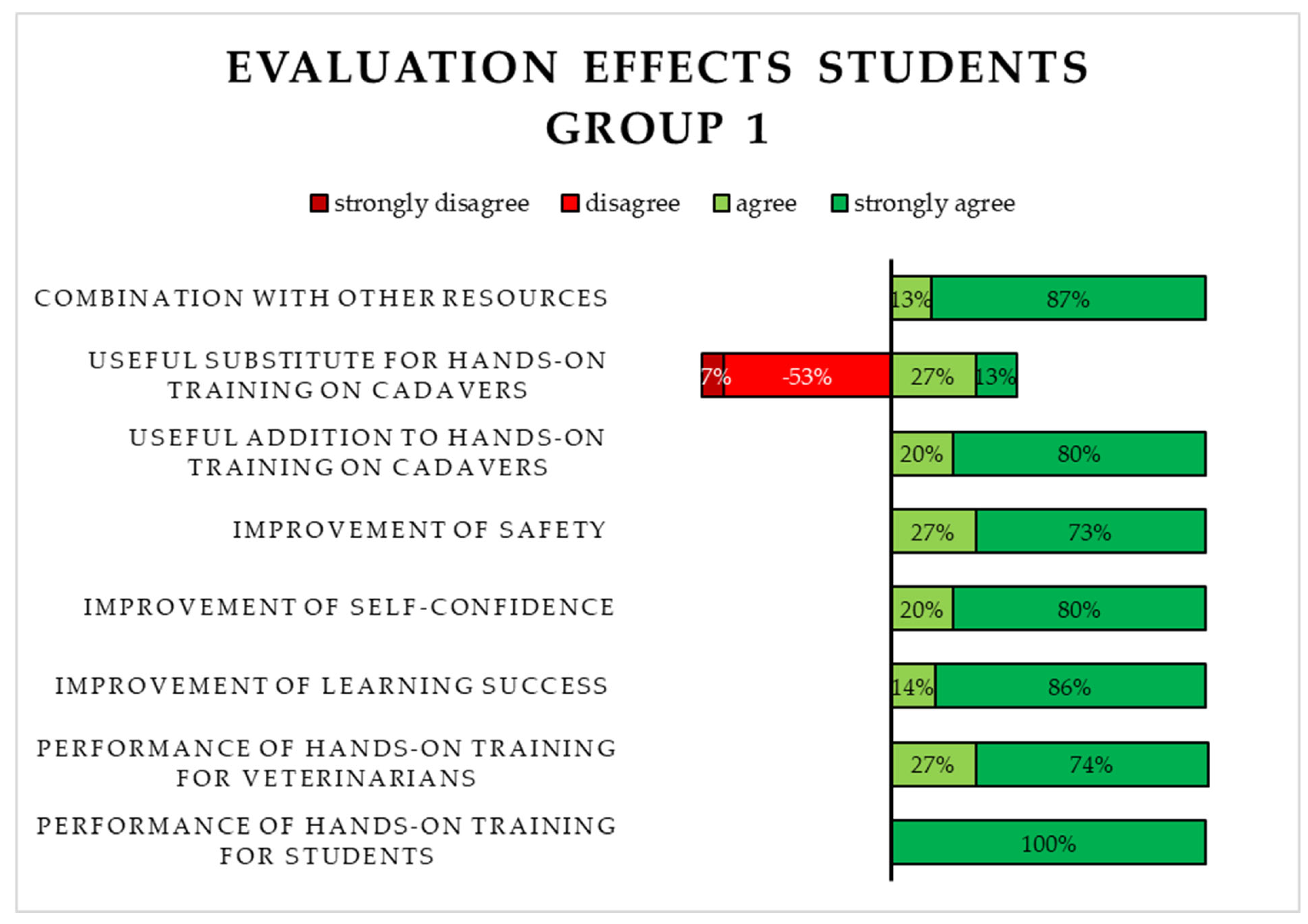
3.2.1. Evaluation of the 3D-Printed Simulator (S1) by Students in Group 1
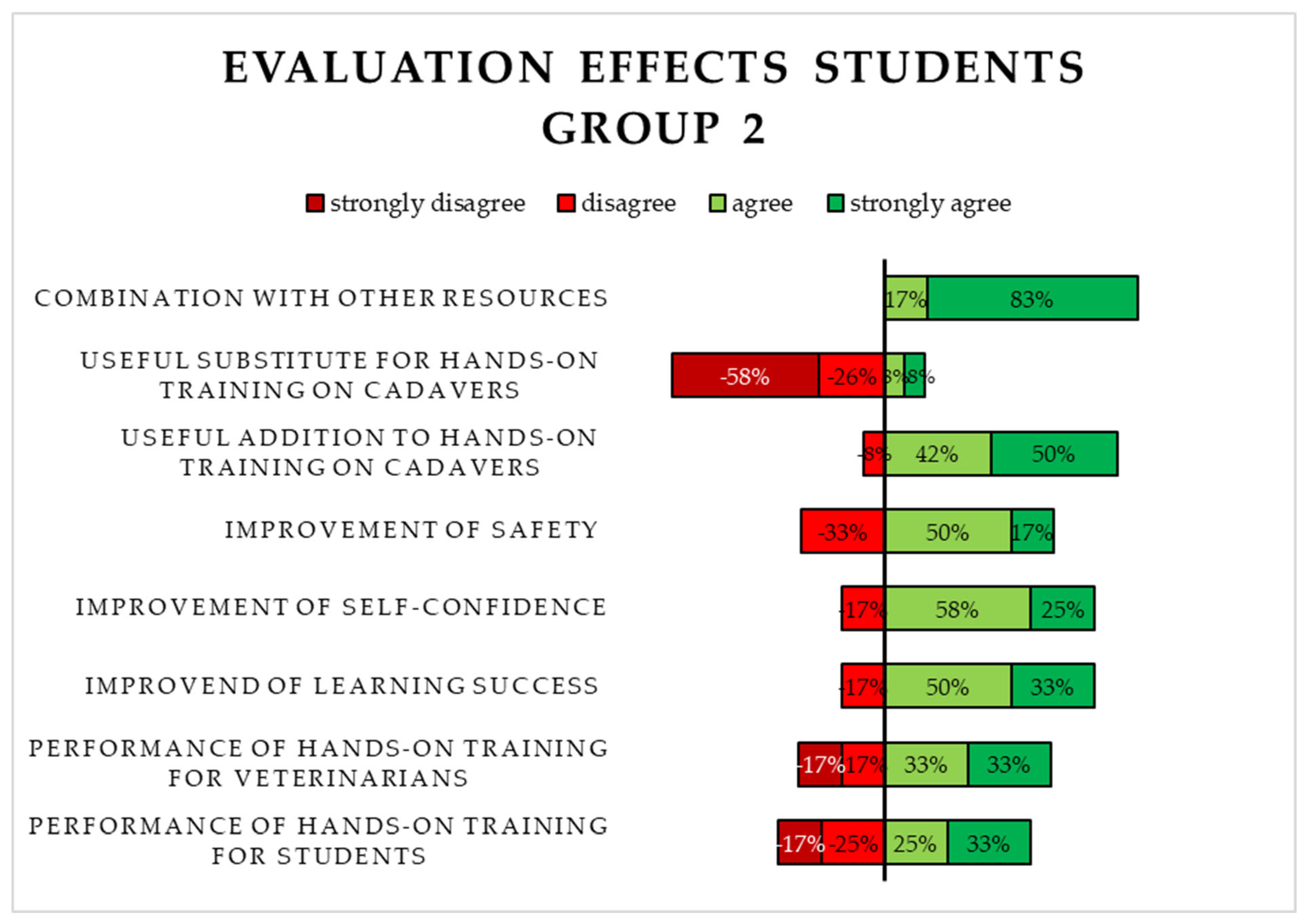
3.2.2. Evaluation of the Virtual 3D Simulation (S2) by Students in Group 2
3.2.3. Evaluation of the 3D-Printed Simulator (S1) by Experts
4. Discussion
4.1. Comparison of Novel with Traditional Educational Resources
4.2. Correlation of Subjective Self-Assessment and Objective Performance
4.3. Limitations and Strengths
4.4. Further Implementation and Perspective of S1
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schuppli, C.A.; Fraser, D.; Bacon, H.J. Welfare of non-traditional pets. Rev. Sci. Tech. 2014, 33, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.; Britsch, G. Grunddaten zur Ernährung von Reptilien. Kleintier Konkret 2016, 19, 25–30. [Google Scholar] [CrossRef]
- Facts & Figures 2021 FEDIAF European Pet Food Annual Report 2022. Available online: https://europeanpetfood.comingsoon.site/wp-content/uploads/2023/02/Annual-Report-2022-2.pdf (accessed on 19 May 2024).
- Facts & Figures 2022 FEDIAF European Pet Food Annual Report 2023. Available online: https://europeanpetfood.comingsoon.site/wp-content/uploads/2023/07/FEDIAF_Annual-Report_2023.pdf (accessed on 19 May 2024).
- pfma Pet Food Manufacturers’ Association Annual Report 2020. Available online: https://ukpetfood-reports.co.uk/annual-report-2020/ (accessed on 15 December 2024).
- pfma Pet Food Manufacturers’ Association Annual Report 2021. Available online: https://ukpetfood-reports.co.uk/annual-report-2021/ (accessed on 15 December 2024).
- pfma Pet Food Manufacturers’ Association Annual Report 2022. Available online: https://ukpetfood-reports.co.uk/annual-report-2022/ (accessed on 15 December 2024).
- UK PET FOOD 2023 ANNUAL REPORT—‘Fit for the Future’. Available online: https://ukpetfood-reports.co.uk/annual-report-2023/ (accessed on 15 December 2024).
- Boyer, T.H.; Innis, C.J. Chelonian Taxonomy Anatomy, and Physiology. In Mader’s Reptile and Amphibian Medicine and Surgery; Elsevier: St. Louis, MO, USA, 2019; pp. 31–49. [Google Scholar]
- Wüst, E.; Divers, S.J. Chelonian Transplastron Coeliotomy. In Mader’s Reptile and Amphibian Medicine and Surgery; Elsevier: St. Louis, MO, USA, 2019; pp. 1057–1061. [Google Scholar]
- Sigirci, B.D.; Ikiz, S.; Celik, B.; Seyyal, A.K. A Survey Study on Self-Evaluations of Small Pet Practitioners about Exotic Pets in Istanbul in 2016. Acta Vet. Eurasia 2019, 46, 7–9. [Google Scholar] [CrossRef]
- Bradley Bays, T.A. Equipping the Reptile Practice. Vet. Clin. N. Am. Exot. Anim. Pract. 2005, 8, 437–461. [Google Scholar] [CrossRef]
- Mayer, J.; Martin, J. Barriers to Exotic Animal Medicine. Vet. Clin. N. Am. Exot. Anim. Pract. 2005, 8, 487–496. [Google Scholar] [CrossRef]
- Ostovic, M.; Sabolek, I.; Piplica, A.; Zaja, I.Z.; Mencik, S.; Nejedli, S.; Mesic, Z. A Survey Study of Veterinary Student Opinions and Knowledge about Pet Reptiles and Their Welfare. Animals 2021, 11, 3185. [Google Scholar] [CrossRef]
- Vučinić, M.; Hajzler, I.; Terzin, J.; Nenadović, K.; Janković, L.; Voslarova, E.; Vučićević, M. Reptile Ownership in Balkan Countries: Demographics and Reliance on Veterinary Advice. Anthrozoos 2019, 32, 129–139. [Google Scholar] [CrossRef]
- Ostovic, M.; Sabolek, I.; Piplica, A.; Zaja, I.Z.; Mikus, T.; Mencik, S.; Matkovic, K.; Pavicic, Z.; Mesic, Z. Opinions and knowledge of veterinary students relating to exotic non-mammal pet animals and their welfare. Veterinarski Arhiv 2022, 92, 349. [Google Scholar] [CrossRef]
- EAEVE List of Subjects and Day One Competences. Available online: https://www.eaeve.org/fileadmin/downloads/eccvt/List_of_subjects_and_Day_One_Competences_approved_on_17_January_2019.pdf (accessed on 19 May 2024).
- AVMA CVTEA Accreditation Policies and Procedures Appendix G—Veterinary Technology Student Essential and Recommended Skills List 2024. Available online: https://www.avma.org/education/center-for-veterinary-accreditation/committee-veterinary-technician-education-activities/cvtea-accreditation-policies-and-procedures-appendix-g (accessed on 29 April 2024).
- Baillie, S.; Dilly, M.; Ciappesoni, J.L.; Read, E.K. The Rapid and International Expansion of Veterinary Clinical Skills Laboratories: A Survey to Establish Recent Developments. J. Vet. Med. Educ. 2014, 51, 215–228. [Google Scholar] [CrossRef]
- Engelskirchen, S.; Ehlers, J.; Kirk, A.T.; Tipold, A.; Dilly, M. Skills lab training in veterinary medicine. Effective preparation for clinical work at the small animal clinic of the University for Veterinary Medicine Hannover, Foundation. Tierärztliche Prax. Ausg. K Kleintiere/Heimtiere 2017, 397–405. [Google Scholar]
- Stiftung Tierärztliche Hochschule Hannover Lehrbericht 2022. Available online: https://www.tiho-hannover.de/fileadmin/01_Verwaltung/Forschung/Lehrbericht_2022.pdf (accessed on 26 January 2025).
- Justus-Liebig Universität-Gießen Fachbereich 10 Veterinärmedizin Skills Lab PETS Veranstaltungsangebot Raum 6. Available online: https://www.uni-giessen.de/de/fbz/fb10/studium-und-prufungen/pets/raeume/raum-6 (accessed on 8 April 2024).
- Universität Leipzig Veterinärmedizinische Fakultät Praktisches Ausbildungs-und Lernzentrum Stationen Vogel und Reptil. Available online: https://www.vetmed.uni-leipzig.de/praktisches-ausbildungs-und-lernzentrum/studium/stationen/stationen-vogel-und-reptil (accessed on 8 April 2024).
- Freie Universität Berlin Fachbereich Veterinärmedizin VETERINARY SKILLS NET Kursangebot. Available online: https://www.vetmed.fu-berlin.de/studium/skills-net/Lehrangebot/Kursangebot/index.html (accessed on 8 April 2024).
- Ludwig-Maximilians-Universität München Tierärztliche Fakultät Kursangebot. Available online: https://www.vetmed.uni-muenchen.de/lehre_vet/vet-skills-lab/kursangebot/index.html (accessed on 8 April 2024).
- TiHo Moodle CSL|Reptilien. Available online: https://moodle.tiho-hannover.de/course/view.php?id=796 (accessed on 8 April 2024).
- YouTube TiHoVideos CSL: Reptilienstationen. Available online: https://www.youtube.com/playlist?list=PLBw3-u7k6HwaU6F1nlJCBU6SCuAMmO1xG (accessed on 8 April 2024).
- Erler Zimmer Weitere Tiere. Available online: https://erler-zimmer.de/Veterinaer-Medizin/weitere-Tiere/ (accessed on 8 April 2024).
- VSI Veterinary Simulator Industries 2024 Product Catalogue. Available online: https://static1.squarespace.com/static/63030b3f3769717a3af5459c/t/661449aeb79f2a148d6f146f/1712605633700/VSI+Brochure+2024+Version+-+FINAL+April+8%2C+2024+-+No+Prices+WEB.pdf (accessed on 4 June 2024).
- Holsim Animal Care & Education SHOP. Available online: https://de.holsim.co.nz/shop (accessed on 22 April 2024).
- Veteffects. Available online: https://veteffects.com/products.htm (accessed on 22 April 2024).
- Rescue Critters Dissection Models. Available online: https://rescuecritters.com/product-category/dissection-models/ (accessed on 22 April 2024).
- Rescue Critters Bone Clones®. Available online: https://rescuecritters.com/product-category/accessories/bone-clones/ (accessed on 22 April 2024).
- Simulaids Search Results for: Veterinary. Available online: https://simulaids.co.uk/?s=dog&jet_ajax_search_settings=%7B%22search_source%22%3A%5B%22page%22%2C%22product%22%5D%2C%22exclude_posts_ids%22%3A%5B%226711%22%2C%226735%22%2C%226753%22%2C%226759%22%2C%2227%22%5D%2C%22custom_fields_source%22%3A%22_sku%22%2C%22results_order_by%22%3A%22relevance%22%2C%22results_order%22%3A%22asc%22%2C%22search_in_taxonomy%22%3A%22yes%22%7D&post_type=page%2Cproduct (accessed on 4 June 2024).
- Realityworks Home/Agriculture/Animal & Veterinary Sciences. Available online: https://www.realityworks.com/product-category/agriculture/animal-veterinary-sciences/?showall=1&v=3a52f3c22ed6 (accessed on 22 April 2024).
- Ruth Lee Dog/Canine Training Manikin. Available online: https://www.ruthlee.com/manikin/dog-manikin (accessed on 22 April 2024).
- Anatomage TableVet. Available online: https://anatomage.com/table-vet/ (accessed on 4 June 2024).
- Hung, T.-F.; Kuo, P.-J.; Tsai, F.-S.; Yu, P.-H.; Nai, Y.-S. A Novel Application of 3D Printing Technology Facilitating Shell Wound Healing of Freshwater Turtle. Animals 2022, 12, 966. [Google Scholar] [CrossRef] [PubMed]
- Blake, C.; Birch, S.; Brandão, J. Medical Three-dimensional printing in zoological medicine. Vet. Clin. N. Am. Exot. Anim. Pract. 2019, 22, 331–348. [Google Scholar] [CrossRef] [PubMed]
- Bulté, G.; Chlebak, R.J.; Dawson, J.W.; Blouin-Demers, G. Studying mate choice in the wild using 3D printed decoys and action cameras: A case of study of male choice in the northern map turtle. Anim. Behav. 2018, 138, 141–143. [Google Scholar] [CrossRef]
- Clark, J.; Clark, C.; Higham, T.E. Tail Control Enhances Gliding in Arboreal Lizards: An Integrative Study Using a 3D Geometric Model and Numerical Simulation. Integr. Comp. Biol. 2021, 62, 579–588. [Google Scholar] [CrossRef]
- Tetzlaff, S.J.; Estrada, A.; DeGregorio, B.A.; Sperry, J.H. Identification of Factors Affecting Predation Risk for Juvenile Turtles Using 3D Printed Models. Animals 2020, 10, 275. [Google Scholar] [CrossRef]
- Song, S.-H.; Kim, M.-S.; Rodrigue, H.; Lee, J.-Y.; Shim, J.-E.; Kim, M.-C.; Chu, W.-S.; Ahn, S.-H. Turtle mimetic soft robot with two swimming gaits. Bioinspir. Biomim. 2016, 11, 036010. [Google Scholar] [CrossRef]
- du Plessis, A.; Broeckhoven, C.; le Roux, S.G. Snake fangs: 3D morphological and mechanical analysis by microCT, simulation, and physical compression testing. GigaScience 2017, 7, 8. [Google Scholar] [CrossRef]
- Holliday, C.M.; Tsai, H.P.; Skiljan, R.J.; George, I.D.; Pathan, S. A 3D interactive model and atlas of the jaw musculature of Alligator mississippiensis. PLoS ONE 2013, 8, e62806. [Google Scholar] [CrossRef]
- Kowalska, M.; Rupik, W. Architecture of the Pancreatic Islets and Endocrine Cell Arrangement in the Embryonic Pancreas of the Grass Snake (Natrix natrix L.). Immunocytochemical Studies and 3D Reconstructions. Int. J. Mol. Sci. 2021, 22, 7601. [Google Scholar] [CrossRef]
- Arencibia, A.; Melian, A.; Oros, J. Anatomic Interactive Atlas of the Loggerhead Sea Turtle (Caretta caretta) Head. Animals 2021, 11, 198. [Google Scholar] [CrossRef]
- Bandura, A. Self-efficacy: Toward a unifying theory of behavioral change. Psychol. Rev. 1977, 84, 191. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C. Untersuchung zum Transfer-Denken von Studierenden der Tiermedizin im Rahmen einer Strukturierten Simulationsbasierten Ausbildung. Ph.D. Thesis, Stiftung Tierärztliche Hochschule Hannover, Hannover, Germany, 2022. [Google Scholar]
- Muehlberg, J.; Tipold, A.; Heppelmann, M.; Wissing, S. Simulator-Assisted Training of Abomasal Surgery—A Pilot Study Using Blended Learning and Face-to-Face Teaching. Animals 2023, 13, 3822. [Google Scholar] [CrossRef] [PubMed]
- Preece, D.; Williams, S.B.; Lam, R.; Weller, R. ’Let’s Get Physical’: Advantages of a physical model over 3D computer models and textbooks in learning imaging anatomy. Anat. Sci. Educ. 2013, 6, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, B.A.; Dos Santos, A.C.; Da Silveira, E.E.; Pereira, H.C.S.; Lisboa Neto, A.F.S.; Alcobaça, M.M.O.; Assis Neto, A.C. Three-Dimensional Digitalized and Printed Tongue Models of the Cow, Dog, Pig, and Horse for Undergraduate Veterinary Education. Int. J. Morphol. 2021, 39, 436–440. [Google Scholar] [CrossRef]
- Lim, K.H.; Loo, Z.Y.; Goldie, S.J.; Adams, J.W.; McMenamin, P.G. Use of 3D printed models in medical education: A randomized control trial comparing 3D prints versus cadaveric materials for learning external cardiac anatomy. Anat. Sci. Educ. 2016, 9, 213–221. [Google Scholar] [CrossRef]
- Kocyigit, A.A.; Hasan, H.; Uslu, B.A. An Insight into Veterinary Students’ Perceptions on the use of 3D-Printed Bone Biomodels in Anatomy Learning. Slov. Vet. Res. 2023, 60, 160–166. [Google Scholar] [CrossRef]
- Hackmann, H.; De Alcântara Leite dos Reis, C.; De Assis Neto, D.; Chaves, A. Digital Revolution in Veterinary Anatomy: Confection of Anatomical Models of Canine Stomach by Scanning and Three-Dimensional Printing (3D). Int. J. Morphol. 2019, 37, 486–490. [Google Scholar] [CrossRef]
- Justiz, B.d. Tierschutzgesetz. Available online: https://www.gesetze-im-internet.de/tierschg/BJNR012770972.html (accessed on 28 February 2025).
- Ultimaker Tough PLA Safety Data Sheet. Available online: https://um-support-files.ultimaker.com/materials/2.85mm/sds/TOUGH-PLA/UM_Tough%20PLA_EU_en_SDSv2.1.pdf (accessed on 2 August 2024).
- Knof, H.; Berndt, M.; Shiozawa, T. Prevalence of Dunning-Kruger effect in first semester medical students: A correlational study of self-assessment and actual academic performance. BMC Med. Educ. 2024, 24, 1210. [Google Scholar] [CrossRef]
- Ehrich, R.A. Untersuchungen zum Erlernen der endoskopischen Untersuchung der oberen Atemwege beim Pferd. Ph.D. Thesis, Stiftung Tierärztliche Hochschule Hannover, Hannover, Germany, 2021. [Google Scholar]
- Bainier, M.; Su, A.; Redondo, R.L. 3D printed rodent skin-skull-brain model: A novel animal-free approach for neurosurgical training. PLoS ONE 2021, 16, e0253477. [Google Scholar] [CrossRef]
- Li, H.; Fan, W.; Zhu, X. Three-dimensional printing: The potential technology widely used in medical fields. J. Biomed. Mater. Res. 2018, 108, 2217–2229. [Google Scholar] [CrossRef]
- Raddatz, L.; Austerjost, J.; Beutel, S. 3D-Druck: Chancen, Möglichkeiten, Risiken. ChiuZ 2018, 52, 42–50. [Google Scholar] [CrossRef]
- YIigör Hurİ, P.; Oto, Ç. 3D Printing in Veterinary Medicine. Ankara Univ. Vet. Fak. Derg. 2022, 69, 111–117. [Google Scholar] [CrossRef]
- Equine Dental Instruments Replacement Teeth Set for Equine Skull Model, Set of 4 Item # 5390. Available online: https://equinedentalinstruments.com/collections/educational/products/replacement-teeth-set-for-equine-skull-model (accessed on 4 November 2024).
- Rodrigues, M.C.; Lima, W.C.; Quessada, A.M.; Silva, F.A.N.; Silva, L.M.C.; de Souza, A.B.; de Moura, C.R.C.; Lima, D.A.S.D. Celiotomy by plastrotomy in a yellow-footed tortoise (Geochelone denticulata). Pesq. Vet. Bras. 2015, 35, 173–176. [Google Scholar] [CrossRef]

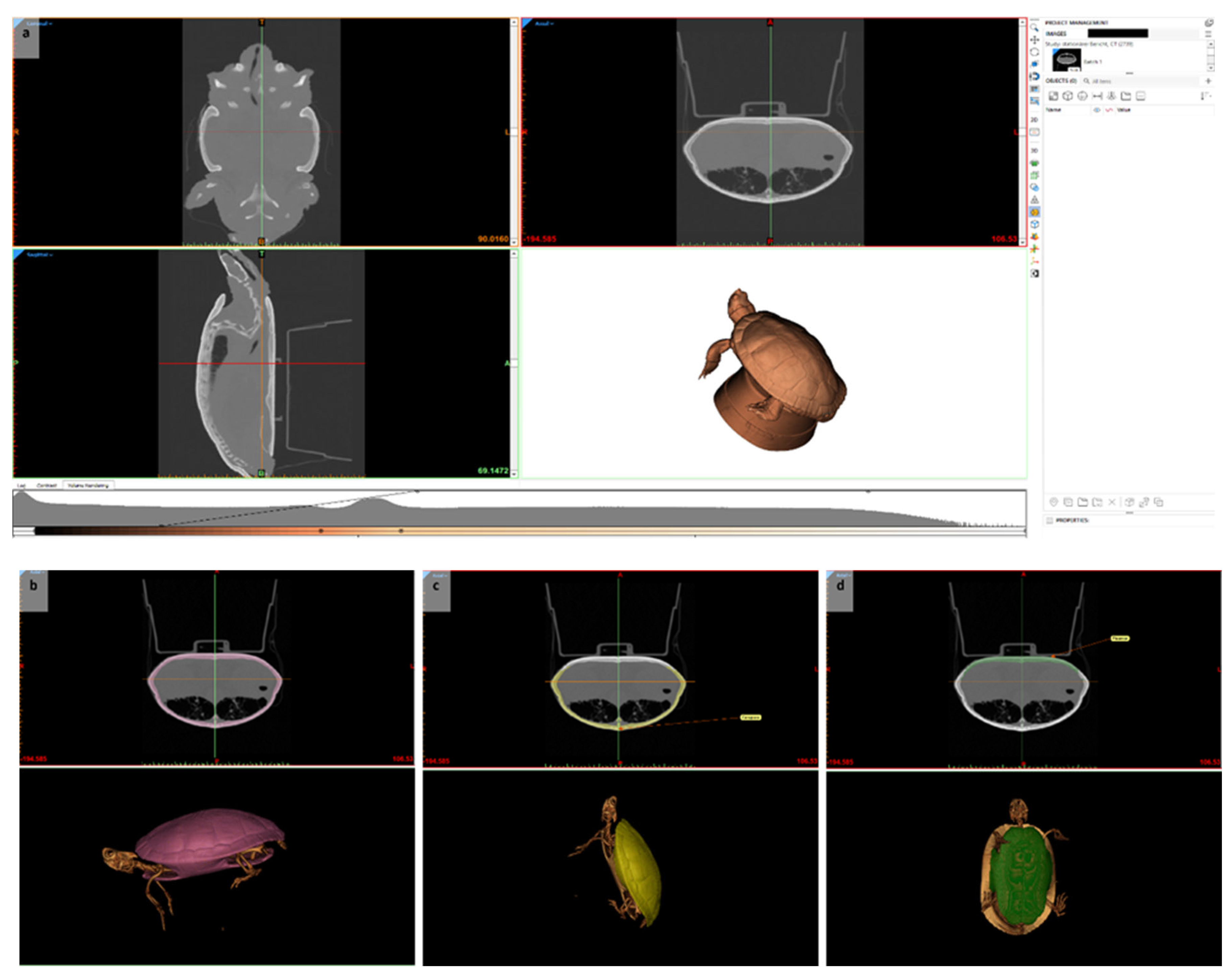


| Group | Professional Training as a Veterinary Assistant | Experience with Reptiles in Private Life | Attendance of Any Courses at the Clinical Skills Lab | Attendance of Elective “Reptile Surgery” | Theoretical Education on Reptile Handling and Diseases During Study Course | Hands-On Experience with Reptiles During Study Course |
|---|---|---|---|---|---|---|
| 1 | 26% | 16% | 73% | 0% | 100% | 80% |
| 2 | 0% | 14% | 78% | 0% | 100% | 92% |
| Group | Coeliotomy with Veterinary Guidance | Coeliotomy Without Veterinary Guidance | Plastrotomy with Veterinary Guidance | Plastrotomy Without Veterinary Guidance | ||
| 1 | 60% | 14% | 16% | 0% | ||
| 2 | 78% | 0% | 0% | 0% | ||
| Group | n | OSCE Result Mean Value | OSCE Result Standard Deviation | OSCE Result Median | OSCE Result Minimum | OSCE Result Maximum | OSCE Result Mean Score | Significance |
|---|---|---|---|---|---|---|---|---|
| 1 | 15 | 78% | 21.66 | 83% | 45% | 98% | 15.7 | p = 0.3933 |
| 2 | 13 | 72% | 21.66 | 73% | 25% | 92% | 13.0 | p = 0.3933 |
| Group | ST Max. | ST Min. | ST Median | ST Standard Deviation | OSCE Result Min. | OSCE Result Max. | OSCE Result Median | Correlation Spearman | Significance |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.6 | 0.3 | 0.4 | 0.11 | 45% | 98% | 83% | −0.31879 | p = 0.3693 |
| 2 | 0.6 | 0.3 | 0.4 | 0.10 | 25% | 92% | 73% | −0.39789 | p = 0.2889 |
| Item | Group | n | SE Mean Score | SE Standard Deviation | SE Sum of Scores | Significance |
|---|---|---|---|---|---|---|
| 7 | 1 | 15 | 12.20 | 19.81 | 183.0 | p = 0.0362 |
| 7 | 2 | 14 | 18 | 252.0 | ||
| 12 | 1 | 15 | 12.50 | 13.16 | 187.50 | p = 0.0250 |
| 12 | 2 | 13 | 16.08 | 218.50 | ||
| 13 | 1 | 15 | 11.70 | 19.27 | 175.50 | p = 0.0313 |
| 13 | 2 | 13 | 17.73 | 230.50 |
| Group | SE t2 Maximum (Sum of Points) | SE t2 Minimum (Sum of Points) | SE t2 Mean Value (Sum of Points) | SE t2 Median (Sum of Points) | SE t2 Standard Deviation | OSCE Result Median | Correlation Spearman | Significance |
|---|---|---|---|---|---|---|---|---|
| 1 | 33 | 19 | 25 | 24 | 4.3 | 83% | 0.14452 | p = 0.6073 |
| 2 | 42 | 20 | 28 | 27 | 6.4 | 73% | −0.25659 | p = 0.3974 |
| Desired Learning Resource | Group 1 | Group 2 | Expert Group |
|---|---|---|---|
| S1 | 33% | 15% | 60% |
| S2 | 0% | 0% | 0% |
| Combination of S1 and S2 | 67% | 85% | 40% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knoll, M.-T.; Tipold, A.; Pees, M.; Wissing, S.; Hetterich, J. Come out of Your Shell—A Comparative Pilot Study for Teaching the Central Plastrotomy in Chelonians Using a 3D-Printed Simulator and a Virtual 3D Simulation. Animals 2025, 15, 824. https://doi.org/10.3390/ani15060824
Knoll M-T, Tipold A, Pees M, Wissing S, Hetterich J. Come out of Your Shell—A Comparative Pilot Study for Teaching the Central Plastrotomy in Chelonians Using a 3D-Printed Simulator and a Virtual 3D Simulation. Animals. 2025; 15(6):824. https://doi.org/10.3390/ani15060824
Chicago/Turabian StyleKnoll, Marie-Therese, Andrea Tipold, Michael Pees, Sandra Wissing, and Johannes Hetterich. 2025. "Come out of Your Shell—A Comparative Pilot Study for Teaching the Central Plastrotomy in Chelonians Using a 3D-Printed Simulator and a Virtual 3D Simulation" Animals 15, no. 6: 824. https://doi.org/10.3390/ani15060824
APA StyleKnoll, M.-T., Tipold, A., Pees, M., Wissing, S., & Hetterich, J. (2025). Come out of Your Shell—A Comparative Pilot Study for Teaching the Central Plastrotomy in Chelonians Using a 3D-Printed Simulator and a Virtual 3D Simulation. Animals, 15(6), 824. https://doi.org/10.3390/ani15060824





