In Vitro Assessment of the Effectiveness of Mineral Adsorbents in Sequestering Boar Taint Compounds
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Adsorbents and Adsorbates
2.2. Dose–Response Curves and Adsorption Assays
2.3. Michaelis–Menten Data Analyses
2.4. Langmuir and Freundlich Isotherm Adsorption Model Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prunier, A.; Bonneau, M.; von Borell, E.H.; Cinotti, S.; Gunn, M.; Fredriksen, B.; Giersing, M.; Morton, D.B.; Tuyttens, F.A.M.; Velarde, A. A review of the welfare consequences of surgical castration in piglets and the evaluation of non-surgical methods. Anim. Welf. 2006, 15, 277–289. [Google Scholar]
- Squires, E.J.; Bone, C.; Cameron, J. Pork production with entire males: Directions for control of boar taint. Animals 2020, 10, 1665. [Google Scholar] [CrossRef]
- Weiler, U.; Font-i-Furnols, M.; Tomasevic, I.; Bonneau, M. Alternatives to piglet castration: From issues to solutions. Animals 2021, 11, 1041. [Google Scholar] [CrossRef]
- Sinclair, P.A.; Squires, E.J. Testicular sulfoconjugation of the 16-androstene steroids by hydroxysteroid sulfotransferase: Its effect on the concentrations of 5alpha-androstenone in plasma and fat of the mature domestic boar. J. Anim. Sci. 2005, 83, 358–365. [Google Scholar]
- Laderoute, H.; Bone, C.; Squires, E.J. The sulfoconjugation of androstenone and dehydroepiandrosterone by human and porcine sulfotransferase enzymes. Steroids 2018, 136, 8–16. [Google Scholar]
- Okour, M.; Brundage, R.C. Modeling enterohepatic circulation. Curr. Pharmacol. Rep. 2017, 3, 301–313. [Google Scholar]
- Jen, K.; Squires, E.J. Efficacy of non-nutritive sorbent materials as intestinal-binding agents for the control of boar taint. Animal 2011, 5, 1814–1820. [Google Scholar]
- Yi, S.W.; Lee, H.G.; So, K.M.; Kim, E.; Jung, Y.H.; Kim, M.; Jeong, J.Y.; Kim, K.H.; Oem, J.K.; Hur, T.Y.; et al. Effect of feeding raw potato starch on the composition dynamics of the piglet intestinal microbiome. Anim. Biosci. 2022, 35, 1698–1710. [Google Scholar]
- Li, X.; Jensen, B.B.; Canibe, N. The mode of action of chicory roots on skatole production in entire male pigs is neither via reducing the population of skatole-producing bacteria nor increased butyrate production in the hindgut. Appl. Environ. Microbiol. 2019, 85, e023278. [Google Scholar]
- Jang, Y.N.; Jung, M.W. Biochemical changes and biological origin of key odor compound generations in pig slurry during indoor storage periods: A pyrosequencing approach. BioMed Res. Int. 2018, 2018, 3503658. [Google Scholar]
- Knarreborg, A.; Beck, J.; Jensen, M.T.; Laue, A.; Agergaard, N.; Jensen, B.B. Effect of non-starch polysaccharides on production and absorption of indolic compounds in entire male pigs. Anim. Sci. 2002, 74, 445–453. [Google Scholar]
- Wesoly, R.; Weiler, U. Nutritional influences on skatole formation and skatole metabolism in the pig. Animals 2012, 2, 221–242. [Google Scholar] [CrossRef]
- Tambyrajah, W.S.; Doran, E.; Wood, J.D.; McGivan, J.D. The pig CYP2E1 promoter is activated by COUP-TF1 and HNF-1 and is inhibited by androstenone. Arch. Biochem. Biophys. 2004, 431, 252–260. [Google Scholar]
- Di Gregorio, M.C.; de Neeff, D.V.; Jager, A.V.; Corassin, C.H.; de Pinho Carão, Á.C.; de Albuquerque, R.; de Azevedo, A.C.; Oliveira, C.A.F. Mineral adsorbents for prevention of mycotoxins in animal feeds. Toxin Rev. 2014, 33, 125–135. [Google Scholar]
- Čolović, R.; Puvača, N.; Cheli, F.; Avantaggiato, G.; Greco, D.; Đuragić, O.; Kos, J.; Pinotti, L. Decontamination of mycotoxin-contaminated feedstuffs and compound feed. Toxins 2019, 11, 617. [Google Scholar] [CrossRef]
- Malekinejad, H.; Maas-Bakker, R.; Fink-Gremmels, J. Species differences in the hepatic biotransformation of zearalenone. Vet. J. 2006, 172, 96–102. [Google Scholar]
- Devreese, M.; De Backer, P.; Croubels, S. Different methods to counteract mycotoxin production and its impact on animal health. Vlaams Diergeneeskd. Tijdschr. 2013, 82, 181–190. [Google Scholar]
- Solfrizzo, M.; Carratu, M.R.; Avantaggiato, G.; Galvano, F.; Pietri, A.; Visconti, A. Ineffectiveness of activated carbon in reducing the alteration of sphingolipid metabolism in rats exposed to fumonisin-contaminated diets. Food Chem. Toxicol. 2001, 39, 507–511. [Google Scholar]
- Choy, J.H.; Choi, S.J.; Oh, J.M.; Park, T. Clay minerals and layered double hydroxides for novel biological applications. Appl. Clay Sci. 2007, 36, 122–132. [Google Scholar]
- Lee, J.; Fratta, D.; Palomino, A.M. The effect of diatom content on the physical, electrical, and mechanical properties of soils. Acta Geotech. 2024, 19, 2251–2271. [Google Scholar]
- Reka, A.A.; Pavlovski, B.; Fazlija, E.; Berisha, A.; Pacarizi, M.; Daghmehchi, M.; Sacalis, C.; Jovanovski, G.; Makreski, P.; Oral, A. Diatomaceous Earth: Characterization, thermal modification, and application. Open Chem. 2021, 19, 451–461. [Google Scholar]
- Jen, K.; Squires, E.J. In vitro assessment of the effectiveness of non-nutritive sorbent materials as binding agents for boar taint compounds. Animal 2011, 5, 1821–1828. [Google Scholar] [PubMed]
- Lanthier, F.; Lou, Y.; Squires, E.J. Skatole metabolism in the intact pre-pubescent male pig: The relationship between hepatic enzyme activity and skatole concentrations in plasma and fat. Livest. Sci. 2007, 106, 145–153. [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar]
- Ho, Y.S.; Porter, J.F.; McKay, G. Equilibrium isotherm studies for the sorption of divalent metal ions onto peat: Copper, nickel and lead single component systems. Water Air Soil Pollut. 2002, 141, 1–33. [Google Scholar]
- Mukasa-Tebandeke, I.; Ssebuwufu, P.; Nyanzi, S.; Nyakairu, G.; Ntale, M.; Lugolobi, F.; Andreas, S. Adsorption behavior of acid-leached clays in bleaching of oil. Am. J. Anal. Chem. 2015, 6, 495–512. [Google Scholar]
- Bolster, C.H.; Hornberger, G.M. On the use of linearized Langmuir equations. Soil Sci. Soc. Am. J. 2007, 71, 1796–1806. [Google Scholar]
- Pinto, R.P.; Mata, F.; Pires, P.; Barros, M.; Araújo, J.P.; Vaz-velho, M. The use of sugar beet pulp in pig diet to control skatole analysed by HPLC. Sci. Agric. 2023, 80, e20220093. [Google Scholar]
- Aly, S.E.; Abdel-Galil, M.M.; Abdel-Wahhab, M.A. Application of adsorbent agents technology in the removal of aflatoxin B1 and fumonisin B1 from malt extract. Food Chem. Toxicol. 2004, 42, 1825–1831. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surface of glass, mica and platinum. J. Am. Chem. Soc. 1916, 40, 1361–1403. [Google Scholar]
- Sohn, S.; Kim, D. Modification of Langmuir isotherm in solution systems—Definition and utilization of concentration dependent factor. Chemosphere 2005, 58, 115–123. [Google Scholar] [PubMed]
- Galvano, F.; Pietri, A.; Bertuzzi, T.; Bognanno, M.; Chies, L.; de Angelis, A.; Galvano, M. Activated carbons: In vitro affinity for fumonisin B1 and relation of adsorption ability to physicochemical parameters. J. Food Prot. 1997, 60, 985–991. [Google Scholar] [PubMed]
- Schubert, D.C.; Chuppava, B.; Witte, F.; Terjung, N.; Visscher, C. Evaluation of coated biochar as an intestinal binding agent for skatole and indole in male intact finishing pigs. Animals 2021, 11, 760. [Google Scholar] [CrossRef] [PubMed]
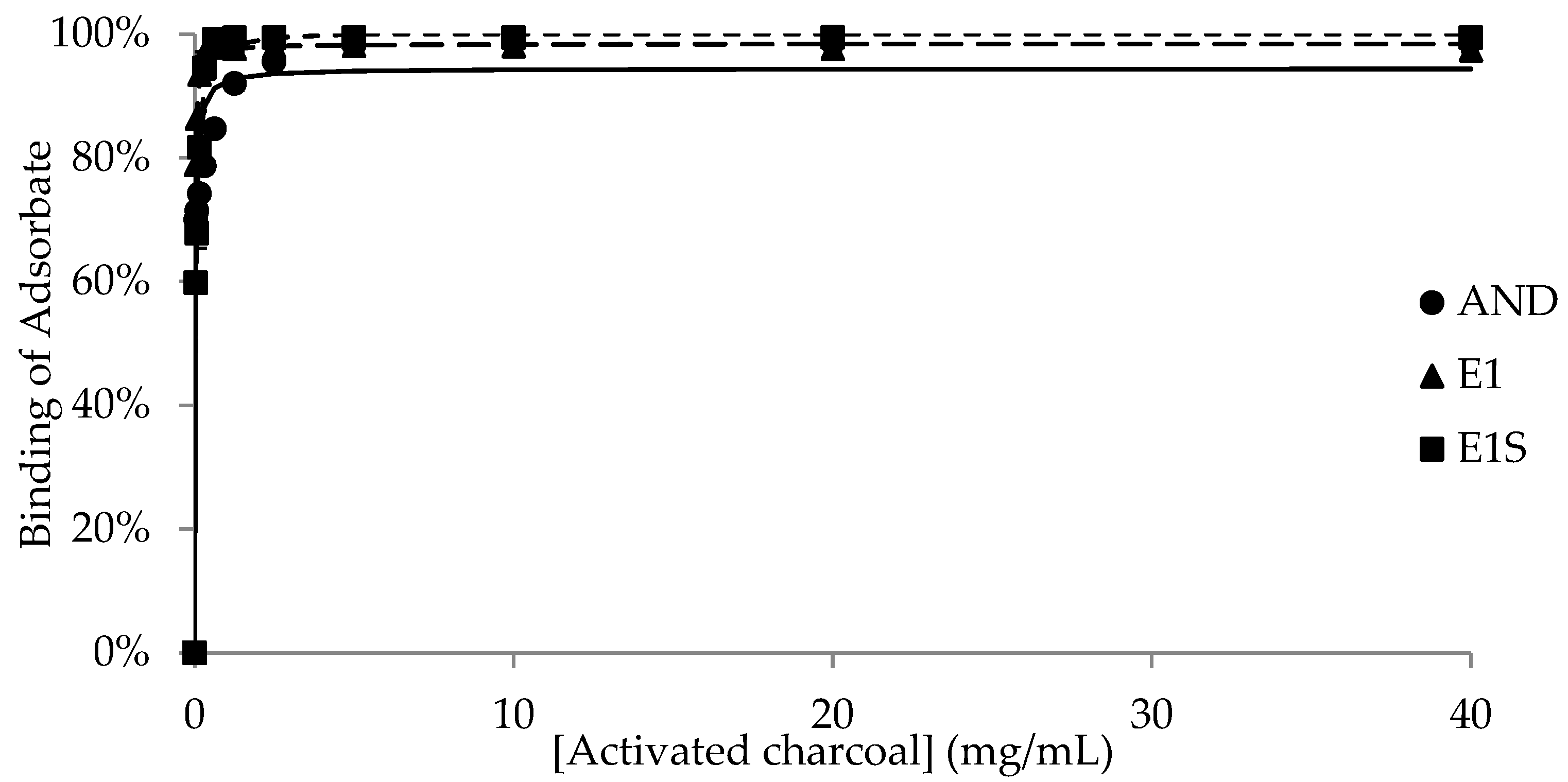
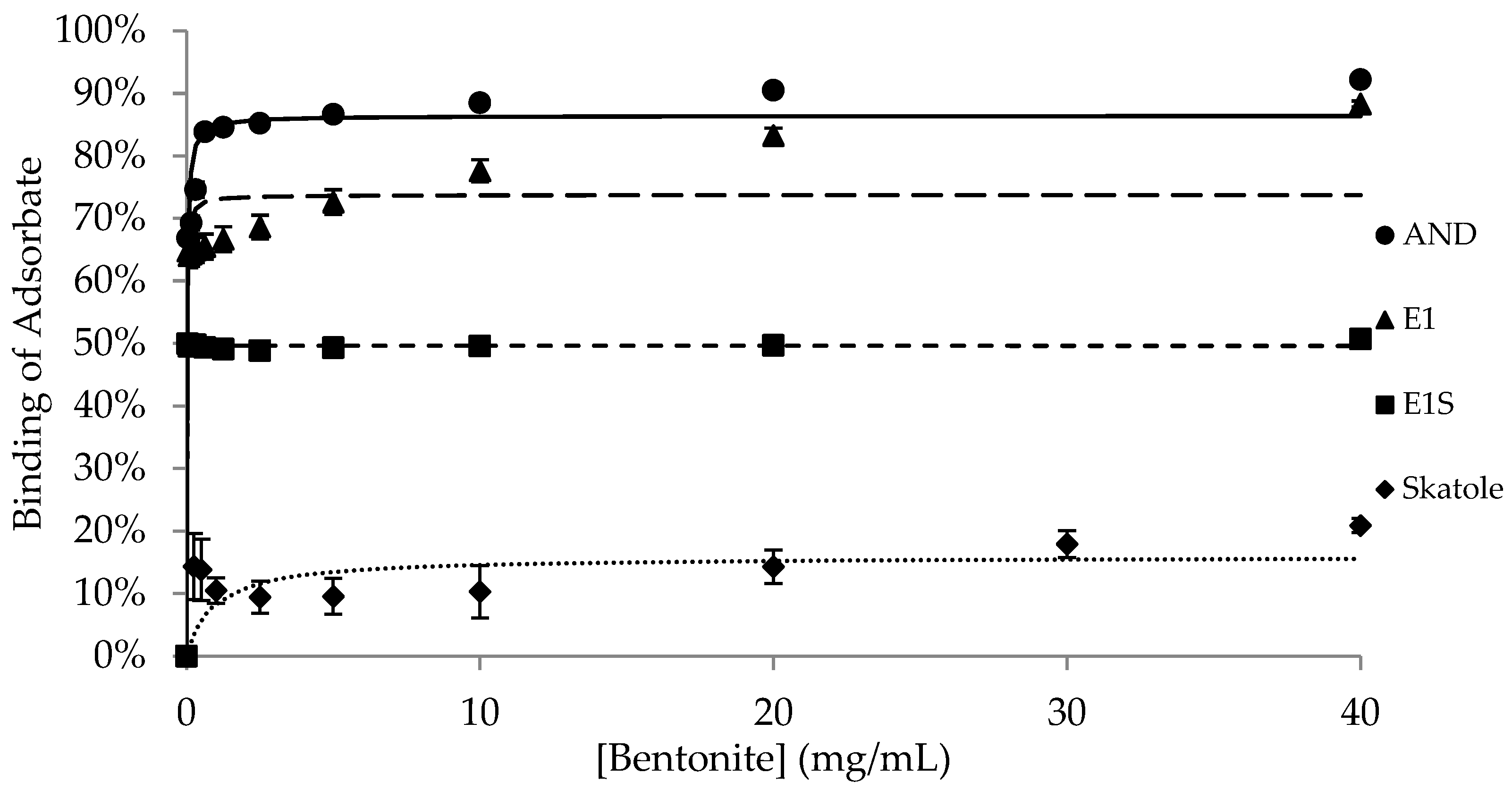
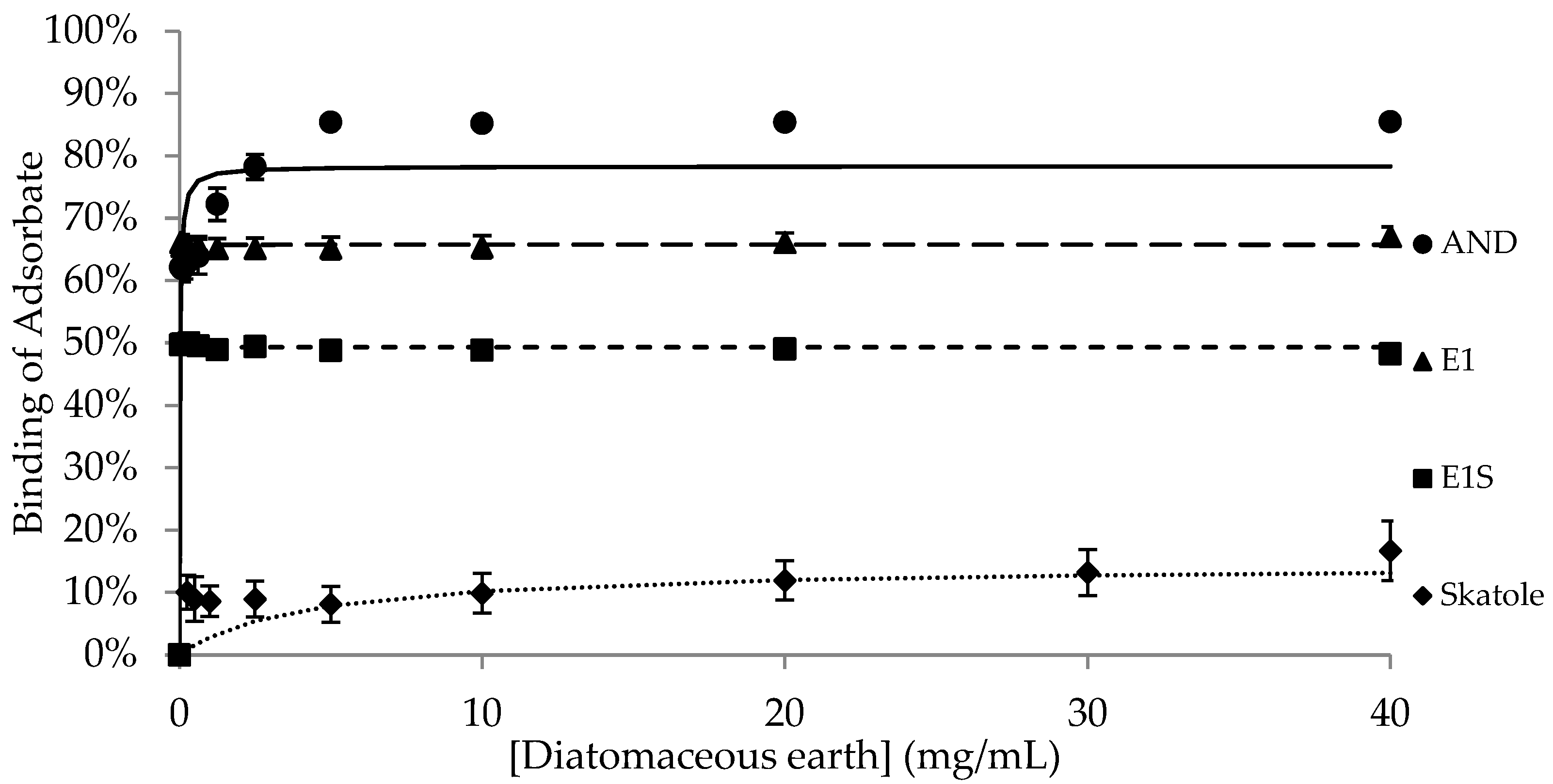

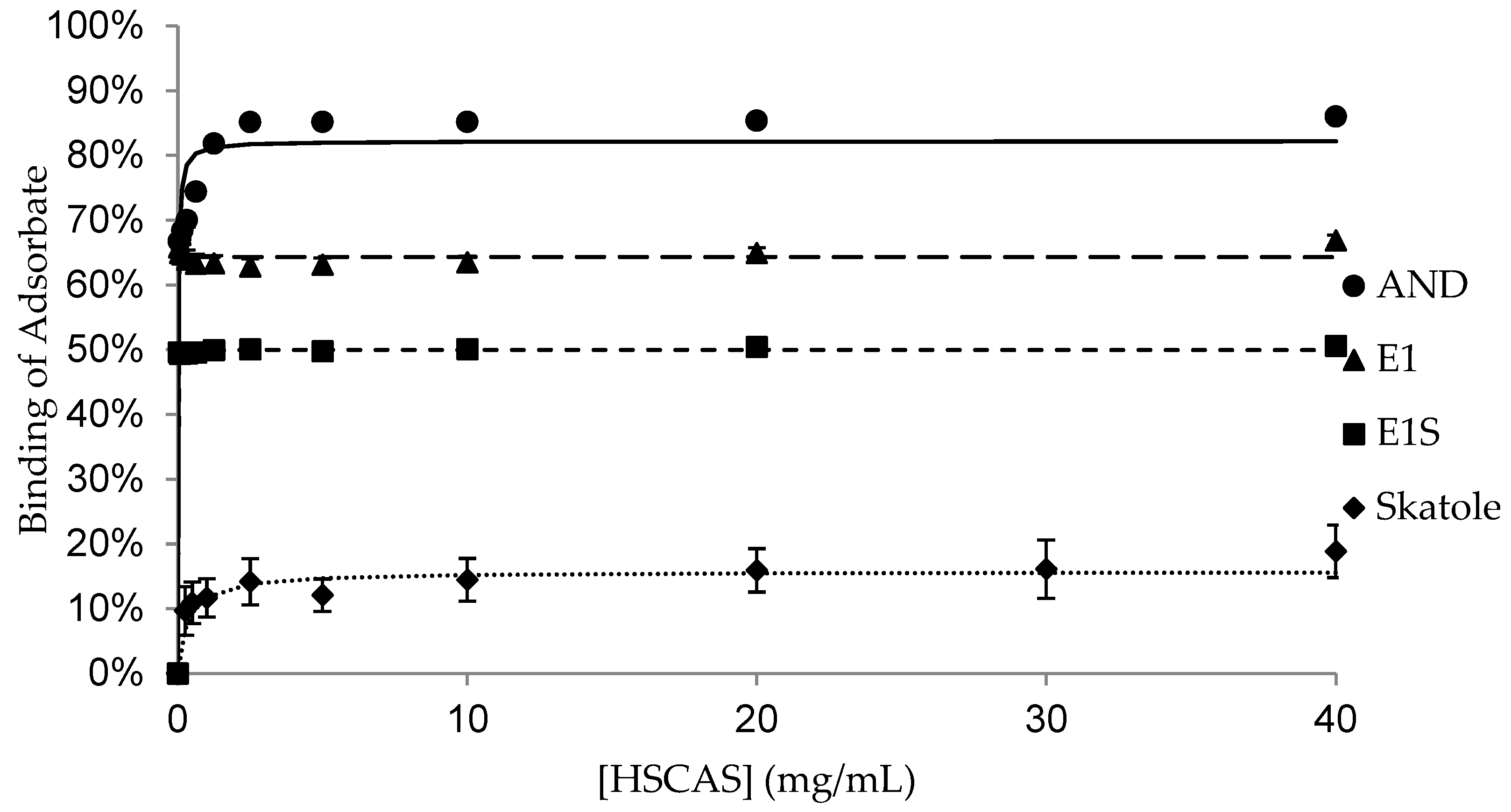
| Adsorbent | |||||
|---|---|---|---|---|---|
| Parameter | BNT | DE | SFA | HSCAS | AC |
| AND | |||||
| Bmax | 77.7 ± 1.12 a,1 | 71.9 ± 1.93 a,1 | 55.0 ± 7.85 b,1 | 69.5 ± 1.44 ab,1 | 95.2 ± 1.39 c,1 |
| K | 0.1 ± 0.03 | 0.8 ± 0.43 | 0.8 ± 0.49 | 0.1 ± 0.05 | 0.1 ± 0.003 1 |
| E1 | |||||
| Bmax | 62.9 ± 7.69 a,1 | 2.6 ± 1.68 b,2 | 62.5 ± 8.29 a,1 | 0.3 ± 0.22 b,2 | 97.3 ± 0.86 c,1 |
| K | 2.3 ± 2.20 | 1.7 ± 1.68 | 1.1 ± 1.02 | N/D § | 0.03 ± 0.003 2 |
| E1S | |||||
| Bmax | 4.3 ± 1.33 a,2 | 0.4 ± 0.25 a,2 | 2.9 ± 2.59 a,2 | 0.8 ± 0.46 a,2 | 103.7 ± 0.35 b,2 |
| K | 26.5 ± 13.73 | 0.02 ± 0.02 | 4.4 ± 4.35 | 0.2 ± 0.15 | 0.1 ± 0.02 1 |
| Skatole | |||||
| Bmax | 15.9 ± 1.55 a,2 | 14.5 ± 3.82 a,3 | 89.9 ± 1.09 b,3 | 15.7 ± 3.24 a,3 | N/D ‡ |
| K | 0.9 ± 0.83 | 4.2 ± 4.15 | 1.8 ± 0.41 | 0.3 ± 0.13 | N/D ‡ |
| LANGMUIR | FREUNDLICH | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Adsorbent | Adsorbate | K1 (mL/mg) | K2 (%) | RSS | AIC | a | KF (%) | RSS | AIC |
| BNT | AND | 9.10 | 77.27 | 0.04 | −52.43 | 0.12 | 58.12 | 0.05 | −49.86 |
| E1 | 7.62 | 52.84 | 0.18 | −35.69 | 0.18 | 37.00 | 0.03 | −57.43 | |
| Skatole | 140.01 | 13.53 | 0.01 | −48.30 | 0.09 | 11.60 | 0.01 | −50.95 | |
| DE | AND | 1.95 | 71.52 | 0.09 | −44.03 | 0.19 | 41.12 | 0.06 | −47.33 |
| Skatole | 10.28 | 11.46 | 0.01 | −55.81 | 0.12 | 8.85 | 0.003 | −62.02 | |
| SFA | AND | 10.70 | 46.63 | 0.20 | −34.76 | 0.18 | 33.71 | 0.04 | −52.14 |
| E1 | 5.14 | 54.59 | 0.18 | −35.78 | 0.20 | 35.18 | 0.03 | −55.74 | |
| Skatole | 0.58 | 89.70 | 0.01 | −51.03 | 0.26 | 36.52 | 0.04 | −37.82 | |
| HSCAS | AND | 9.26 | 69.11 | 0.05 | −49.49 | 0.12 | 52.46 | 0.05 | −50.67 |
| Skatole | 4.66 | 15.67 | 0.002 | −63.34 | 0.11 | 11.51 | 0.001 | −73.10 | |
| AC | AND | 7.89 | 95.17 | 0.06 | −47.08 | 0.12 | 70.30 | 0.08 | −44.76 |
| E1 | 41.27 | 97.17 | 0.004 | −76.55 | 0.04 | 88.01 | 0.08 | −45.21 | |
| E1S | 9.57 | 103.56 | 0.04 | −51.91 | 0.11 | 78.16 | 0.37 | −27.94 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Squires, J. In Vitro Assessment of the Effectiveness of Mineral Adsorbents in Sequestering Boar Taint Compounds. Animals 2025, 15, 765. https://doi.org/10.3390/ani15060765
Park S, Squires J. In Vitro Assessment of the Effectiveness of Mineral Adsorbents in Sequestering Boar Taint Compounds. Animals. 2025; 15(6):765. https://doi.org/10.3390/ani15060765
Chicago/Turabian StylePark, Sanghyuk, and James Squires. 2025. "In Vitro Assessment of the Effectiveness of Mineral Adsorbents in Sequestering Boar Taint Compounds" Animals 15, no. 6: 765. https://doi.org/10.3390/ani15060765
APA StylePark, S., & Squires, J. (2025). In Vitro Assessment of the Effectiveness of Mineral Adsorbents in Sequestering Boar Taint Compounds. Animals, 15(6), 765. https://doi.org/10.3390/ani15060765






