A New Research Tool for Use in Sharks and Rays: Relevance of Reproductive Hormone Levels in the Skin of Small-Spotted Catshark (Scyliorhinus canicula)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sampling
2.2. Hormone Extraction
2.3. Hormone Analysis and Assay Validation
2.4. Statistical Analysis
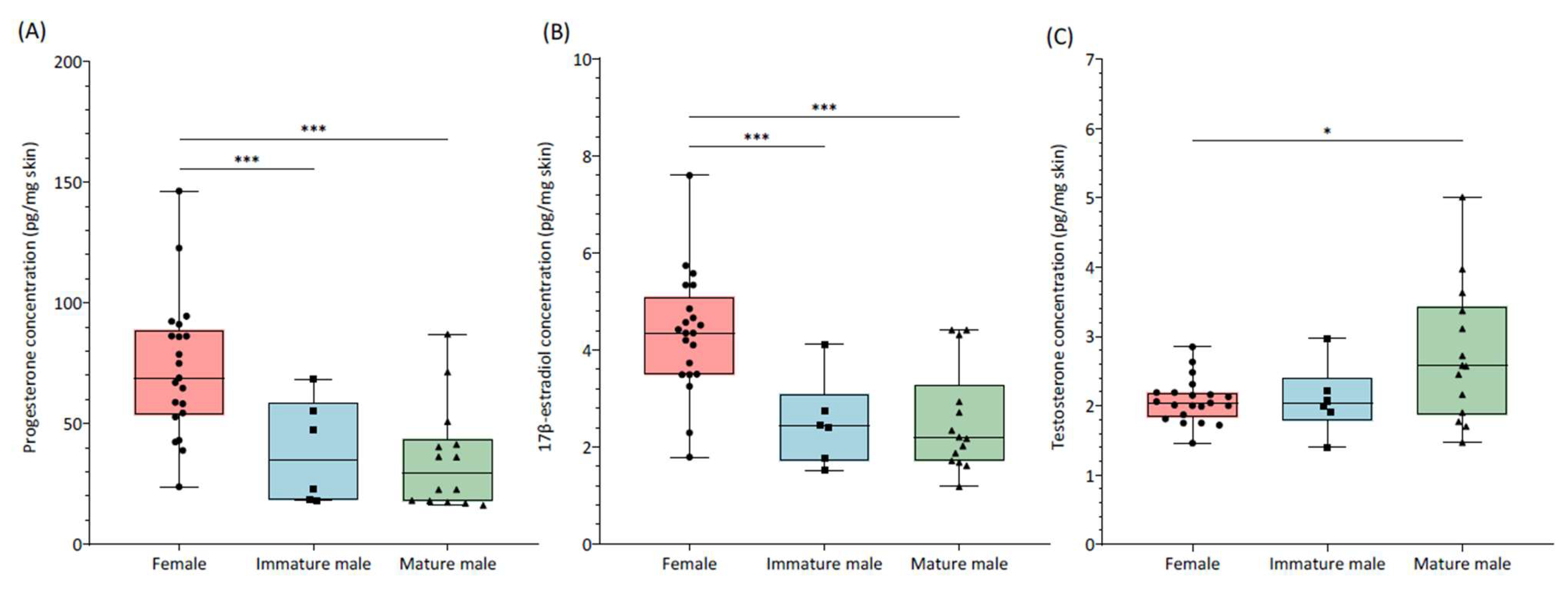
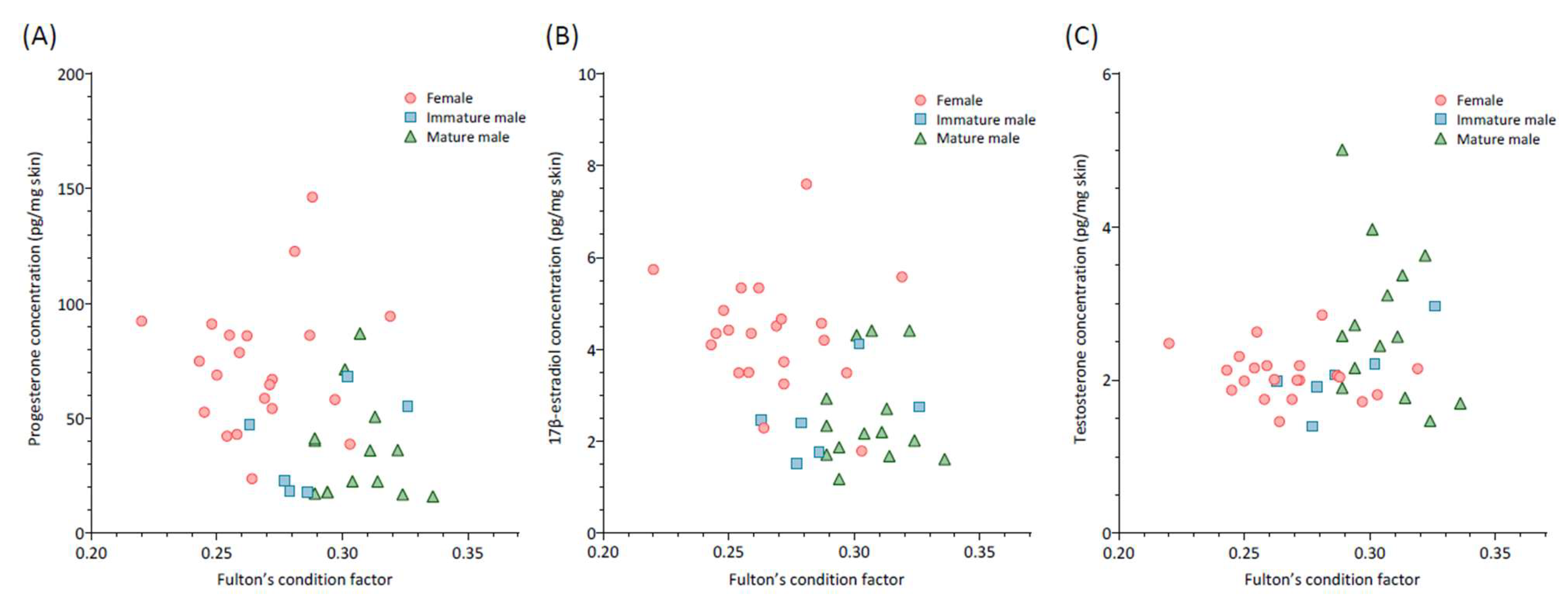
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EIA | Enzyme immunoassay |
| CV | Coefficient of variation |
References
- Dulvy, N.K.; Fowler, S.L.; Musick, J.A.; Cavanagh, R.D.; Kyne, P.M.; Harrison, L.R.; Carlson, J.K.; Davidson, L.N.; Fordham, S.V.; Francis, M.P.; et al. Extinction Risk and Conservation of the World’s Sharks and Rays. Elife 2014, 3, e00590. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.M.; Cooke, S.J. Trends in Shark Bycatch Research: Current Status and Research Needs. Rev. Fish Biol. Fish. 2012, 22, 719–737. [Google Scholar] [CrossRef]
- Pegado, M.R.; Santos, C.P.; Raffoul, D.; Konieczna, M.; Sampaio, E.; Luísa Maulvault, A.; Diniz, M.; Rosa, R. Impact of a Simulated Marine Heatwave in the Hematological Profile of a Temperate Shark (Scyliorhinus canicula). Ecol. Indic. 2020, 114, 106327. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Pacoureau, N.; Rigby, C.L.; Pollom, R.A.; Jabado, R.W.; Ebert, D.A.; Finucci, B.; Pollock, C.M.; Cheok, J.; Derrick, D.H.; et al. Overfishing Drives over One-Third of All Sharks and Rays toward a Global Extinction Crisis. Curr. Biol. 2021, 31, 4773–4787. [Google Scholar] [CrossRef]
- McClenachan, L.; Cooper, A.B.; Carpenter, K.E.; Dulvy, N.K. Extinction Risk and Bottlenecks in the Conservation of Charismatic Marine Species. Conserv. Lett. 2012, 5, 73–80. [Google Scholar] [CrossRef]
- Garnier, D.H.; Sourdaine, P.; Jégou, B. Seasonal Variations in Sex Steroids and Male Sexual Characteristics in Scyliorhinus Canicula. Gen. Comp. Endocrinol. 1999, 116, 281–290. [Google Scholar] [CrossRef]
- Inoue, T.; Shimoyama, K.; Saito, M.; Wong, M.K.S.; Ikeba, K.; Nozu, R.; Matsumoto, R.; Murakumo, K.; Sato, K.; Tokunaga, K.; et al. Long-Term Monitoring of Egg-Laying Cycle Using Ultrasonography Reveals the Reproductive Dynamics of Circulating Sex Steroids in an Oviparous Catshark, Scyliorhinus torazame. Gen. Comp. Endocrinol. 2022, 327, 114076. [Google Scholar] [CrossRef]
- Moberg, J.; Mench, G.P. The Biology of Animal Stress. Basic Principles and Implications for Animal Welfare; Moberg, G.P., Mench, J.A., Eds.; CABI Pu: New York, NY, USA, 2000; ISBN 0851993591. [Google Scholar]
- Möstl, E.; Palme, R. Hormones as Indicators of Stress. Domest. Anim. Endocrinol. 2002, 23, 67–74. [Google Scholar] [CrossRef]
- Zemanova, M.A. Towards More Compassionate Wildlife Research through the 3Rs Principles: Moving from Invasive to Non-Invasive Methods. Wildlife Biol. 2020, 2020. [Google Scholar] [CrossRef]
- Cook, N.J. Review: Minimally Invasive Sampling Media and the Measurement of Corticosteroids as Biomarkers of Stress in Animals. Can. J. Anim. Sci. 2012, 92, 227–259. [Google Scholar] [CrossRef]
- Palme, R. Non-Invasive Measurement of Glucocorticoids: Advances and Problems. Physiol. Behav. 2019, 199, 229–243. [Google Scholar] [CrossRef]
- Gormally, B.M.G.; Romero, L.M. What Are You Actually Measuring? A Review of Techniques That Integrate the Stress Response on Distinct Time-Scales. Funct. Ecol. 2020, 34, 2030–2044. [Google Scholar] [CrossRef]
- Jaime-Rivera, M.; Caraveo-Patiño, J.; Hoyos-Padilla, M.; Galván-Magaña, F. Evaluation of Biopsy Systems for Sampling White Shark Carcharodon Carcharias (Lamniformes: Lamnidae) Muscle for Stable Isotope Analysis. Rev. Biol. Mar. Oceanogr. 2013, 48, 345–351. [Google Scholar] [CrossRef]
- Marsili, L.; Coppola, D.; Giannetti, M.; Casini, S.; Fossi, M.; van Wyk, J.; Sperone, E.; Tripepi, S.; Micarelli, P.; Rizzuto, S. Skin Biopsies as a Sensitive Non-Lethal Technique for the Ecotoxicological Studies of Great White Shark(Carcharodon carcharias) Sampled in South Africa. Expert Opin. Environ. Biol. 2016, 4. [Google Scholar] [CrossRef]
- Montemagno, F.; Romano, C.; Bastoni, D.; Cordone, A.; De Castro, O.; Stefanni, S.; Sperone, E.; Giovannelli, D. Shark Microbiome Analysis Demonstrates Unique Microbial Communities in Two Distinct Mediterranean Sea Shark Species. Microorganisms 2024, 12, 557. [Google Scholar] [CrossRef]
- Meyer, L.; Fox, A.; Huveneers, C. Simple Biopsy Modification to Collect Muscle Samples from Free-Swimming Sharks. Biol. Conserv. 2018, 228, 142–147. [Google Scholar] [CrossRef]
- Carbajal, A.; Hua-Monclús, J.; Serres-Corral, P.; Lobató, I.; Muñoz-Baquero, M.; López-Béjar, M. Toward the validation of an alternative method for endocrine monitoring in sharks: Insights from testosterone analyses in the skin of bycatch individuals. Integr. Zool. 2024, 1–7. [Google Scholar] [CrossRef]
- Kersey, D.C.; Dehnhard, M. The Use of Noninvasive and Minimally Invasive Methods in Endocrinology for Threatened Mammalian Species Conservation. Gen. Comp. Endocrinol. 2014, 203, 296–306. [Google Scholar] [CrossRef]
- Palme, R. Measuring Fecal Steroids: Guidelines for Practical Application. Ann. N. Y. Acad. Sci. 2005, 1046, 75–80. [Google Scholar] [CrossRef]
- Naresh, M.D.; Arumugam, V.; Sanjeevi, R. Mechanical Behaviour of Shark Skin. J. Biosci. 1997, 22, 431–437. [Google Scholar] [CrossRef]
- Meyer, W.; Seegers, U. Basics of Skin Structure and Function in Elasmobranchs: A Review. J. Fish Biol. 2012, 80, 1940–1967. [Google Scholar] [CrossRef]
- Koob, T.J.; Callard, I.P. Reproductive Endocrinology of Female Elasmobranchs: Lessons from the Little Skate (Raja erinacea) and Spiny Dogfish (Squalus acanthias). J. Exp. Zool. 1999, 284, 557–574. [Google Scholar] [CrossRef]
- Tricas, T.C.; Maruska, K.P.; Rasmussen, L.E.L. Annual Cycles of Steroid Hormone Production, Gonad Development, and Reproductive Behavior in the Atlantic Stingray. Gen. Comp. Endocrinol. 2000, 118, 209–225. [Google Scholar] [CrossRef]
- Awruch, C.A. Reproductive Endocrinology in Chondrichthyans: The Present and the Future. Gen. Comp. Endocrinol. 2013, 192, 60–70. [Google Scholar] [CrossRef]
- Maruska, K.P.; Gelsleichter, J. Hormones and Reproduction in Chondrichthyan Fishes. Horm. Reprod. Vertebr. Fishes 2010, 1, 209–237. [Google Scholar] [CrossRef]
- Serena, F.; Ellis, J.; Abella, A.; Mancusi, C.; Haka, F.; Guallart, J.; Ungaro, N.; Coelho, R.P.; Schembri, T.; Kirsteen, M. Scyliorhinus Canicula. The IUCN Red List of Threatened Species; IUCN: Gland, Switzerland, 2015. [Google Scholar]
- Mancia, A.; Chenet, T.; Bono, G.; Geraci, M.L.; Vaccaro, C.; Munari, C.; Mistri, M.; Cavazzini, A.; Pasti, L. Adverse Effects of Plastic Ingestion on the Mediterranean Small-Spotted Catshark (Scyliorhinus canicula). Mar. Environ. Res. 2020, 155, 104876. [Google Scholar] [CrossRef]
- Musa, S.M.; Ripley, D.M.; Moritz, T.; Shiels, H.A. Ocean Warming and Hypoxia Affect Embryonic Growth, Fitness and Survival of Small-Spotted Catsharks, Scyliorhinus canicula. J. Fish Biol. 2020, 97, 257–264. [Google Scholar] [CrossRef]
- Jeffree, R.A.; Warnau, M.; Teyssié, J.L.; Markich, S.J. Comparison of the Bioaccumulation from Seawater and Depuration of Heavy Metals and Radionuclides in the Spotted Dogfish Scyliorhinus canicula (Chondrichthys) and the Turbot Psetta Maxima (Actinopterygii: Teleostei). Sci. Total Environ. 2006, 368, 839–852. [Google Scholar] [CrossRef]
- Kumar, V.; Umapathy, G. Non-Invasive Monitoring of Steroid Hormones in Wildlife for Conservation and Management of Endangered Species-A Review. Indian, J. Exp. Biol. 2019, 57, 307–314. [Google Scholar]
- Becerril-García, E.E.; Arellano-Martínez, M.; Bernot-Simon, D.; Hoyos-Padilla, E.M.; Galván-Magaña, F.; Godard-Codding, C. Steroid Hormones and Chondrichthyan Reproduction: Physiological Functions, Scientific Research, and Implications for Conservation. PeerJ 2020, 8, e9686. [Google Scholar] [CrossRef]
- Barton, B.A.; Morgan, J.D.; Vljayan, M. Physiological and Condition-Related Indicators of Environmental Stress in Fish. In Biological Indicators of Aquatic Ecosystem Stress; American Fisheries Society: Bethesda, MD, USA, 1998; pp. 111–148. [Google Scholar]
- Goodbred, S.L.; Patiño, R.; Torres, L.; Echols, K.R.; Jenkins, J.A.; Rosen, M.R.; Orsak, E. Are Endocrine and Reproductive Biomarkers Altered in Contaminant-Exposed Wild Male Largemouth Bass (Micropterus salmoides) of Lake Mead, Nevada/Arizona, USA? Gen. Comp. Endocrinol. 2015, 219, 125–135. [Google Scholar] [CrossRef]
- Gonzalez De Acevedo, M.; Frazier, B.S.; Belcher, C.; Gelsleichter, J. Reproductive Cycle and Fecundity of the Bonnethead Sphyrna Tiburo L. from the Northwest Atlantic Ocean. J. Fish Biol. 2020, 97, 1733–1747. [Google Scholar] [CrossRef] [PubMed]
- Manire, C.A.; Rasmussen, L.E.L.; Gross, T.S. Serum Steroid Hormones Including 11-Ketotestosterone, 11-Ketoandrostenedione, and Dihydroprogesterone in Juvenile and Adult Bonnethead Sharks, Sphyrna tiburo. J. Exp. Zool. 1999, 284, 595–603. [Google Scholar] [CrossRef]
- Sulikowski, J.A.; Tsang, P.C.W.; Howell, W.H. Age and Size at Sexual Maturity for the Winter Skate, Leucoraja Ocellata, in the Western Gulf of Maine Based on Morphological, Histological and Steroid Hormone Analyses. Environ. Biol. Fishes 2005, 72, 429–441. [Google Scholar] [CrossRef]
- Penfold, L.M.; Wyffels, J.T. Reproductive Science in Sharks and Rays. Adv. Exp. Med. Biol. 2019, 1200, 465–488. [Google Scholar] [CrossRef]
- Fuzzen, M.; Bernier, N.J.; Van Der Kraak, G. Stress and Reproduction. In Hormones and Reproduction in Vertebrates; Norris, D.O., Lopez, K.H., Eds.; Academic Press: New York, NY, USA, 2011; pp. 103–117. [Google Scholar]
- Norris, D.O. Chapter 10. The Endocrinology of Mammalian Reproduction. In Vertebrate Endocrinology; Elsevier Science & Technology: San Diego, CA, USA, 2006; pp. 322–370. [Google Scholar]
- Koob, T.J.; Tsang, P.; Callard, I.P. Plasma Estradiol, Testosterone, and Progesterone Levels during the Ovulatory Cycle of the Skate (Raja erinacea). Biol. Reprod. 1986, 35, 267–275. [Google Scholar] [CrossRef]
- Muñoz-Baquero, M.; Marco-Jiménez, F.; García-Domínguez, X.; Ros-Santaella, J.L.; Pintus, E.; Jiménez-Movilla, M.; García-Párraga, D.; García-Vazquez, F.A. Comparative Study of Semen Parameters and Hormone Profile in Small-Spotted Catshark (Scyliorhinus canicula): Aquarium-Housed vs. Wild-Captured. Animals 2021, 11, 2884. [Google Scholar] [CrossRef]
- Hammerschlag, N.; Skubel, R.A.; Sulikowski, J.; Irschick, D.J.; Gallagher, A.J. A Comparison of Reproductive and Energetic States in a Marine Apex Predator (the Tiger Shark, Galeocerdo cuvier). Physiol. Biochem. Zool. 2018, 91, 933942. [Google Scholar] [CrossRef]
- Wyffels, J.T.; George, R.; Adams, L.; Adams, C.; Clauss, T.; Newton, A.; Hyatt, M.W.; Yach, C.; Penfold, L.M. Testosterone and Semen Seasonality for the Sand Tiger Shark Carcharias Taurus. Biol. Reprod. 2020, 102, 876–887. [Google Scholar] [CrossRef]
- Prisco, M.; Salvatore, V.; Maddalena Di Fiore, M.; Raucci, F.; Del Giudice, G.; Romano, M.; Laforgia, V.; Limantola, E.; Andreucetti, P. Effect of 17β-Estradiol and Progesterone on Vitellogenesis in the Spotted Ray Torpedo Marmorata Risso 1810 (Elasmobranchii: Torpediniformes): Studies on Females and on Estrogen-Treated Males. Gen. Comp. Endocrinol. 2008, 157, 125–132. [Google Scholar] [CrossRef]
- Henningsen, A.D.; Murru, F.L.; Rasmussen, L.E.L.; Whitaker, B.R.; Violetta, G.C. Serum Levels of Reproductive Steroid Hormones in Captive Sand Tiger Sharks, Carcharias taurus (Rafinesque), and Comments on Their Relation to Sexual Conflicts. Fish Physiol. Biochem. 2008, 34, 437–446. [Google Scholar] [CrossRef]
- Gelsleichter, J.; Rasmussen, L.E.L.; Manire, C.A.; Tyminski, J.; Chang, B.; Lombardi-Carlson, L. Serum Steroid Concentrations and Development of Reproductive Organs during Puberty in Male Bonnethead Sharks, Sphyrna tiburo. Fish Physiol. Biochem. 2002, 26, 389–401. [Google Scholar] [CrossRef]
- Rasmussen, L.E.L.; Hess, D.L.; Luer, C.A. Alterations in Serum Steroid Concentrations in the Clearnose Skate, Raja eglanteria: Correlations with Season and Reproductive Status. J. Exp. Zool. 1999, 284, 575–585. [Google Scholar] [CrossRef]
- Carrier, J.C.; Pratt, H.L.; Martin, L.K.; Martin, L.K. Group Reproductive Behaviors in Free-Living Nurse Sharks, Ginglymostoma cirratum. Copeia 1994, 1994, 646–656. Available online: https://www.jstor.org/stable/1447180 (accessed on 14 June 2024).
- Whittamore, J.M.; Bloomer, C.; Hanna, G.M.; McCarthy, I.D. Evaluating Ultrasonography as a Non-Lethal Method for the Assessment of Maturity in Oviparous Elasmobranchs. Mar. Biol. 2010, 157, 2613–2624. [Google Scholar] [CrossRef]
- Manire, C.A.; Rasmussen, L.E.L.; Maruska, K.P.; Tricas, T.C. Sex, Seasonal, and Stress-Related Variations in Elasmobranch Corticosterone Concentrations. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 148, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.E.L.; Crow, G.L. Serum Corticosterone Concentrations in Immature Captive Whitetip Reef Sharks, Triaenodon obesus. J. Exp. Zool. 1993, 267, 283–287. [Google Scholar] [CrossRef]
- McClusky, L.M. Cadmium Accumulation and Binding Characteristics in Intact Sertoli/Germ Cell Units, and Associated Effects on Stage-Specific Functions in Vitro: Insights from a Shark Testis Model. J. Appl. Toxicol. 2007, 28, 112–121. [Google Scholar] [CrossRef]
- Pethybridge, H.; Butler, E.C.V.; Cossa, D.; Daley, R.; Boudou, A. Trophic Structure and Biomagnification of Mercury in an Assemblage of Deepwater Chondrichthyans from Southeastern Australia. Mar. Ecol. Prog. Ser. 2012, 451, 163–174. [Google Scholar] [CrossRef]
| Sex | Total Length (cm) | Body Weight (g) | GSI | K | N | |
|---|---|---|---|---|---|---|
| Female | 39.1 ± 2.2 | 162.2 ± 36.1 | - | 0.27 ± 0.02 | 21 | |
| Male | Immature | 35.8 ± 2.0 | 133.2 ± 19.5 | 1.1 ± 0.5 | 0.29 ± 0.02 | 6 |
| Mature | 41.5 ± 2.0 | 219.9 ± 37.9 | 3.7 ± 0.6 | 0.31 ± 0.01 | 14 | |
| Sex | Hormone | Intra-Assay CV (%) | Dilution | Spike-and-Recovery | Sensitivity (ng/mL) | |
|---|---|---|---|---|---|---|
| R2 (%) | Mean Error (%) | Mean Recovery (±SD) | ||||
| Male | Testosterone * | 6.2 (±5.2) | 0.99 | 107.5 (±7.5) | 102.2 (±9.7) | 0.008 |
| Progesterone | 15.4 (±10.7) | 0.99 | 85.6 (±14.4) | 99.2 (±16.3) | 0.345 | |
| 17β-estradiol | 10.2 (±10.7) | 0.99 | 111.5 (±11.5) | 87.5 (±19.9) | 0.020 | |
| Female | Testosterone | 4.91 (±2.5) | 0.99 | 109.4 (±9.4) | 100.6 (±8.0) | 0.012 |
| Progesterone | 10.8 (±5.5) | 0.99 | 111.8 (±11.8) | 93.2 (±16.8) | 0.196 | |
| 17β-estradiol | 8.7 (±6.8) | 0.99 | 109.3 (±9.3) | 91.5 (±11.9) | 0.021 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbajal, A.; Lobato, I.G.; Agustí, C.; Muñoz-Baquero, M.; Serres-Corral, P.; López-Béjar, M. A New Research Tool for Use in Sharks and Rays: Relevance of Reproductive Hormone Levels in the Skin of Small-Spotted Catshark (Scyliorhinus canicula). Animals 2025, 15, 762. https://doi.org/10.3390/ani15050762
Carbajal A, Lobato IG, Agustí C, Muñoz-Baquero M, Serres-Corral P, López-Béjar M. A New Research Tool for Use in Sharks and Rays: Relevance of Reproductive Hormone Levels in the Skin of Small-Spotted Catshark (Scyliorhinus canicula). Animals. 2025; 15(5):762. https://doi.org/10.3390/ani15050762
Chicago/Turabian StyleCarbajal, Annaïs, Isabel González Lobato, Clara Agustí, Marta Muñoz-Baquero, Paula Serres-Corral, and Manel López-Béjar. 2025. "A New Research Tool for Use in Sharks and Rays: Relevance of Reproductive Hormone Levels in the Skin of Small-Spotted Catshark (Scyliorhinus canicula)" Animals 15, no. 5: 762. https://doi.org/10.3390/ani15050762
APA StyleCarbajal, A., Lobato, I. G., Agustí, C., Muñoz-Baquero, M., Serres-Corral, P., & López-Béjar, M. (2025). A New Research Tool for Use in Sharks and Rays: Relevance of Reproductive Hormone Levels in the Skin of Small-Spotted Catshark (Scyliorhinus canicula). Animals, 15(5), 762. https://doi.org/10.3390/ani15050762







