Genetic Diversity in the Diminazene Resistance-Associated P2 Adenosine Transporter-1 (AT-1) Gene of Trypanosoma evansi
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Field Sample, and Genomic DNA Extraction
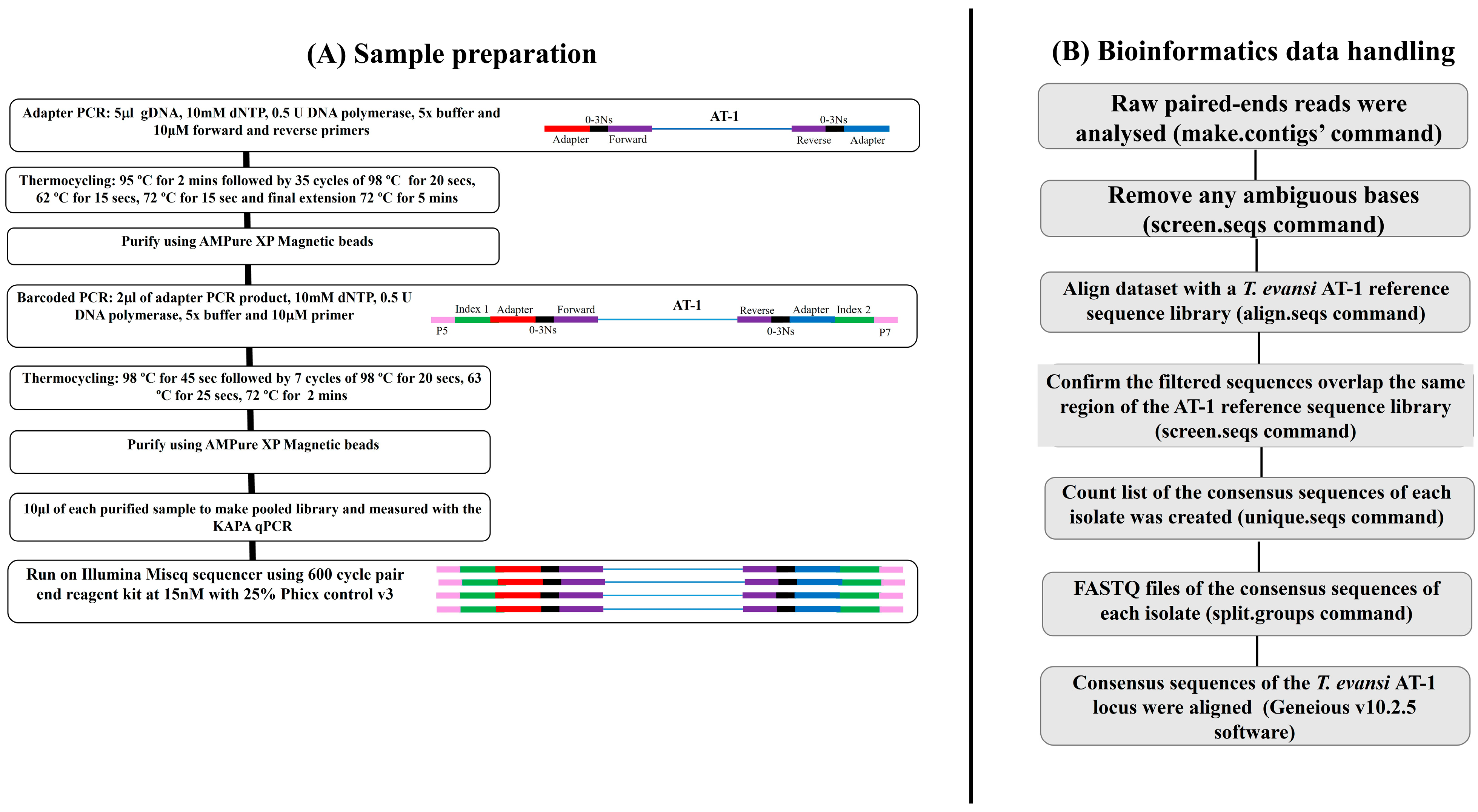
2.2. Adapter and Barcoded PCR Amplification
2.3. Amplicon Deep Sequencing
2.4. Bioinformatic Analyses
2.5. Statistical Analyses of the Amplicon Variance
3. Results
3.1. Genetic Diversity in the T. evansi P2 Adenosine Transporter-1 (AT-1) Locus
3.2. Potential Diminazene Resistance-Type Mutations
3.3. Allele Frequencies of Diminazene Resistance-Type Mutations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cayla, M.; Rojas, F.; Silvester, E.; Venter, F.; Matthews, K.R. African Trypanosomes. Parasit. Vectors 2019, 12, 190. [Google Scholar] [CrossRef] [PubMed]
- Desquesnes, M.; Holzmuller, P.; Lai, D.-H.; Dargantes, A.; Lun, Z.-R.; Jittaplapong, S. Trypanosoma Evansi and Surra: A Review and Perspectives on Origin, History, Distribution, Taxonomy, Morphology, Hosts, and Pathogenic Effects. Biomed. Res. Int. 2013, 2013, 194176. [Google Scholar] [CrossRef] [PubMed]
- Ventura, R.M.; Takeda, G.F.; Silva, R.A.M.S.; Nunes, V.L.B.; Buck, G.A.; Teixeira, M.M.G. Genetic Relatedness among Trypanosoma Evansi Stocks by Random Amplification of Polymorphic DNA and Evaluation of a Synapomorphic DNA Fragment for Species-Specific Diagnosis. Int. J. Parasitol. 2002, 32, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Fairlamb, A.H.; Horn, D. Melarsoprol Resistance in African Trypanosomiasis. Trends Parasitol. 2018, 34, 481–492. [Google Scholar] [CrossRef]
- Barrett, M.P.; Zhang, Z.Q.; Denise, H.; Giroud, C.; Baltz, T. A Diamidine-Resistant Trypanosoma Equiperdum Clone Contains a P2 Purine Transporter with Reduced Substrate Affinity. Mol. Biochem. Parasitol. 1995, 73, 223–229. [Google Scholar] [CrossRef]
- Gehrig, S.; Efferth, T. Development of Drug Resistance in Trypanosoma Brucei Rhodesiense and Trypanosoma Brucei Gambiense. Treatment of Human African Trypanosomiasis with Natural Products (Review). Int. J. Mol. Med. 2008, 22, 411–419. [Google Scholar]
- Delespaux, V.; Geysen, D.; Van den Bossche, P.; Geerts, S. Molecular Tools for the Rapid Detection of Drug Resistance in Animal Trypanosomes. Trends Parasitol. 2008, 24, 236–242. [Google Scholar] [CrossRef]
- Kazibwe, A.J.; Nerima, B.; de Koning, H.P.; Mäser, P.; Barrett, M.P.; Matovu, E. Genotypic Status of the TbAT1/P2 Adenosine Transporter of Trypanosoma Brucei Gambiense Isolates from Northwestern Uganda Following Melarsoprol Withdrawal. PLoS Negl. Trop. Dis. 2009, 3, e523. [Google Scholar] [CrossRef]
- Mäser, P.; Sütterlin, C.; Kralli, A.; Kaminsky, R. A Nucleoside Transporter from Trypanosoma Brucei Involved in Drug Resistance. Science 1999, 285, 242–244. [Google Scholar] [CrossRef]
- Carter, N.S.; Barrett, M.P.; de Koning, H.P. A Drug Resistance Determinant in Trypanosoma Brucei. Trends Microbiol. 1999, 7, 469–471. [Google Scholar] [CrossRef]
- Matovu, E.; Geiser, F.; Schneider, V.; Mäser, P.; Enyaru, J.C.; Kaminsky, R.; Gallati, S.; Seebeck, T. Genetic Variants of the TbAT1 Adenosine Transporter from African Trypanosomes in Relapse Infections Following Melarsoprol Therapy. Mol. Biochem. Parasitol. 2001, 117, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Witola, W.H.; Inoue, N.; Ohashi, K.; Onuma, M. RNA-Interference Silencing of the Adenosine Transporter-1 Gene in Trypanosoma Evansi Confers Resistance to Diminazene Aceturate. Exp. Parasitol. 2004, 107, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Witola, W.H.; Tsuda, A.; Inoue, N.; Ohashi, K.; Onuma, M. Acquired Resistance to Berenil in a Cloned Isolate of Trypanosoma Evansi Is Associated with Upregulation of a Novel Gene, TeDR40. Parasitology 2005, 131 Pt 5, 635–646. [Google Scholar] [CrossRef]
- Chaudhry, U.; Ali, Q.; Zheng, L.; Rashid, I.; Shabbir, M.Z.; Numan, M.; Ashraf, K.; Evans, M.; Rafiq, S.; Oneeb, M.; et al. Contrasting Population Genetics of Co-Endemic Cattle- and Buffalo- Derived Theileria Annulata. Ticks Tick. Borne Dis. 2021, 12, 101595. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Chen, M.-X.; Alasaad, S.; Elsheikha, H.M.; Li, J.; Li, H.-L.; Lin, R.-Q.; Zou, F.-C.; Zhu, X.-Q.; Chen, J.-X. Genetic Characterization, Species Differentiation and Detection of Fasciola spp. by Molecular Approaches. Parasites Vectors 2011, 4, 101. [Google Scholar] [CrossRef]
- Rehman, Z.; Tashibu, A.; Tashiro, M.; Rashid, I.; Ali, Q.; Zahid, O.; Ashraf, K.; Shehzad, W.; Chaudhry, U.; Ichikawa-Seki, M. Molecular Characterization of Fasciola Gigantica in Punjab, Pakistan to Infer the Dispersal Route among the Neighbouring Countries of the Indian Subcontinent. bioRxiv 2020. [Google Scholar] [CrossRef]
- Shaukat, A.; Ali, Q.; Connelley, T.; Khan, M.A.U.; Saleem, M.A.; Evans, M.; Rashid, I.; Sargison, N.D.; Chaudhry, U. Selective Sweep and Phylogenetic Models for the Emergence and Spread of Pyrimethamine Resistance Mutations in Plasmodium Vivax. Infect. Genet. Evol. 2019, 68, 221–230. [Google Scholar] [CrossRef]
- Ali, Q.; Zahid, O.; Mhadhbi, M.; Jones, B.; Darghouth, M.A.; Raynes, G.; Afshan, K.; Birtles, R.; Sargison, N.D.; Betson, M.; et al. Genetic Characterisation of the Theileria Annulata Cytochrome b Locus and Its Impact on Buparvaquone Resistance in Bovine. Int. J. Parasitol. Drugs Drug Resist. 2022, 20, 65–75. [Google Scholar] [CrossRef]
- Yasein, G.; Zahid, O.; Minter, E.; Ashraf, K.; Rashid, I.; Shabbir, M.Z.; Betson, M.; Sargison, N.D.; Chaudhry, U. A Novel Metabarcoded Deep Amplicon Sequencing Tool for Disease Surveillance and Determining the Species Composition of Trypanosoma in Cattle and Other Farm Animals. Acta Trop. 2022, 230, 106416. [Google Scholar] [CrossRef]
- Chaudhry, U.; Ali, Q.; Rashid, I.; Shabbir, M.Z.; Ijaz, M.; Abbas, M.; Evans, M.; Ashraf, K.; Morrison, I.; Morrison, L.; et al. Development of a Deep Amplicon Sequencing Method to Determine the Species Composition of Piroplasm Haemoprotozoa. Ticks Tick. Borne Dis. 2019, 10, 101276. [Google Scholar] [CrossRef]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a Dual-Index Sequencing Strategy and Curation Pipeline for Analyzing Amplicon Sequence Data on the MiSeq Illumina Sequencing Platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J. Introducing Mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Librado, P.; Rozas, J. DnaSP v5: A Software for Comprehensive Analysis of DNA Polymorphism Data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed]
| Field Isolates | Total No of Illumina Mi-Seq Reads | Susceptible-Type Reads | Resistance-Type Reads | Heterozygosity (He) | Nucleotide Diversity (π) | Segregating Sites (S) | Pairwise Differences (k) | Host | Endemic Region |
|---|---|---|---|---|---|---|---|---|---|
| Pop203 | 2675 | 1954 | 721 | 0.0011 | 0.01326 | 94 | 7.362 | Camel | Rahim Yar Khan |
| Pop207 | 2261 | 1676 | 585 | 0.0014 | 0.01415 | 106 | 7.853 | Camel | Rahim Yar Khan |
| Pop266 | 2716 | 1851 | 865 | 0.0031 | 0.01784 | 97 | 9.902 | Camel | Multan |
| Pop277 | 2560 | 1771 | 508 | 0.0021 | 0.01587 | 111 | 8.806 | Buffalo | Layyah |
| Pop278 | 1426 | 1103 | 323 | 0.0014 | 0.01408 | 101 | 7.812 | Buffalo | Layyah |
| Pop279 | 1409 | 1230 | 179 | 0.0120 | 0.01464 | 53 | 8.123 | Cattle | Layyah |
| Pop287 | 1954 | 1440 | 514 | 0.0150 | 0.04122 | 78 | 8.156 | Goat | Rahim Yar Khan |
| Pop111 | 1262 | 1262 | 0.2720 | 0.00601 | 5 | 3.333 | Cattle | Bahawalpur | |
| Pop202 | 1397 | 1397 | 0.1770 | 0.00885 | 9 | 4.500 | Cattle | Bahawalpur | |
| Pop208 | 1346 | 1346 | 0.3140 | 0.00601 | 5 | 3.242 | Cattle | Lodhraan | |
| Pop210 | 2361 | 2361 | 0.0770 | 0.00955 | 15 | 3.000 | Camel | Rahim Yar Khan | |
| Pop268 | 2733 | 2733 | 0.0450 | 0.00529 | 21 | 5.600 | Camel | Rahim Yar Khan | |
| Pop283 | 1960 | 1960 | 0.0270 | 0.00367 | 42 | 8.538 | Camel | Rahim Yar Khan | |
| Pop284 | 1509 | 1509 | 0.0390 | 0.00461 | 37 | 8.109 | Cattle | Layyah | |
| Pop253 | 1708 | 1708 | N/A | Sheep | Rahim Yar Khan | ||||
| Pop199 | 1444 | 1444 | N/A | Sheep | Rahim Yar Khan | ||||
| Pop234 | 1614 | 1614 | N/A | Cattle | Muzafargar | ||||
| Pop217 | 2261 | 2261 | N/A | Camel | Lodhraan | ||||
| Pop220 | 2221 | 2221 | N/A | Cattle | Bahawalpur | ||||
| Pop237 | 1289 | 1289 | N/A | Cattle | Dera Ghazi Khan | ||||
| Pop230 | 2282 | 2282 | N/A | Cattle | Dera Ghazi Khan | ||||
| Pop238 | 1247 | 1247 | N/A | Cattle | Bahawalpur | ||||
| Pop25 | 2267 | 2267 | N/A | Cattle | Dera Ghazi Khan | ||||
| Pop114 | 2843 | 2843 | N/A | Cattle | Dera Ghazi Khan | ||||
| Pop100 | 3087 | 3087 | N/A | Cattle | Muzafargar | ||||
| Pop112 | 3276 | 3276 | N/A | Cattle | Rahim Yar Khan | ||||
| Nucleotide | 532–534 (GCA/GAA/TCA) | 541–543 (GGA/GAA) | 715–717 (GAT/GGT/GCT/GAG) | 856–858 (AAC/AGC/CAC/ATC/GAC/ACC) | Host | Endemic Region | |
|---|---|---|---|---|---|---|---|
| Codon | 178 A/E/S | 181 G/E | 239 D/Y/A/E | 286 N/S/H/I/D/T | |||
| Pop203 | A | G | D/Y | N/S/H | Camel | Rahim Yar Khan | |
| Pop207 | A | G | D | N/H/I | Camel | Rahim Yar Khan | |
| Pop266 | A | G | D/A | N/I/D/T | Camel | Multan | |
| Pop277 | A/E/S | G | D/E/A | N | Buffalo | Layyah | |
| Pop278 | A | G | D/Y | N/S | Buffalo | Layyah | |
| Pop279 | A | G | D | N/D | Cattle | Layyah | |
| Pop287 | A | G | D/Y | N/T/D | Goat | Rahim Yar Khan | |
| Pop100 | A | G | D | N | Cattle | Bahawalpur | |
| Pop111 | A | G | D | N | Cattle | Bahawalpur | |
| Pop112 | A | G | D | N | Cattle | Lodhraan | |
| Pop202 | A | G | D | N | Camel | Rahim Yar Khan | |
| Pop208 | A | G | D | N | Camel | Rahim Yar Khan | |
| Pop210 | A | G | D | N | Camel | Rahim Yar Khan | |
| Pop268 | A | G | D | N | Cattle | Layyah | |
| Pop283 | A | G | D | N | Sheep | Rahim Yar Khan | |
| Pop284 | A | G | D | N | Sheep | Rahim Yar Khan | |
| Pop253 | A | G | D | N | Cattle | Muzafargar | |
| Pop199 | A | G | D | N | Camel | Lodhraan | |
| Pop234 | A | G | D | N | Cattle | Bahawalpur | |
| Pop217 | A | G | D | N | Cattle | Dera Ghazi Khan | |
| Pop220 | A | G | D | N | Cattle | Dera Ghazi Khan | |
| Pop237 | A | G | D | N | Cattle | Bahawalpur | |
| Pop230 | A | G | D | N | Cattle | Dera Ghazi Khan | |
| Pop238 | A | G | D | N | Cattle | Dera Ghazi Khan | |
| Pop25 | A | G | D | N | Cattle | Muzafargar | |
| Pop114 | A | G | D | N | Cattle | Rahim Yar Khan |
| Field Isolates | Susceptible Type Mutations % | Resistant Type Mutations % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| GCA(A178) GGA(G181) GAT(D239) AAC(N286) | GAA(178E) | TCA(178S) | GGT(239Y) | GCT(239A) | GAG(239E) | AGC(286S) | CAC(286H) | ATC(286I) | GAC(286D) | ACC(286T) | |
| Pop203 | 73.05 | 7.07 | 9.16 | 10.73 | |||||||
| Pop207 | 74.13 | 14.55 | 11.32 | ||||||||
| Pop266 | 68.15 | 9.02 | 10.16 | 6.08 | 6.59 | ||||||
| Pop277 | 69.18 | 6.17 | 4.80 | 5.59 | 14.26 | ||||||
| Pop278 | 77.35 | 10.17 | 12.48 | ||||||||
| Pop279 | 87.30 | 12.70 | |||||||||
| Pop287 | 73.69 | 10.18 | 8.50 | 7.63 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashraf, S.; Yasein, G.; Ali, Q.; Afshan, K.; Betson, M.; Sargison, N.; Chaudhry, U. Genetic Diversity in the Diminazene Resistance-Associated P2 Adenosine Transporter-1 (AT-1) Gene of Trypanosoma evansi. Animals 2025, 15, 756. https://doi.org/10.3390/ani15050756
Ashraf S, Yasein G, Ali Q, Afshan K, Betson M, Sargison N, Chaudhry U. Genetic Diversity in the Diminazene Resistance-Associated P2 Adenosine Transporter-1 (AT-1) Gene of Trypanosoma evansi. Animals. 2025; 15(5):756. https://doi.org/10.3390/ani15050756
Chicago/Turabian StyleAshraf, Shoaib, Ghulam Yasein, Qasim Ali, Kiran Afshan, Martha Betson, Neil Sargison, and Umer Chaudhry. 2025. "Genetic Diversity in the Diminazene Resistance-Associated P2 Adenosine Transporter-1 (AT-1) Gene of Trypanosoma evansi" Animals 15, no. 5: 756. https://doi.org/10.3390/ani15050756
APA StyleAshraf, S., Yasein, G., Ali, Q., Afshan, K., Betson, M., Sargison, N., & Chaudhry, U. (2025). Genetic Diversity in the Diminazene Resistance-Associated P2 Adenosine Transporter-1 (AT-1) Gene of Trypanosoma evansi. Animals, 15(5), 756. https://doi.org/10.3390/ani15050756







