Consequences of Domestication on Gut Microbiome: A Comparative Analysis Between Wild Boars and Domestic Pigs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sample Collection
2.2. Total Bacterial Genomic DNA (gDNA) Extraction
2.3. PCR Amplification and Sequencing
2.4. Bioinformatics Analysis
3. Results
3.1. Statistics for Sequencing Data
3.2. Bacterial Diversity
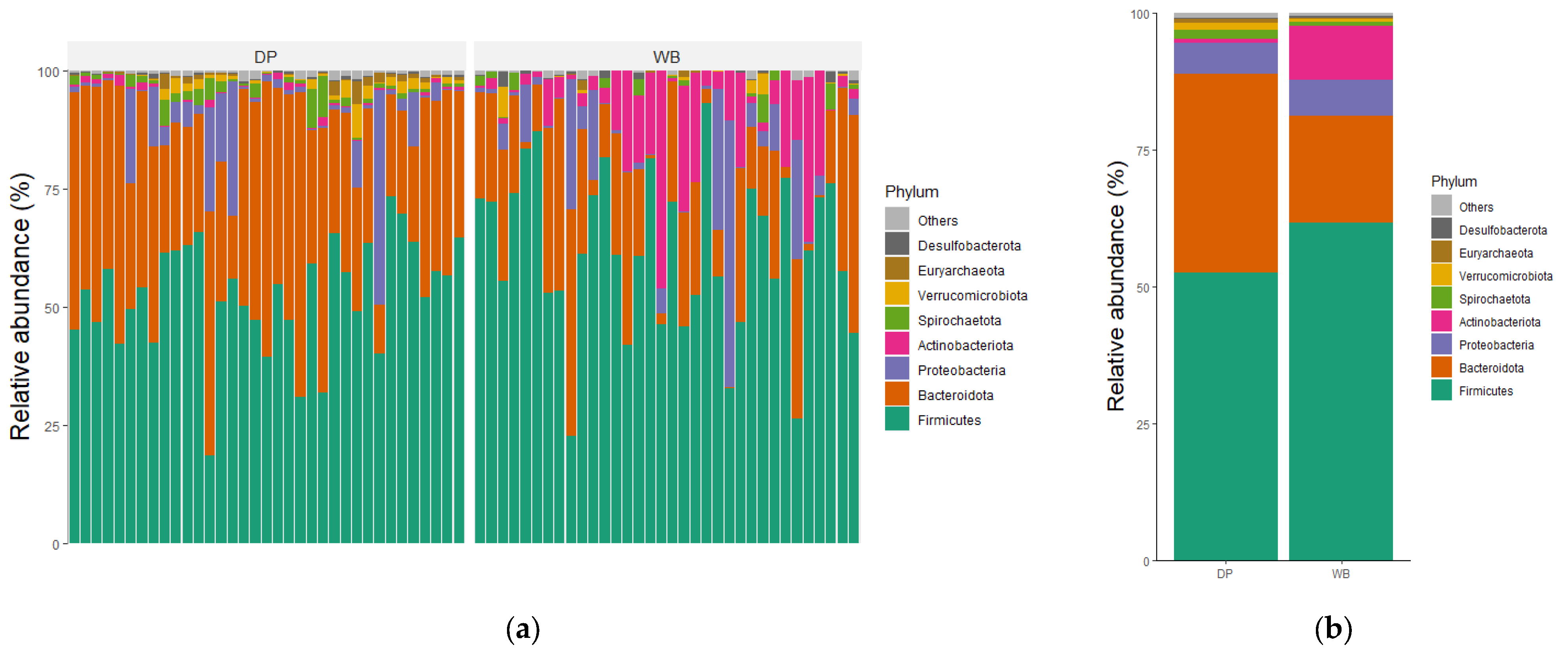
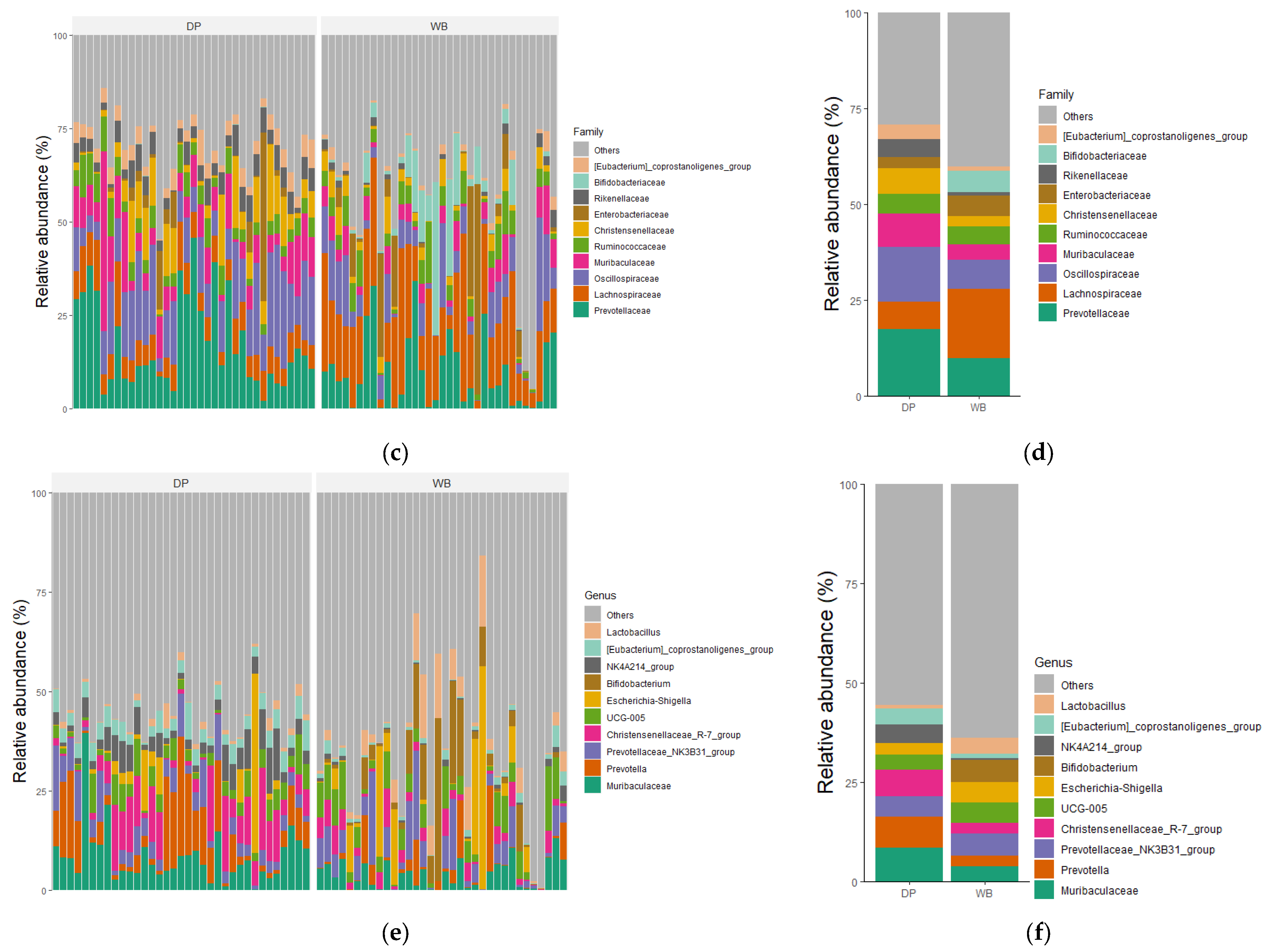
3.3. Taxonomic Profiles
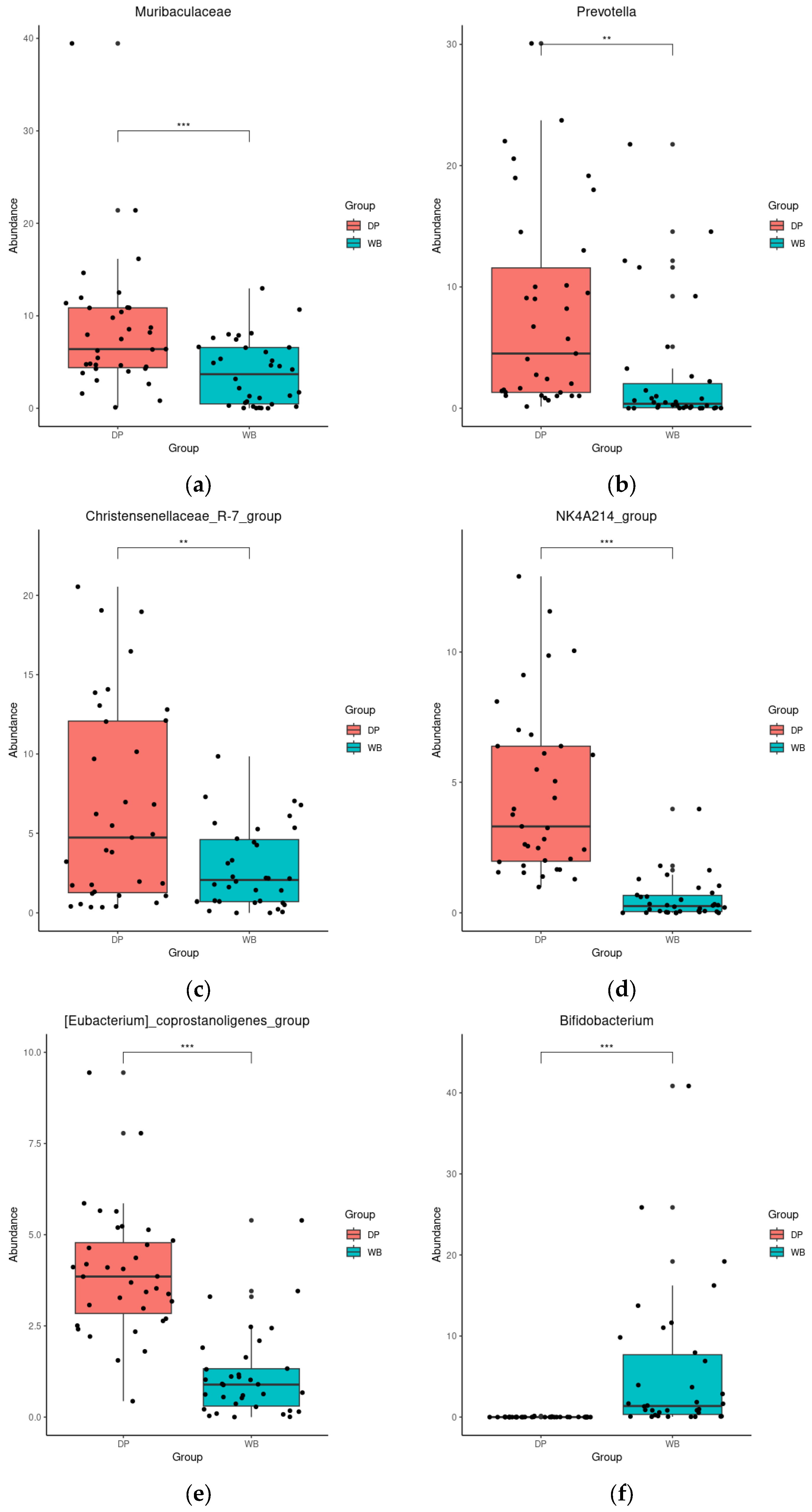
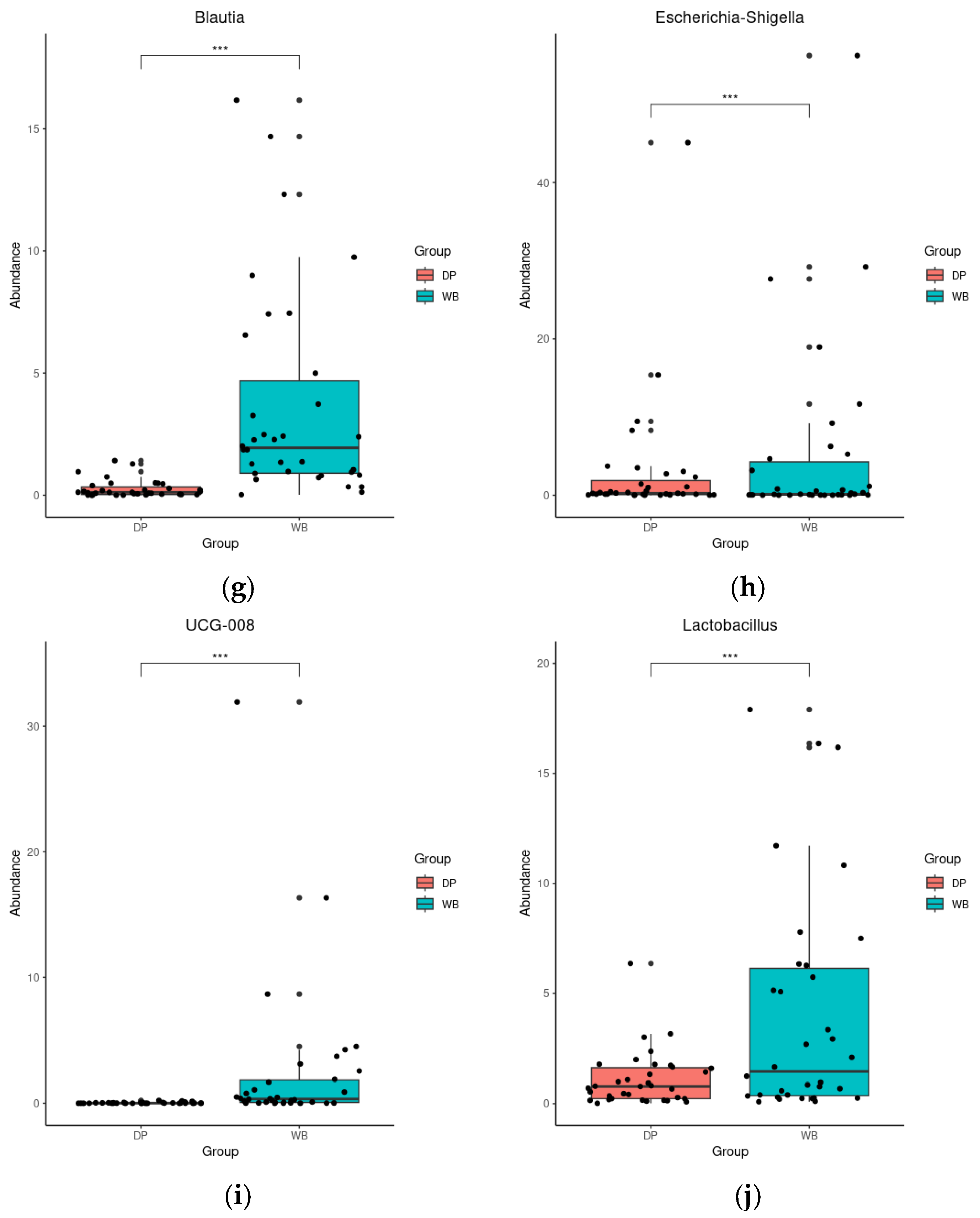
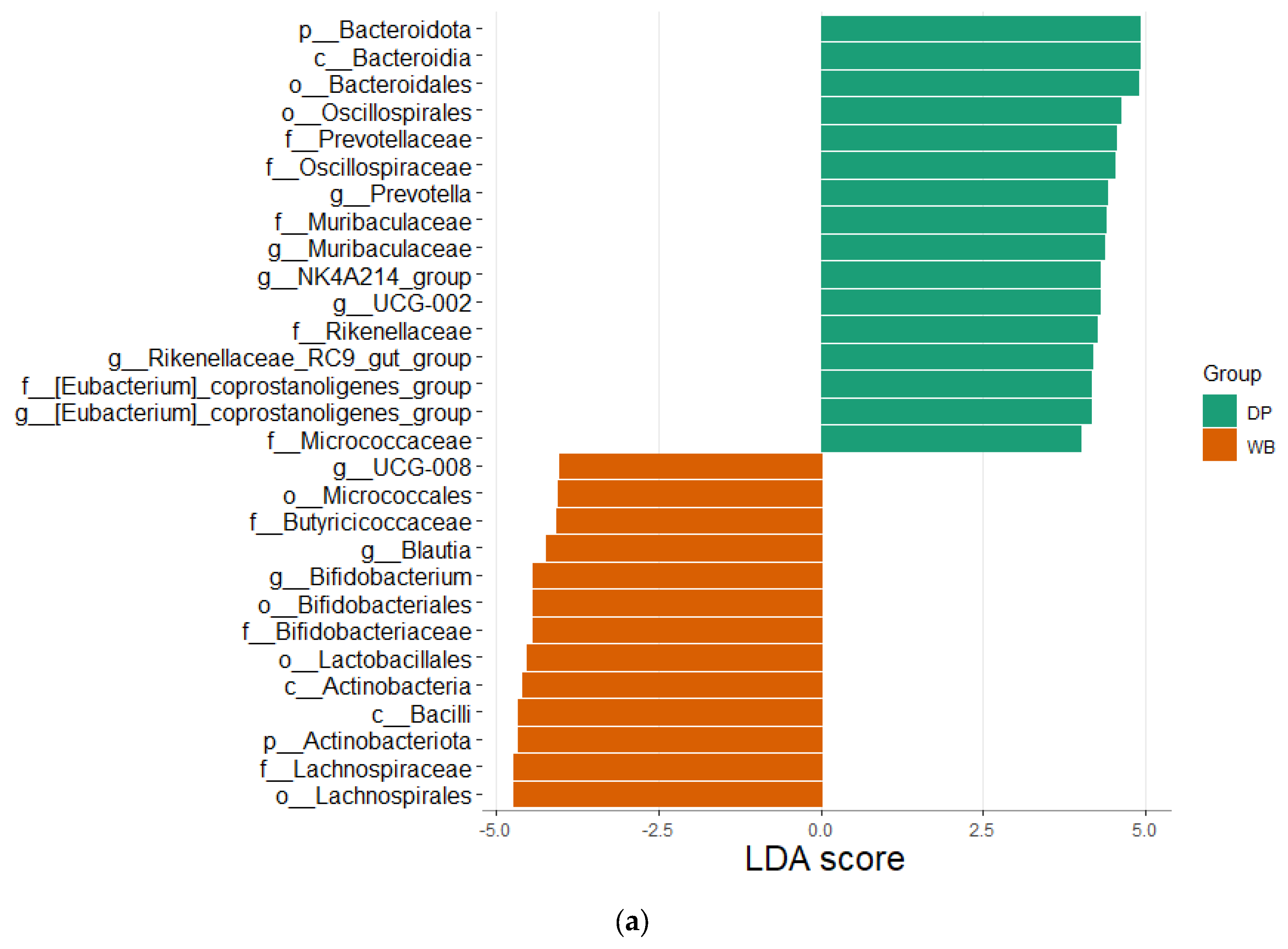
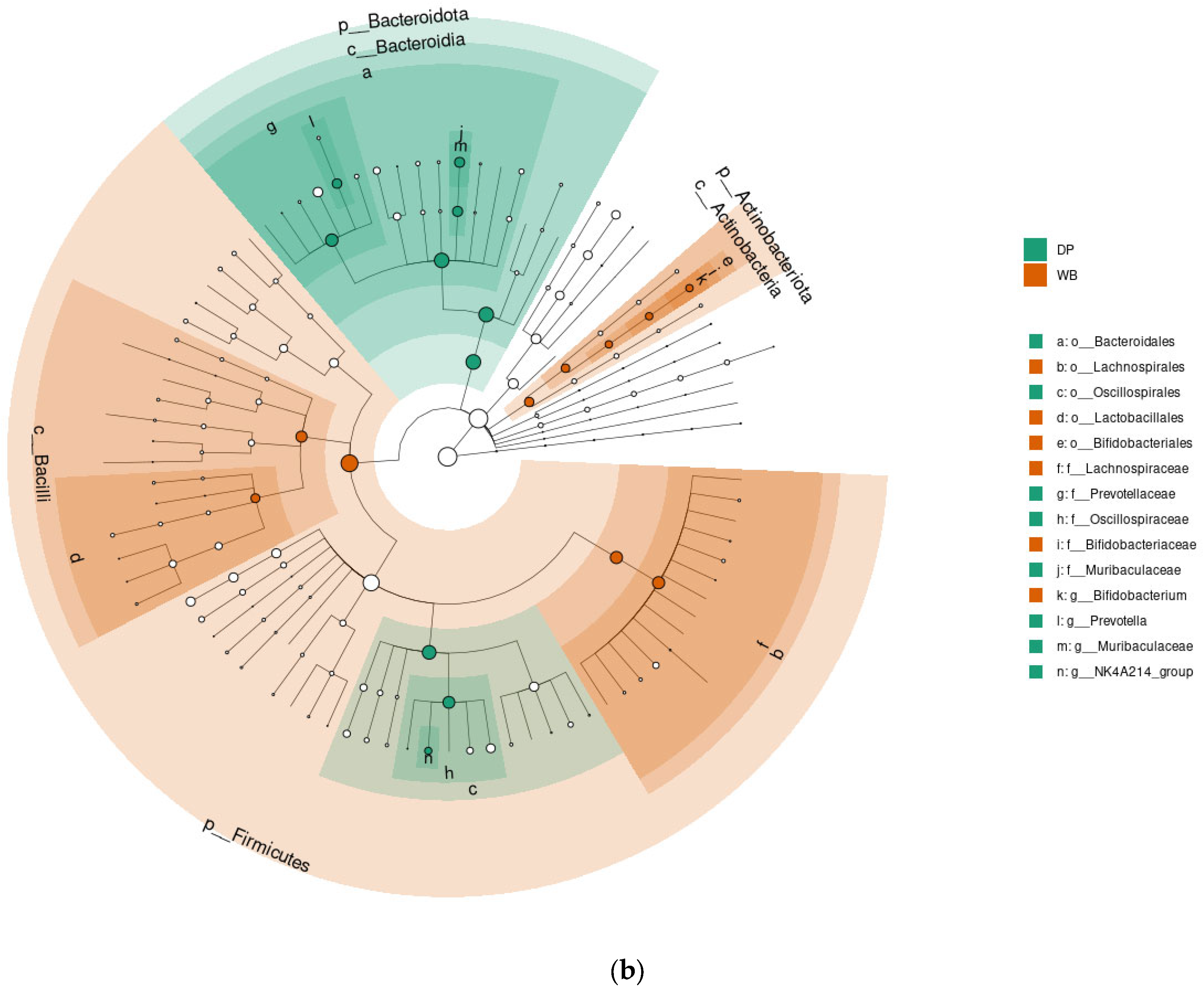
3.4. Linear Discriminant Analysis Effect Size (LEfSe) Analysis
3.5. Predicted Functions of Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lamendella, R.; Santo Domingo, J.W.; Ghosh, S.; Martinson, J.; Oerther, D.B. Comparative fecal metagenomics unveils unique functional capacity of the swine gut. BMC Microbiol. 2011, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, R.; Kim, H.B. The intestinal microbiome of the pig. Anim. Health Res. Rev. 2012, 13, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Neu, J.; Douglas-Escobar, M.; Lopez, M. Microbes and the developing gastrointestinal tract. Nutr. Clin. Pract. 2007, 22, 174–182. [Google Scholar] [CrossRef]
- Wan, X.; Yang, Q.; Wang, X.; Bai, Y.; Liu, Z. Isolation and Cultivation of Human Gut Microorganisms: A Review. Microorganisms 2023, 11, 1080. [Google Scholar] [CrossRef]
- Gupta, S.; Mortensen, M.S.; Schjørring, S.; Trivedi, U.; Vestergaard, G.; Stokholm, J.; Bisgaard, H.; Krogfelt, K.A.; Sørensen, S.J. Amplicon sequencing provides more accurate microbiome information in healthy children compared to culturing. Commun. Biol. 2019, 2, 291. [Google Scholar] [CrossRef]
- Kim, H.B.; Isaacson, R.E. The pig gut microbial diversity: Understanding the pig gut microbial ecology through the next generation high throughput sequencing. Vet. Microbiol. 2015, 177, 242–251. [Google Scholar] [CrossRef]
- Yang, L.; Bian, G.; Su, Y.; Zhu, W. Comparison of Faecal Microbial Community of Lantang, Bama, Erhualian, Meishan, Xiaomeishan, Duroc, Landrace, and Yorkshire Sows. Asian-Australas. J. Anim. Sci. 2014, 27, 898–906. [Google Scholar] [CrossRef]
- Zhao, J.B.; Liu, P.; Huang, C.F.; Liu, L.; Li, E.K.; Zhang, G.; Zhang, S. Effect of wheat bran on apparent total tract digestibility, growth performance, fecal microbiota and their metabolites in growing pigs. Anim. Feed Sci. Technol. 2018, 239, 14–26. [Google Scholar] [CrossRef]
- Giuffra, E.; Kijas, J.M.H.; Amarger, V.; Carlborg, Ö.; Jeon, J.-T.; Andersson, L. The Origin of the Domestic Pig: Independent Domestication and Subsequent Introgression. Genetics 2000, 154, 1785–1791. [Google Scholar] [CrossRef]
- Schley, L.; Roper, T.J. Diet of wild boar Sus scrofa in Western Europe, with particular reference to consumption of agricultural crops. Mammal Rev. 2003, 33, 43–56. [Google Scholar] [CrossRef]
- Montes-Sánchez, J.J.; Huato-Soberanis, L.; Buntinx-Dios, S.E.; León-de la Luz, J.L. The Feral Pig in a Low Impacted Ecosystem: Analysis of Diet Composition and Its Utility. Rangel. Ecol. Manag. 2020, 73, 703–711. [Google Scholar] [CrossRef]
- Uhr, G. The intestinal tract and the Peyer’s patch dimensions of wild boars (Sus scrofa L., 1758) and domestic pigs (Sus scrofa f. domestica). An allometric comparison. J. Mt. Ecol. 1995, 3, 77–82. [Google Scholar]
- Ushida, K.; Tsuchida, S.; Ogura, Y.; Toyoda, A.; Maruyama, F. Domestication and cereal feeding developed domestic pig-type intestinal microbiota in animals of suidae. Anim. Sci. J. 2016, 87, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Francioli, D.; Lentendu, G.; Lewin, S.; Kolb, S. DNA metabarcoding for the characterization of terrestrial microbiota-pitfalls and solutions. Microorganisms 2021, 9, 361. [Google Scholar] [CrossRef]
- CJ Bioscience, Inc. 16S rRNA Microbiome Protocol for Illumina iSeq 100. Available online: https://help.ezbiocloud.net/16s-mtp-protocol-for-illumina-iseq-100/ (accessed on 13 September 2024).
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef]
- Liu, C.; Cui, Y.; Li, X.; Yao, M. microeco: An R package for data mining in microbial community ecology. FEMS Microbiol. Ecol. 2021, 97, fiaa255. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Kuthyar, S.; Diaz, J.; Avalos-Villatoro, F.; Maltecca, C.; Tiezzi, F.; Dunn, R.R.; Reese, A.T. Domestication shapes the pig gut microbiome and immune traits from the scale of lineage to population. J. Evol. Biol. 2023, 36, 1695–1711. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, H.; Zhang, C.; Wang, G.; Shi, C.; Li, Z.; Gao, F.; Cui, Y.; Li, M.; Yang, G. Composition and evolutionary characterization of the gut microbiota in pigs. Int. Microbiol. 2023, 27, 993–1008. [Google Scholar] [CrossRef]
- Petrelli, S.; Buglione, M.; Rivieccio, E.; Ricca, E.; Baccigalupi, L.; Scala, G.; Fulgione, D. Reprogramming of the gut microbiota following feralization in Sus scrofa. Anim. Microbiome 2023, 5, 14. [Google Scholar] [CrossRef]
- Nguyen, D.; Nyachoti, C.; Kim, I. Evaluation of effect of probiotics mixture supplementation on growth performance, nutrient digestibility, faecal bacterial enumeration, and noxious gas emission in weaning pigs. Ital. J. Anim. Sci. 2019, 18, 466–473. [Google Scholar] [CrossRef]
- Suo, C.; Yin, Y.; Wang, X.; Lou, X.; Song, D.; Wang, X.; Gu, Q. Effects of Lactobacillus plantarum ZJ316 on pig growth and pork quality. BMC Vet. Res. 2012, 8, 89. [Google Scholar] [CrossRef]
- Pluske, J.R. Feed- and feed additives-related aspects of gut health and development in weanling pigs. J. Anim. Sci. Biotechnol. 2013, 4, 1. [Google Scholar] [CrossRef]
- Guo, X.; Li, D.; Lu, W.; Piao, X.; Chen, X. Screening of Bacillus strains as potential probiotics and subsequent confirmation of the in vivo effectiveness of Bacillus subtilis MA139 in pigs. Antonie Van Leeuwenhoek 2006, 90, 139–146. [Google Scholar] [CrossRef]
- Rahman, R.; Fouhse, J.M.; Ju, T.; Fan, Y.; Marcolla, C.S.; Pieper, R.; Brook, R.K.; Willing, B.P. A comparison of wild boar and domestic pig microbiota does not reveal a loss of microbial species but an increase in alpha diversity and opportunistic genera in domestic pigs. Microbiol. Spectr. 2024, 12, e0084324. [Google Scholar] [CrossRef]
- Wei, L.; Zhou, W.; Zhu, Z. Comparison of Changes in Gut Microbiota in Wild Boars and Domestic Pigs Using 16S rRNA Gene and Metagenomics Sequencing Technologies. Animals 2022, 12, 2270. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, W.; Fan, R.; Liu, Z.; Huang, T.; Li, J.; Du, T.; Xiong, T. Composition and functional diversity of fecal bacterial community of wild boar, commercial pig and domestic native pig as revealed by 16S rRNA gene sequencing. Arch. Microbiol. 2020, 202, 843–857. [Google Scholar] [CrossRef]
- Gareau, M.G.; Sherman, P.M.; Walker, W.A. Probiotics and the gut microbiota in intestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Ray, K. Infection: Lactobacillus probiotic could prevent recurrent UTI. Nat. Rev. Urol. 2011, 8, 292. [Google Scholar] [CrossRef] [PubMed]
- Valeriano, V.; Balolong, M.; Kang, D.K. Probiotic roles of Lactobacillus sp. in swine: Insights from gut microbiota. J. Appl. Microbiol. 2017, 122, 554–567. [Google Scholar] [CrossRef]
- Mallo, J.J.; Rioperez, J.; Honrubia, P. The addition of Enterococcus faecium to diet improves piglet’s intestinal microbiota and performance. Livest. Sci. 2010, 133, 176–178. [Google Scholar] [CrossRef]
- Patil, A.; Kumar, S.; Verma, A.; Baghel, R. Probiotics as feed additives in weaned pigs: A review. Livest. Res. Int. 2015, 3, 31–39. [Google Scholar]
- Upadhaya, S.D.; Kim, I.H. The Impact of Weaning Stress on Gut Health and the Mechanistic Aspects of Several Feed Additives Contributing to Improved Gut Health Function in Weanling Piglets—A Review. Animals 2021, 11, 2418. [Google Scholar] [CrossRef]
- Khattab, M.S.; Abd El Tawab, A.M.; Fouad, M.T. Isolation and characterization of anaerobic bacteria from frozen rumen liquid and its potential characterizations. Int. J. Dairy Sci. 2016, 12, 47–51. [Google Scholar] [CrossRef][Green Version]
- Kalyana Chakravarthy, S.; Jayasudha, R.; Sai Prashanthi, G.; Ali, M.H.; Sharma, S.; Tyagi, M.; Shivaji, S. Dysbiosis in the Gut Bacterial Microbiome of Patients with Uveitis, an Inflammatory Disease of the Eye. Indian J. Microbiol. 2018, 58, 457–469. [Google Scholar] [CrossRef]
- Dibner, J.; Richards, J.D. Antibiotic growth promoters in agriculture: History and mode of action. Poult. Sci. 2005, 84, 634–643. [Google Scholar] [CrossRef]
- Tasnim, N.; Abulizi, N.; Pither, J.; Hart, M.M.; Gibson, D.L. Linking the gut microbial ecosystem with the environment: Does gut health depend on where we live? Front. Microbiol. 2017, 8, 299664. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Paul, S.; Dutta, C. Geography, ethnicity or subsistence-specific variations in human microbiome composition and diversity. Front. Microbiol. 2017, 8, 237451. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.E.; Brown, A.E.; Williams, C.L. The role of diet and host species in shaping the seasonal dynamics of the gut microbiome. FEMS Microbiol. Ecol. 2023, 99, fiad156. [Google Scholar] [CrossRef]
- Zeng, Y.; Zeng, D.; Zhou, Y.; Niu, L.; Deng, J.; Li, Y.; Pu, Y.; Lin, Y.; Xu, S.; Liu, Q.; et al. Microbial Biogeography Along the Gastrointestinal Tract of a Red Panda. Front. Microbiol. 2018, 9, 1411. [Google Scholar] [CrossRef]
- Li, S.; Zheng, J.; He, J.; Liu, H.; Huang, Y.; Huang, L.; Wang, K.; Zhao, X.; Feng, B.; Che, L.; et al. Dietary fiber during gestation improves lactational feed intake of sows by modulating gut microbiota. J. Anim. Sci. Biotechnol. 2023, 14, 65. [Google Scholar] [CrossRef]
- Chen, W.; Chen, X.; Zhang, Y.; Wu, H.; Zhao, D. Variation on gut microbiota diversity of endangered red pandas (Ailurus fulgens) living in captivity acrosss geographical latitudes. Front. Microbiol. 2024, 15, 1420305. [Google Scholar] [CrossRef]
- Ma, Y.; Han, X.; Fang, J.; Jiang, H. Role of dietary amino acids and microbial metabolites in the regulation of pig intestinal health. Anim. Nutr. 2022, 9, 1–6. [Google Scholar] [CrossRef]




| Domestic Pig | Total (n = 35) | Wild Boar | Total (n = 34) | |
|---|---|---|---|---|
| Stage/Age (years) | ||||
| Weanling | 8 | Juvernile (<1 year) | 7 | |
| Grower | 8 | Subadult (1–2 year) | 12 | |
| Gestating sows | 8 | Adult (>2 year) | 15 | |
| Lactating sows | 7 | |||
| Finisher | 4 | |||
| Sex | ||||
| Male | 15 | 15 | ||
| Female | 20 | 19 | ||
| Farm/Location of capture (Province) | ||||
| Farm A | 15 | Gyeongnam | 15 | |
| Farm B | 20 | Jeonnam | 12 | |
| Gangwon | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, D.-Y.; Moon, S.-H.; Lee, T.G.; Ko, Y.-S.; Cho, Y.-C.; Kang, H.; Park, C.-S.; Kang, J.-S.; Oh, Y.; Cho, H.-S. Consequences of Domestication on Gut Microbiome: A Comparative Analysis Between Wild Boars and Domestic Pigs. Animals 2025, 15, 747. https://doi.org/10.3390/ani15050747
Bae D-Y, Moon S-H, Lee TG, Ko Y-S, Cho Y-C, Kang H, Park C-S, Kang J-S, Oh Y, Cho H-S. Consequences of Domestication on Gut Microbiome: A Comparative Analysis Between Wild Boars and Domestic Pigs. Animals. 2025; 15(5):747. https://doi.org/10.3390/ani15050747
Chicago/Turabian StyleBae, Da-Yun, Sung-Hyun Moon, Taek Geun Lee, Young-Seung Ko, Yun-Chae Cho, Hamin Kang, Chan-Soo Park, Jung-Sun Kang, Yeonsu Oh, and Ho-Seong Cho. 2025. "Consequences of Domestication on Gut Microbiome: A Comparative Analysis Between Wild Boars and Domestic Pigs" Animals 15, no. 5: 747. https://doi.org/10.3390/ani15050747
APA StyleBae, D.-Y., Moon, S.-H., Lee, T. G., Ko, Y.-S., Cho, Y.-C., Kang, H., Park, C.-S., Kang, J.-S., Oh, Y., & Cho, H.-S. (2025). Consequences of Domestication on Gut Microbiome: A Comparative Analysis Between Wild Boars and Domestic Pigs. Animals, 15(5), 747. https://doi.org/10.3390/ani15050747







