Multi-Omics Analysis Reveals the Negative Effects of High-Concentrate Diets on the Colonic Epithelium of Dumont Lambs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Feeding and Experimental Design
2.2. Sample Collection
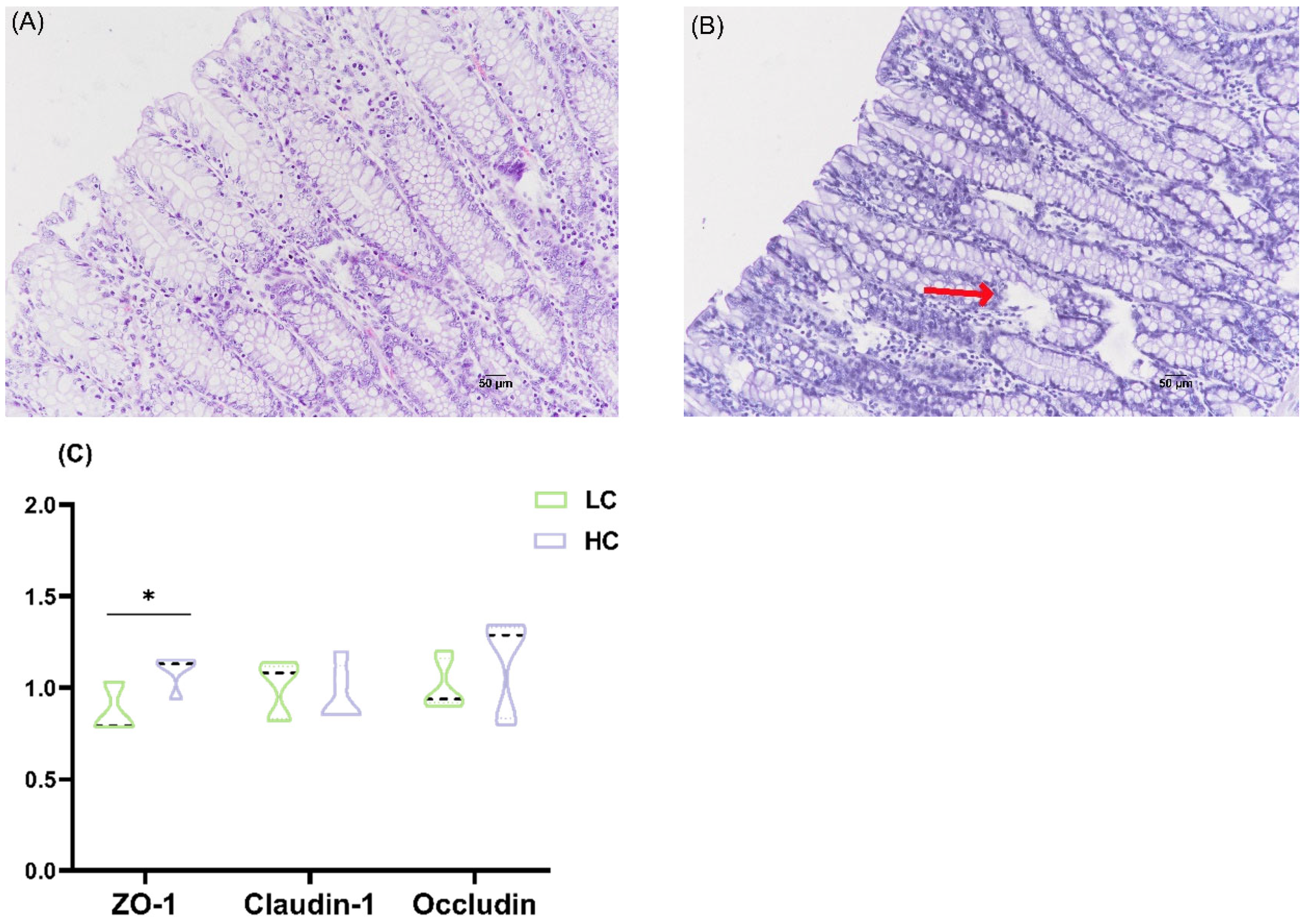
2.3. Histological Analysis of Colon Tissue
2.4. Measurement of Colonic Epithelial LPS Content and Serum Parameters
2.5. Quantitative Real-Time PCR Analysis
2.6. Measurement of VFA Concentration
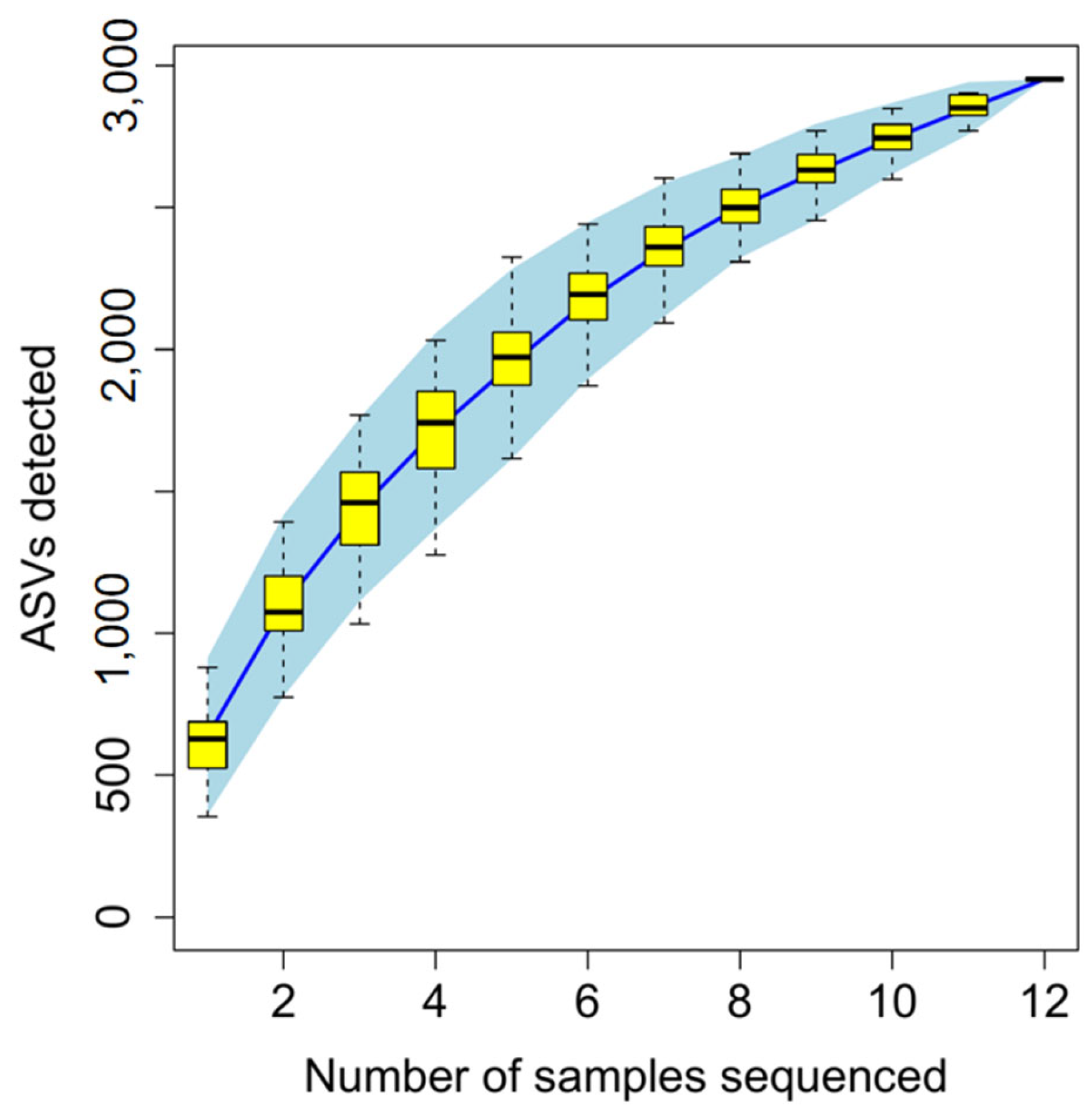
2.7. Colon Microbial Diversity Analysis
2.8. Metabolomics Analysis of Colon Content
2.9. Colon Transcriptome Sequencing
2.10. Statistical Analyses
3. Results
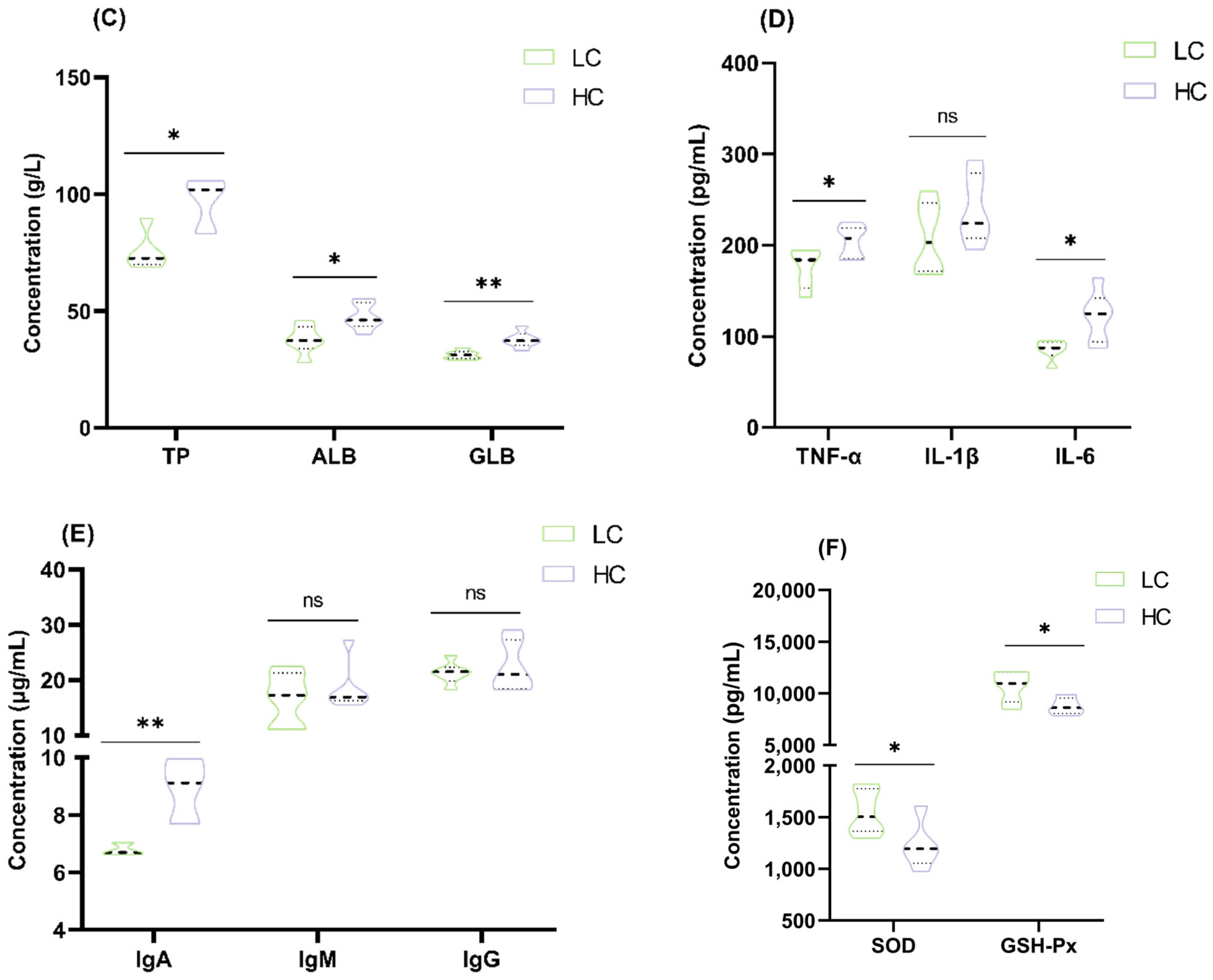
3.1. Effect of HC Diet on Colon Epithelial Lipopolysaccharide Content and Serum Parameters in Dumont Lambs
3.2. Effects of HC Diet on Colonic Epithelium Morphology and Tight Junction mRNA Expression in Dumont Lambs
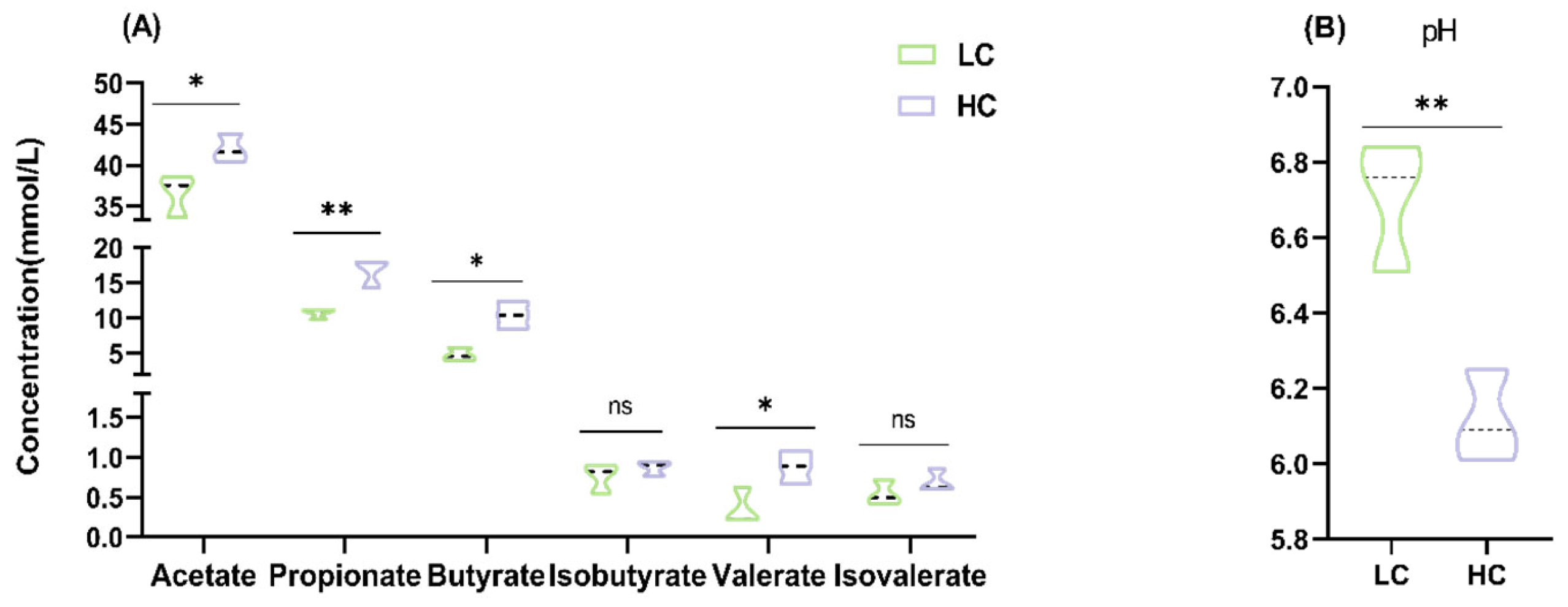
3.3. Effect of HC Diet on Colon Fermentation Parameters and Microbial Composition in Dumont Lambs
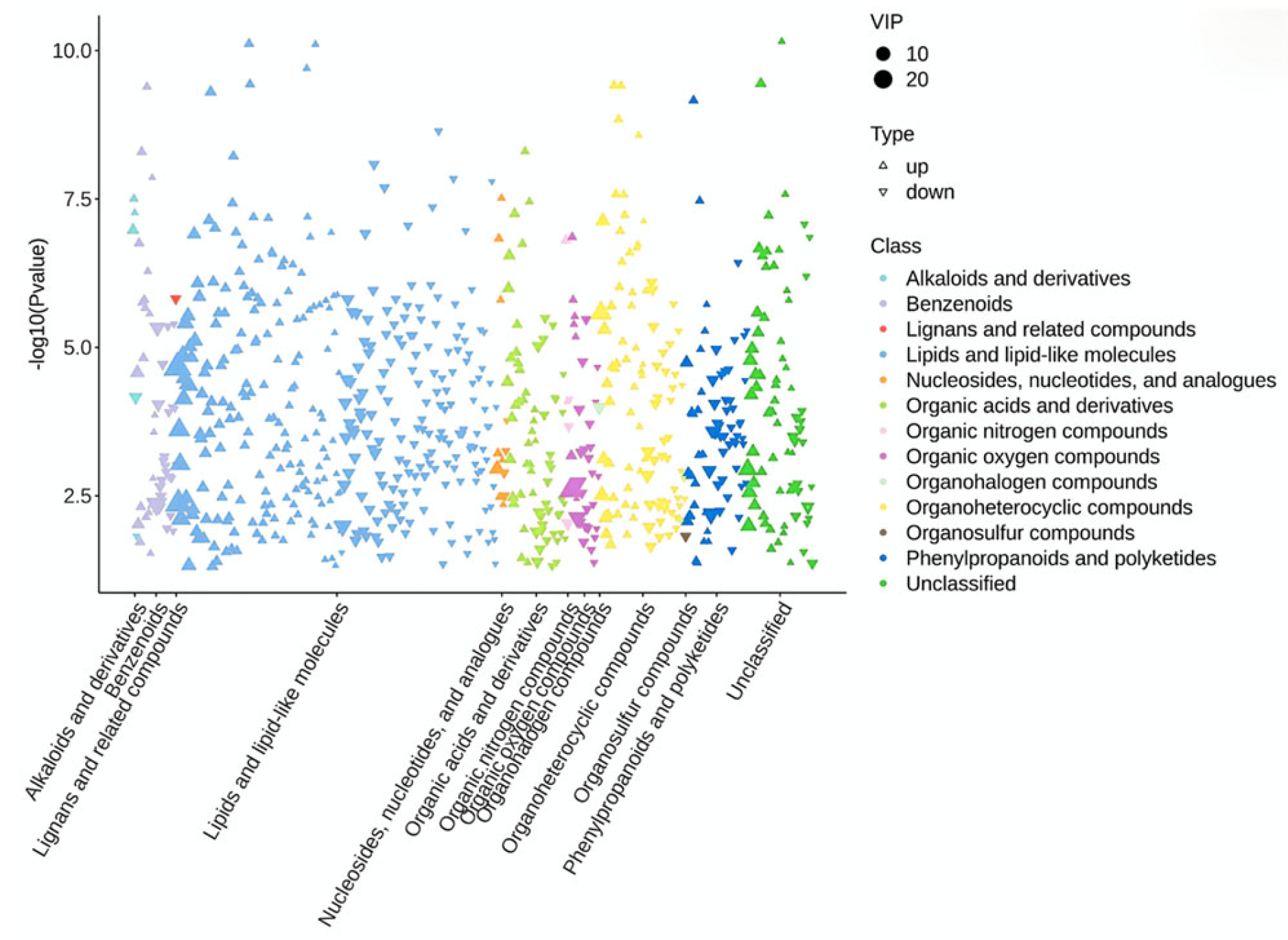
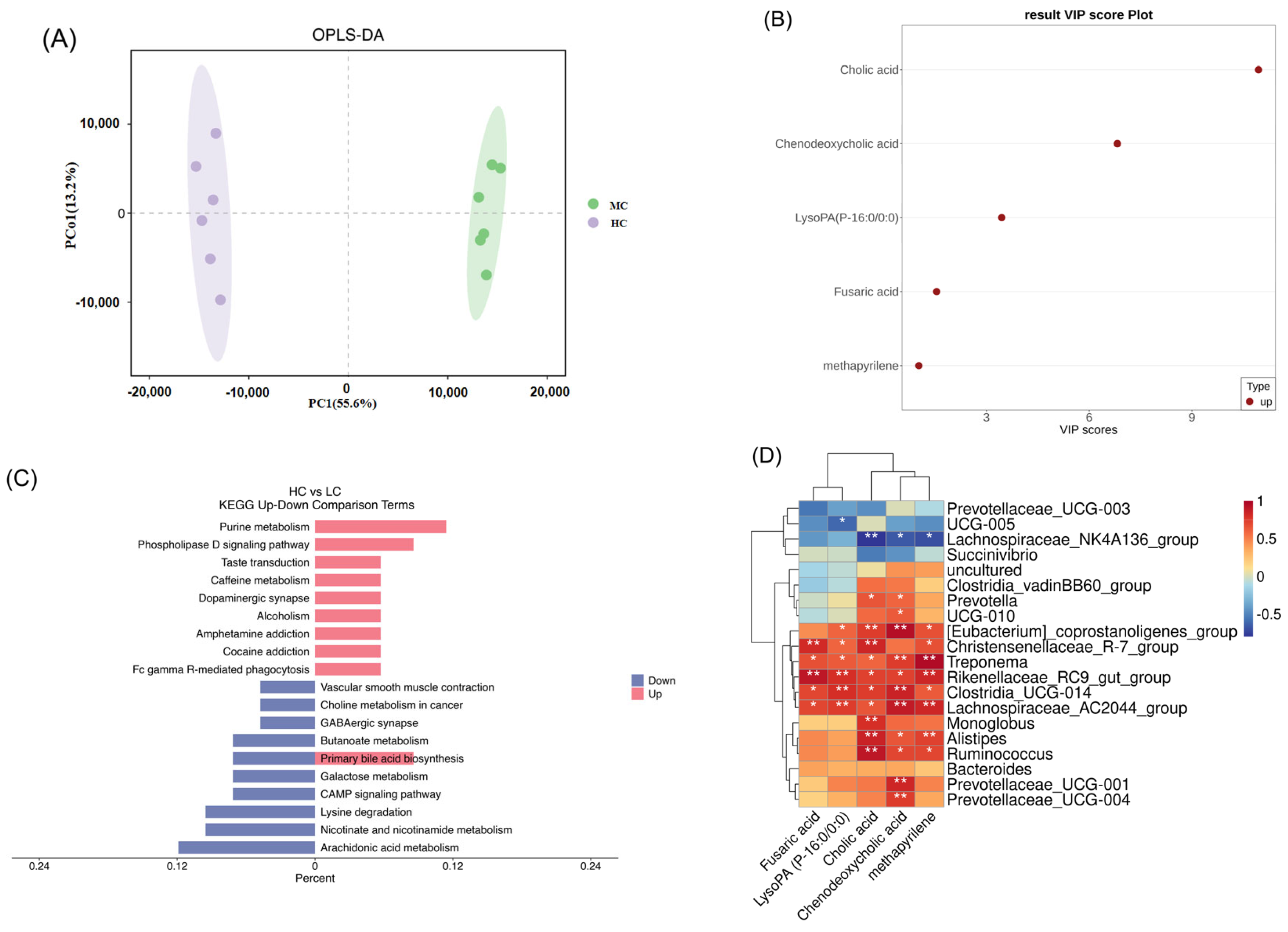
3.4. Effect of HC Diet on Metabolites in Colon Content of Dumont Lambs
3.5. Effect of HC Diet on the Transcription Profile of the Colon Epithelium
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VFAs | Volatile fatty acids |
| ASVs | Amplicon sequence variants |
| PCoA | Principal coordinate analysis |
| LEfSe | Linear discriminant analysis (LDA) coupled with effect size measurements |
| OPLS-DA | Orthogonal partial least squares discrimination analysis |
| VIP | Variable important in projection |
| KEGG | Kyoto encyclopedia of genes and genomes |
| DEGs | Differentially expressed genes |
Appendix A
Appendix A.1
| Items | LC | HC |
|---|---|---|
| Ingredients (% of DM) | ||
| Mixed hay | 70.00 | 30.00 |
| Corn | 28.80 | 53.00 |
| Soybean meal | 0.00 | 14.9 |
| Calcium phosphate dibasic | 0.00 | 0.10 |
| Salt | 0.70 | 0.50 |
| Premix1 | 0.50 | 0.50 |
| Sodium bicarbonate | 0.00 | 1.00 |
| Total | 100.00 | 100.00 |
| Nutrient levels (%) | ||
| Crude protein | 10.70 | 14.40 |
| Soluble carbohydrates | 37.50 | 49.90 |
| Neutral detergent fiber | 36.90 | 23.30 |
| Calcium | 1.40 | 0.70 |
| Phosphorus | 0.30 | 0.30 |
| Acid detergent fiber | 27.90 | 15.00 |
| Soluble carbohydrates/NDF ratio | 1.02 | 2.14 |
| Metabolizable energy (MJ/kg) | 9.00 | 10.40 |
Appendix A.2
| Genes | Primer sequence (5′-3′) | Product Size/bp | Tm/°C |
|---|---|---|---|
| Claudin-1 | F: GTGGATGTCGTGCGTGTC R: TAGTCCCAGCAGGATGCC | 127 | 58 |
| Occludin | F: AGCAGCAGTGGTAACTTGG R: TCCCGTCGTGTAGTCTGTT | 111 | 58 |
| ZO-1 | F: CGAGCAGACGCAGAAAA R: GGCAGAAGATTGTGGTTGA | 123 | 55 |
| β-actin | F: CATCGTCCACCGCAAAT R: GCCATGCCAATCTCATCTC | 103 | 56 |
| GAPDH | F: GGTCGGAGTGAACGGATTTG R: TGGCAACGATGTCCACTTTG | 83 | 59 |
Appendix B

References
- Plaizier, J.C.; Danesh Mesgaran, M.; Derakhshani, H.; Golder, H.; Khafipour, E.; Kleen, J.L.; Lean, I.; Loor, J.; Penner, G.; Zebeli, Q. Review: Enhancing gastrointestinal health in dairy cows. Anim. Int. J. Anim. Biosci. 2018, 12, s399–s418. [Google Scholar] [CrossRef] [PubMed]
- Gray, F.V. The digestion of cellulose by sheep; the extent of cellulose digestion at successive levels of the alimentary tract. J. Exp. Biol. 1947, 24, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Myers, L.L.; Jackson, H.D.; Packett, L.V. Absorption of volatle fatty acids from the cecum of sheep. J. Anim. Sci. 1967, 26, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef]
- Matthé, A.; Lebzien, P.; Hric, I.; Flachowsky, G. Influence of prolonged adaptation periods on starch degradation in the digestive tract of dairy cows. Anim. Feed. Sci. Technol. 2003, 103, 15–27. [Google Scholar] [CrossRef]
- Plascencia, A.; Bermúdez, R.M.; Cervantes, M.; Corona, L.; Dávila-Ramos, H.; López-Soto, M.A.; May, D.; Torrentera, N.G.; Zinn, R.A. Influence of processing method on comparative digestion of white corn versus conventional steam-flaked yellow dent corn in finishing diets for feedlot cattle. J. Anim. Sci. 2011, 89, 136–141. [Google Scholar] [CrossRef]
- Tao, S.; Duanmu, Y.; Dong, H.; Tian, J.; Ni, Y.; Zhao, R. A high-concentrate diet induced colonic epithelial barrier disruption is associated with the activating of cell apoptosis in lactating goats. BMC Vet. Res. 2014, 10, 235. [Google Scholar] [CrossRef]
- Gressley, T.F.; Hall, M.B.; Armentano, L.E. Ruminant Nutrition Symposium: Productivity, digestion, and health responses to hindgut acidosis in ruminants. J. Anim. Sci. 2011, 89, 1120–1130. [Google Scholar] [CrossRef]
- McNeil, N.I.; Ling, K.L.; Wager, J. Mucosal surface pH of the large intestine of the rat and of normal and inflamed large intestine in man. Gut 1987, 28, 707–713. [Google Scholar] [CrossRef]
- Jose, V.L.; Appoothy, T.; More, R.P.; Arun, A.S. Metagenomic insights into the rumen microbial fibrolytic enzymes in Indian crossbred cattle fed finger millet straw. AMB Express 2017, 7, 13. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xie, W.; Zhou, S.; Ma, N.; Wang, Y.; Huang, J.; Shen, X.; Chang, G. A high-concentrate diet induces colonic inflammation and barrier damage in Hu sheep. J. Dairy Sci. 2023, 106, 9644–9662. [Google Scholar] [CrossRef]
- Liu, J.; Xu, T.; Zhu, W.; Mao, S. High-grain feeding alters caecal bacterial microbiota composition and fermentation and results in caecal mucosal injury in goats. Br. J. Nutr. 2014, 112, 416–427. [Google Scholar] [CrossRef]
- Lin, L.; Trabi, E.B.; Xie, F.; Mao, S. Comparison of the fermentation and bacterial community in the colon of Hu sheep fed a low-grain, non-pelleted, or pelleted high-grain diet. Appl. Microbiol. Biotechnol. 2021, 105, 2071–2080. [Google Scholar] [CrossRef]
- Wang, B. Effects of Feeding Regimens on Gastrointestinal Microbiota, FattyAcid Metabolism and Meat Quality of Sunit Sheep and Its Underlying Mechanism. Ph.D. Dissertation, Inner Mongolia Agricultural University, Hohhot, China, 2019. [Google Scholar]
- Lyte, M.; Villageliú, D.N.; Crooker, B.A.; Brown, D.R. Symposium review: Microbial endocrinology-Why the integration of microbes, epithelial cells, and neurochemical signals in the digestive tract matters to ruminant health. J. Dairy Sci. 2018, 101, 5619–5628. [Google Scholar] [CrossRef]
- Spor, A.; Koren, O.; Ley, R. Unravelling the effects of the environment and host genotype on the gut microbiome. Nat. Rev. Microbiol. 2011, 9, 279–290. [Google Scholar] [CrossRef]
- Jin, W.; Li, Y.; Cheng, Y.; Mao, S.; Zhu, W. The bacterial and archaeal community structures and methanogenic potential of the cecal microbiota of goats fed with hay and high-grain diets. Antonie Van Leeuwenhoek 2018, 111, 2037–2049. [Google Scholar] [CrossRef]
- Tao, S.; Tian, P.; Luo, Y.; Tian, J.; Hua, C.; Geng, Y.; Cong, R.; Ni, Y.; Zhao, R. Microbiome-Metabolome Responses to a High-Grain Diet Associated with the Hind-Gut Health of Goats. Front. Microbiol. 2017, 8, 1764. [Google Scholar] [CrossRef]
- Diez-Gonzalez, F.; Callaway, T.R.; Kizoulis, M.G.; Russell, J.B. Grain feeding and the dissemination of acid-resistant Escherichia coli from cattle. Science 1998, 281, 1666–1668. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Hall, M.; Beiko, R.G. 16S rRNA Gene Analysis with QIIME2. Methods Mol. Biol. 2018, 1849, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.X.; Dai, Z.L.; Zhu, W.Y. Important impacts of intestinal bacteria on utilization of dietary amino acids in pigs. Amino Acids 2014, 46, 2489–2501. [Google Scholar] [CrossRef]
- Hoover, W.H. Digestion and absorption in the hindgut of ruminants. J. Anim. Sci. 1978, 46, 1789–1799. [Google Scholar] [CrossRef]
- LI, H.; Liu, J.; Huo, W.; Zhu, W.; Mao, S. Effects of high concentrate diet on microbial fermentation and biogenic amine formation and absorption in the rumen and cecum of goats. Acta Pratacult. Sin. 2017, 26, 210–216. [Google Scholar]
- Ye, H.; Liu, J.; Feng, P.; Zhu, W.; Mao, S. Grain-rich diets altered the colonic fermentation and mucosa-associated bacterial communities and induced mucosal injuries in goats. Sci. Rep. 2016, 6, 20329. [Google Scholar] [CrossRef]
- Petri, R.M.; Aditya, S.; Humer, E.; Zebeli, Q. Effect of an intramammary lipopolysaccharide challenge on the hindgut microbial composition and fermentation of dairy cattle experiencing intermittent subacute ruminal acidosis. J. Dairy Sci. 2021, 104, 5417–5431. [Google Scholar] [CrossRef]
- Plaizier, J.C.; Li, S.; Le Sciellour, M.; Schurmann, B.L.; Górka, P.; Penner, G.B. Effects of duration of moderate increases in grain feeding on endotoxins in the digestive tract and acute phase proteins in peripheral blood of yearling calves. J. Dairy Sci. 2014, 97, 7076–7084. [Google Scholar] [CrossRef]
- Stephens, M.; von der Weid, P.Y. Lipopolysaccharides modulate intestinal epithelial permeability and inflammation in a species-specific manner. Gut Microbes 2020, 11, 421–432. [Google Scholar] [CrossRef]
- Han, X.; Lei, X.; Yang, X.; Shen, J.; Zheng, L.; Jin, C.; Cao, Y.; Yao, J. A Metagenomic Insight Into the Hindgut Microbiota and Their Metabolites for Dairy Goats Fed Different Rumen Degradable Starch. Front. Microbiol. 2021, 12, 651631. [Google Scholar] [CrossRef]
- Opal, S.M. The host response to endotoxin, antilipopolysaccharide strategies, and the management of severe sepsis. Int. J. Med. Microbiol. IJMM 2007, 297, 365–377. [Google Scholar] [CrossRef]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Gao, Z.; Yang, X.; Guo, S.; Li, S.; Wang, H. Effects of Forage with Varied Concentrate/Roughage Ratios on Growth, Serum Biochemistry, and Immunity of Dumeng Lambs. Fujian J. Agric. Sci. 2022, 37, 1519–1527. [Google Scholar] [CrossRef]
- Yang, X. Effect of Adding Sophora alopecuroides L. in High-Concentration Diet on Growth Performance, Serum Biochemical and Immune Indexes of Mongolian Lambs. Master’s Thesis, Inner Mongolia University, Hohhot, China, 2021. [Google Scholar]
- Ibrahim, S.; Zhu, X.; Luo, X.; Feng, Y.; Wang, J. PIK3R3 regulates ZO-1 expression through the NF-kB pathway in inflammatory bowel disease. Int. Immunopharmacol. 2020, 85, 106610. [Google Scholar] [CrossRef]
- Shi, H.; Sha, Y.; Wei, H.; Lv, H.; Wen, Y.; Liu, X.; Luo, Y.; Wang, J.; Li, S.; Hu, J. Interaction between Rumen Microbial Density and Short Chain Fatty Acid Concentration in Tibetan Sheep during Warm and Cold Seasons. Chin. J. Anim. Nutr. 2021, 33, 1191–1200. [Google Scholar]
- Fan, Q.; Cui, X.; Wang, Z.; Chang, S.; Wanapat, M.; Yan, T.; Hou, F. Rumen Microbiota of Tibetan Sheep (Ovis aries) Adaptation to Extremely Cold Season on the Qinghai-Tibetan Plateau. Front. Vet. Sci. 2021, 8, 673822. [Google Scholar] [CrossRef]
- Riva, A.; Kuzyk, O.; Forsberg, E.; Siuzdak, G.; Pfann, C.; Herbold, C.; Daims, H.; Loy, A.; Warth, B.; Berry, D. A fiber-deprived diet disturbs the fine-scale spatial architecture of the murine colon microbiome. Nat. Commun. 2019, 10, 4366. [Google Scholar] [CrossRef]
- Thomas, F.; Hehemann, J.H.; Rebuffet, E.; Czjzek, M.; Michel, G. Environmental and gut bacteroidetes: The food connection. Front. Microbiol. 2011, 2, 93. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Kim, Y.S.; Milner, J.A. Dietary modulation of colon cancer risk. J. Nutr. 2007, 137, 2576s–2579s. [Google Scholar] [CrossRef]
- Binda, C.; Lopetuso, L.R.; Rizzatti, G.; Gibiino, G.; Cennamo, V.; Gasbarrini, A. Actinobacteria: A relevant minority for the maintenance of gut homeostasis. Dig. Liver Dis. 2018, 50, 421–428. [Google Scholar] [CrossRef]
- Pitta, D.W.; Pinchak, E.; Dowd, S.E.; Osterstock, J.; Gontcharova, V.; Youn, E.; Dorton, K.; Yoon, I.; Min, B.R.; Fulford, J.D.; et al. Rumen bacterial diversity dynamics associated with changing from bermudagrass hay to grazed winter wheat diets. Microb. Ecol. 2010, 59, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Freier, T.A.; Beitz, D.C.; Li, L.; Hartman, P.A. Characterization of Eubacterium coprostanoligenes sp. nov., a cholesterol-reducing anaerobe. Int. J. Syst. Bacteriol. 1994, 44, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.J.; Brown, J.M.; Murray, R.D.; Getty, B.; Birtles, R.J.; Hart, C.A.; Carter, S.D. Characterization of novel bovine gastrointestinal tract Treponema isolates and comparison with bovine digital dermatitis treponemes. Appl. Environ. Microbiol. 2011, 77, 138–147. [Google Scholar] [CrossRef]
- Marizzoni, M.; Mirabelli, P.; Mombelli, E.; Coppola, L.; Festari, C.; Lopizzo, N.; Luongo, D.; Mazzelli, M.; Naviglio, D.; Blouin, J.L.; et al. A peripheral signature of Alzheimer’s disease featuring microbiota-gut-brain axis markers. Alzheimer’s Res. Ther. 2023, 15, 101. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Zhang, Q.; Shi, K.; Zhang, Y.; Zhu, B.; Bi, Y.; Wang, X. Gut microbiota dysbiosis with hepatitis B virus liver disease and association with immune response. Front. Cell. Infect. Microbiol. 2023, 13, 1152987. [Google Scholar] [CrossRef]
- Leibovitzh, H.; Lee, S.H.; Xue, M.; Raygoza Garay, J.A.; Hernandez-Rocha, C.; Madsen, K.L.; Meddings, J.B.; Guttman, D.S.; Espin-Garcia, O.; Smith, M.I.; et al. Altered Gut Microbiome Composition and Function Are Associated with Gut Barrier Dysfunction in Healthy Relatives of Patients with Crohn’s Disease. Gastroenterology 2022, 163, 1364–1376.e1310. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria with Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef]
- Zhou, S.; Luo, R.; Gong, G.; Wang, Y.; Gesang, Z.; Wang, K.; Xu, Z.; Suolang, S. Characterization of Metagenome-Assembled Genomes and Carbohydrate-Degrading Genes in the Gut Microbiota of Tibetan Pig. Front. Microbiol. 2020, 11, 595066. [Google Scholar] [CrossRef]
- Mamuad, L.L.; Seo, B.J.; Faruk, M.S.A.; Espiritu, H.M.; Jin, S.J.; Kim, W.I.; Lee, S.S.; Cho, Y.I. Treponema spp., the dominant pathogen in the lesion of bovine digital dermatitis and its characterization in dairy cattle. Vet. Microbiol. 2020, 245, 108696. [Google Scholar] [CrossRef]
- Paraschou, G.; Cook, J.M.; Priestnall, S.L.; Evans, N.J.; Staton, G.J.; Paterson, G.K.; Winkler, B.; Whitbread, T.J. Treponema spp. spirochetes and keratinopathogenic fungi isolated from keratomas in donkeys. Vet. Pathol. 2023, 60, 190–198. [Google Scholar] [CrossRef]
- Liang, J.; Fang, W.; Chang, J.; Zhang, G.; Ma, W.; Nabi, M.; Zubair, M.; Zhang, R.; Chen, L.; Huang, J.; et al. Long-term rumen microorganism fermentation of corn stover in vitro for volatile fatty acid production. Bioresour. Technol. 2022, 358, 127447. [Google Scholar] [CrossRef] [PubMed]
- La Reau, A.J.; Suen, G. The Ruminococci: Key symbionts of the gut ecosystem. J. Microbiol. 2018, 56, 199–208. [Google Scholar] [CrossRef]
- Henke, M.T.; Kenny, D.J.; Cassilly, C.D.; Vlamakis, H.; Xavier, R.J.; Clardy, J. Ruminococcus gnavus, a member of the human gut microbiome associated with Crohn’s disease, produces an inflammatory polysaccharide. Proc. Natl. Acad. Sci. USA 2019, 116, 12672–12677. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, H.; Chen, W.; Liu, C.; Meng, Q.; Zhou, Z. Rumen Microbiome and Metabolome of High and Low Residual Feed Intake Angus Heifers. Front. Vet. Sci. 2022, 9, 812861. [Google Scholar] [CrossRef]
- Pang, K.; Dai, D.; Yang, Y.; Wang, X.; Liu, S.; Huang, W.; Xue, B.; Chai, S.; Wang, S. Effects of high concentrate rations on ruminal fermentation and microbiota of yaks. Front. Microbiol. 2022, 13, 957152. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Lu, Z.; Chai, L.; Zhang, X.; Xu, P.; Li, Q.; Wang, S.; Shen, C.; Shi, J.; Xu, Z. Differences of the structure, succession and function of Clostridial communities between jiupei and pit mud during Luzhou-flavour baijiu fermentation. Chin. J. Biotechnol. 2020, 36, 1190–1197. [Google Scholar] [CrossRef]
- Rajilić-Stojanović, M.; de Vos, W.M. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol. Rev. 2014, 38, 996–1047. [Google Scholar] [CrossRef]
- Wahlström, A.; Sayin, S.I.; Marschall, H.U.; Metabolism, F.B.J.C. Intestinal Crosstalk between Bile Acids and Microbiota and Its Impact on Host Metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef]
- de Aguiar Vallim, T.Q.; Tarling, E.J.; Edwards, P.A.; Metabolism, E.J.C. Pleiotropic Roles of Bile Acids in Metabolism. Cell Metab. 2013, 17, 657–669. [Google Scholar] [CrossRef]
- Stellwag, E.J.; Hylemon, P.B. Purification and characterization of bile salt hydrolase from Bacteroides fragilis subsp. fragilis. Biochim. Et Biophys. Acta 1976, 452, 165–176. [Google Scholar] [CrossRef]
- Coleman, J.P.; Hudson, L.L. Cloning and characterization of a conjugated bile acid hydrolase gene from Clostridium perfringens. Appl. Environ. Microbiol. 1995, 61, 2514–2520. [Google Scholar] [CrossRef] [PubMed]
- Doden, H.L.; Wolf, P.G.; Gaskins, H.R.; Anantharaman, K.; Alves, J.M.P.; Ridlon, J.M. Completion of the gut microbial epi-bile acid pathway. Gut Microbes 2021, 13, 1907271. [Google Scholar] [CrossRef] [PubMed]
- Gebeyew, K.; Chen, K.; Wassie, T.; Azad, M.A.K.; He, J.; Jiang, W.; Song, W.; He, Z.; Tan, Z. Dietary Amylose/Amylopectin Ratio Modulates Cecal Microbiota and Metabolites in Weaned Goats. Front. Nutr. 2021, 8, 774766. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Stoll, B.; Burrin, D.G.; Holst, J.J.; Moore, D.D. Enteral bile acid treatment improves parenteral nutrition-related liver disease and intestinal mucosal atrophy in neonatal pigs. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G218–G224. [Google Scholar] [CrossRef]
- Bilz, S.; Samuel, V.; Morino, K.; Savage, D.; Choi, C.S.; Shulman, G.I. Activation of the farnesoid X receptor improves lipid metabolism in combined hyperlipidemic hamsters. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E716–E722. [Google Scholar] [CrossRef]
- Krauss, R.M.; Blanche, P.J.; Rawlings, R.S.; Fernstrom, H.S.; Williams, P.T. Separate effects of reduced carbohydrate intake and weight loss on atherogenic dyslipidemia. Am. J. Clin. Nutr. 2006, 83, 1025–1031. [Google Scholar] [CrossRef]
- Yang, B.; Huang, S.; Zhao, G.; Ma, Q. Dietary supplementation of porcine bile acids improves laying performance, serum lipid metabolism and cecal microbiota in late-phase laying hens. Anim. Nutr. 2022, 11, 283–292. [Google Scholar] [CrossRef]
- Zhang, X.; Choi, F.F.; Zhou, Y.; Leung, F.P.; Tan, S.; Lin, S.; Xu, H.; Jia, W.; Sung, J.J.; Cai, Z.; et al. Metabolite profiling of plasma and urine from rats with TNBS-induced acute colitis using UPLC-ESI-QTOF-MS-based metabonomics—A pilot study. FEBS J. 2012, 279, 2322–2338. [Google Scholar] [CrossRef]
- Chen, L.; Jiao, T.; Liu, W.; Luo, Y.; Wang, J.; Guo, X.; Tong, X.; Lin, Z.; Sun, C.; Wang, K.; et al. Hepatic cytochrome P450 8B1 and cholic acid potentiate intestinal epithelial injury in colitis by suppressing intestinal stem cell renewal. Cell Stem Cell 2022, 29, 1366–1381.e1369. [Google Scholar] [CrossRef]
- Zheng, M.; Zhai, Y.; Yu, Y.; Shen, J.; Chu, S.; Focaccia, E.; Tian, W.; Wang, S.; Liu, X.; Yuan, X.; et al. TNF compromises intestinal bile-acid tolerance dictating colitis progression and limited infliximab response. Cell Metab. 2024, 36, 2086–2103.e2089. [Google Scholar] [CrossRef]
- Tan, S.T.; Ramesh, T.; Toh, X.R.; Nguyen, L.N. Emerging roles of lysophospholipids in health and disease. Prog. Lipid Res. 2020, 80, 101068. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; van Putten, J.P.M.; Wösten, M. Biological functions of bacterial lysophospholipids. Adv. Microb. Physiol. 2023, 82, 129–154. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wei, H.; Zhou, Y.; Szeto, C.H.; Li, C.; Lin, Y.; Coker, O.O.; Lau, H.C.H.; Chan, A.W.H.; Sung, J.J.Y.; et al. High-Fat Diet Promotes Colorectal Tumorigenesis Through Modulating Gut Microbiota and Metabolites. Gastroenterology 2022, 162, 135–149.e132. [Google Scholar] [CrossRef] [PubMed]
- Ratra, G.S.; Morgan, W.A.; Mullervy, J.; Powell, C.J.; Wright, M.C. Methapyrilene hepatotoxicity is associated with oxidative stress, mitochondrial disfunction and is prevented by the Ca2+ channel blocker verapamil. Toxicology 1998, 130, 79–93. [Google Scholar] [CrossRef]
- Dolenšek, T.; Švara, T.; Knific, T.; Gombač, M.; Luzar, B.; Jakovac-Strajn, B. The Influence of Fusarium Mycotoxins on the Liver of Gilts and Their Suckling Piglets. Animals 2021, 11, 2534. [Google Scholar] [CrossRef]
- Ghazi, T.; Nagiah, S.; Tiloke, C.; Sheik Abdul, N.; Chuturgoon, A.A. Fusaric Acid Induces DNA Damage and Post-Translational Modifications of p53 in Human Hepatocellular Carcinoma (HepG(2)) Cells. J. Cell. Biochem. 2017, 118, 3866–3874. [Google Scholar] [CrossRef]
- Sheik Abdul, N.; Nagiah, S.; Chuturgoon, A.A. Fusaric acid induces mitochondrial stress in human hepatocellular carcinoma (HepG2) cells. Toxicon 2016, 119, 336–344. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, Y.; Yang, Y.; Guo, M.; Zhang, T.; Zong, B.; Huang, S.; Suo, L.; Ma, B.; Wang, X.; et al. Gut microbiota-derived metabolites contribute negatively to hindgut barrier function development at the early weaning goat model. Anim. Nutr. 2022, 10, 111–123. [Google Scholar] [CrossRef]
- Mu, Y.; Qi, W.; Zhang, T.; Zhang, J.; Mao, S. Multi-omics Analysis Revealed Coordinated Responses of Rumen Microbiome and Epithelium to High-Grain-Induced Subacute Rumen Acidosis in Lactating Dairy Cows. mSystems 2022, 7, e0149021. [Google Scholar] [CrossRef]
- Ramos, S.C.; Jeong, C.D.; Mamuad, L.L.; Kim, S.H.; Kang, S.H.; Kim, E.T.; Cho, Y.I.; Lee, S.S.; Lee, S.S. Diet Transition from High-Forage to High-Concentrate Alters Rumen Bacterial Community Composition, Epithelial Transcriptomes and Ruminal Fermentation Parameters in Dairy Cows. Animals 2021, 11, 838. [Google Scholar] [CrossRef]
- Forcina, G.C.; Dixon, S.J. GPX4 at the Crossroads of Lipid Homeostasis and Ferroptosis. Proteomics 2019, 19, e1800311. [Google Scholar] [CrossRef] [PubMed]
- Balsera, M.; Buchanan, B.B. Evolution of the thioredoxin system as a step enabling adaptation to oxidative stress. Free Radic. Biol. Med. 2019, 140, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Powis, G.; Montfort, W.R. Properties and biological activities of thioredoxins. Annu. Rev. Biophys. Biomol. Struct. 2001, 30, 421–455. [Google Scholar] [CrossRef] [PubMed]
- Coskun, M. The role of CDX2 in inflammatory bowel disease. Dan. Med. J. 2014, 61, B4820. [Google Scholar]
- Chewchuk, S.; Jahan, S.; Lohnes, D. Cdx2 regulates immune cell infiltration in the intestine. Sci. Rep. 2021, 11, 15841. [Google Scholar] [CrossRef]
- Kirschning, C.J.; Schumann, R.R. TLR2: Cellular sensor for microbial and endogenous molecular patterns. Curr. Top. Microbiol. Immunol. 2002, 270, 121–144. [Google Scholar] [CrossRef]
- Yuan, B.; Luo, S.; Feng, L.; Wang, J.; Mao, J.; Luo, B. Resveratrol regulates the inflammation and oxidative stress of granulosa cells in PCOS via targeting TLR2. J. Bioenerg. Biomembr. 2022, 54, 191–201. [Google Scholar] [CrossRef]
- Walrath, T.; Malizia, R.A.; Zhu, X.; Sharp, S.P.; D’Souza, S.S.; Lopez-Soler, R.; Parr, B.; Kartchner, B.; Lee, E.C.; Stain, S.C.; et al. IFN-γ and IL-17A regulate intestinal crypt production of CXCL10 in the healthy and inflamed colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2020, 318, G479–G489. [Google Scholar] [CrossRef]
- Overcast, G.R.; Meibers, H.E.; Eshleman, E.M.; Saha, I.; Waggoner, L.; Patel, K.N.; Jain, V.G.; Haslam, D.B.; Alenghat, T.; VanDussen, K.L.; et al. IEC-intrinsic IL-1R signaling holds dual roles in regulating intestinal homeostasis and inflammation. J. Exp. Med. 2023, 220, e20212523. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Wang, H.; Li, B.; Lu, H.; Zhao, J.; Gao, A.; An, Y.; Yang, J.; Ma, T. Multi-Omics Analysis Reveals the Negative Effects of High-Concentrate Diets on the Colonic Epithelium of Dumont Lambs. Animals 2025, 15, 749. https://doi.org/10.3390/ani15050749
Li S, Wang H, Li B, Lu H, Zhao J, Gao A, An Y, Yang J, Ma T. Multi-Omics Analysis Reveals the Negative Effects of High-Concentrate Diets on the Colonic Epithelium of Dumont Lambs. Animals. 2025; 15(5):749. https://doi.org/10.3390/ani15050749
Chicago/Turabian StyleLi, Shufang, Hairong Wang, Boyang Li, Henan Lu, Jianxin Zhao, Aiwu Gao, Yawen An, Jinli Yang, and Tian Ma. 2025. "Multi-Omics Analysis Reveals the Negative Effects of High-Concentrate Diets on the Colonic Epithelium of Dumont Lambs" Animals 15, no. 5: 749. https://doi.org/10.3390/ani15050749
APA StyleLi, S., Wang, H., Li, B., Lu, H., Zhao, J., Gao, A., An, Y., Yang, J., & Ma, T. (2025). Multi-Omics Analysis Reveals the Negative Effects of High-Concentrate Diets on the Colonic Epithelium of Dumont Lambs. Animals, 15(5), 749. https://doi.org/10.3390/ani15050749








