Invited Review: Inflammation and Health in the Transition Period Influence Reproductive Function in Dairy Cows
Simple Summary
Abstract
1. Introduction
2. Transition Health
2.1. Parturition and Transition Physiology
2.2. Lipid Mobilization
2.3. Hypocalcemia
2.4. Systemic Inflammation
2.5. Uterine Disease
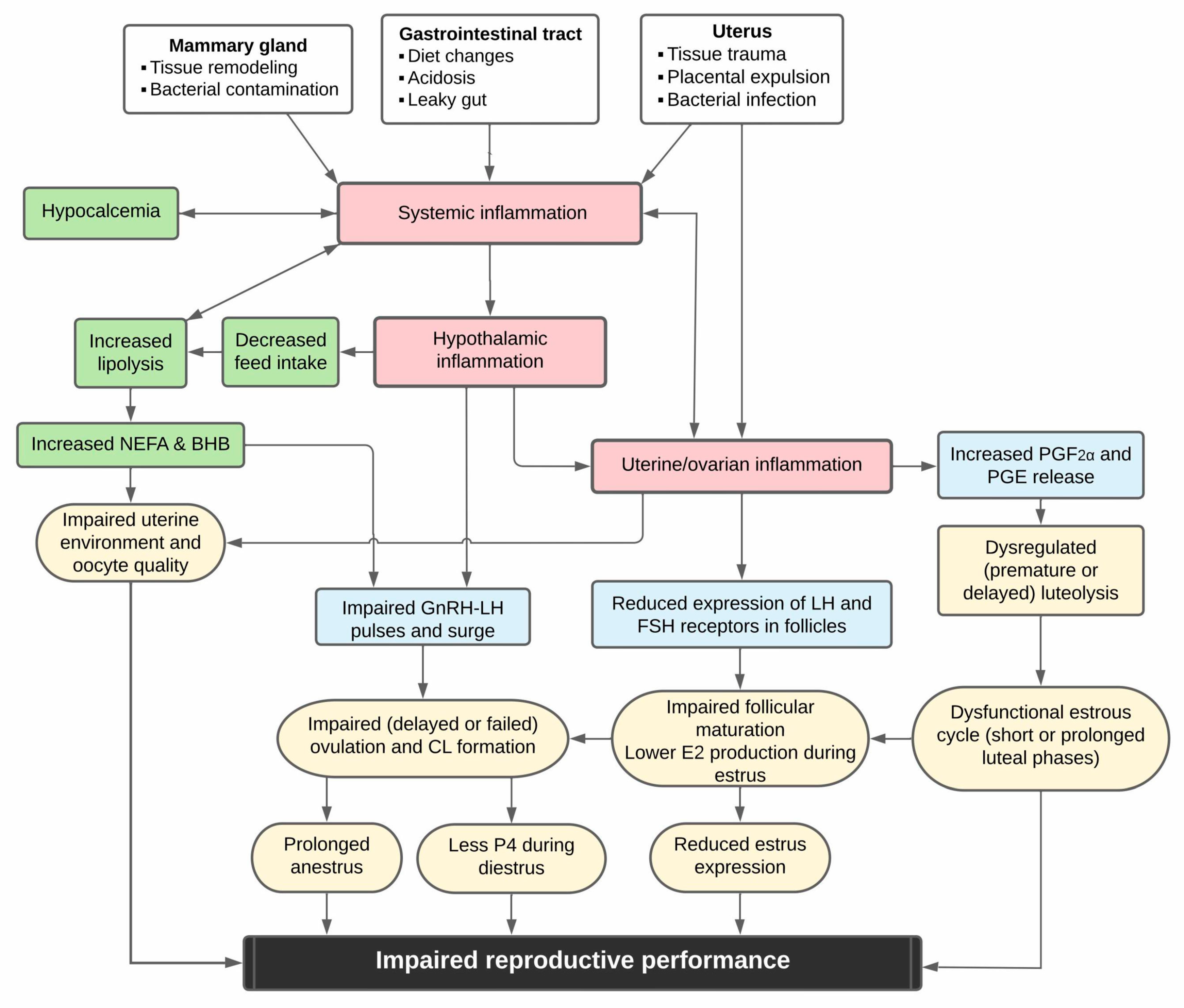
3. Mechanisms Linking Disease and Reproductive Function
3.1. Dominant Follicle Function, Estrus, and Ovulation
3.2. Inflammation and Luteal Function
3.3. Effects of Endotoxins on Oocyte Competence or Uterine Environment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- NASS-USDA. Milk Production February 23, 2022; National Agricultural Statistics Service (NASS), Agricultural Statistics Board, United States Department of Agriculture (USDA): Washington, DC, USA, 2022; pp. 1–20.
- Lactanet-AAFC. Average Production per Cow and Breed—Selected Countries (Milk Recording) Dairy; Lactanet: Guelph, ON, Canada, 2022. [Google Scholar]
- LeBlanc, S.J. Interactions of Metabolism, Inflammation, and Reproductive Tract Health in the Postpartum Period in Dairy Cattle. Reprod. Domest. Anim. 2012, 47, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Horst, E.A.; Kvidera, S.K.; Baumgard, L.H. Invited Review: The Influence of Immune Activation on Transition Cow Health and Performance—A Critical Evaluation of Traditional Dogmas. J. Dairy Sci. 2021, 104, 8380–8410. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, S.J. Review: Postpartum Reproductive Disease and Fertility in Dairy Cows. Animal 2023, 17, 100781. [Google Scholar] [CrossRef] [PubMed]
- Bradford, B.J.; Yuan, K.; Farney, J.K.; Mamedova, L.K.; Carpenter, A.J. Invited Review: Inflammation during the Transition to Lactation: New Adventures with an Old Flame. J. Dairy Sci. 2015, 98, 6631–6650. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, S.J. Relationship of Peripartum Inflammation with Reproductive Health in Dairy Cows. JDS Commun. 2023, 4, 230–234. [Google Scholar] [CrossRef]
- Bogado Pascottini, O.; LeBlanc, S.J. Metabolic Markers for Purulent Vaginal Discharge and Subclinical Endometritis in Dairy Cows. Theriogenology 2020, 155, 43–48. [Google Scholar] [CrossRef]
- Bruinjé, T.C.; Bogado Pascottini, O.; LeBlanc, S.J. Inflammatory and Metabolic Profiles in Cows with Postpartum Reproductive Tract Disease: A Case-Control Study (Abstract #2305). In Proceedings of the 2024 American Dairy Science Association (ADSA) Meeting, West Palm Beach, FL, USA, 16 June 2024. [Google Scholar]
- Ribeiro, E.S.; Lima, F.S.; Greco, L.F.; Bisinotto, R.S.; Monteiro, A.P.A.; Favoreto, M.; Ayres, H.; Marsola, R.S.; Martinez, N.; Thatcher, W.W.; et al. Prevalence of Periparturient Diseases and Effects on Fertility of Seasonally Calving Grazing Dairy Cows Supplemented with Concentrates. J. Dairy Sci. 2013, 96, 5682–5697. [Google Scholar] [CrossRef]
- Pinedo, P.; Santos, J.E.P.; Chebel, R.C.; Galvão, K.N.; Schuenemann, G.M.; Bicalho, R.C.; Gilbert, R.O.; Seabury, C.M.; Rosa, G.; Thatcher, W. Associations of Reproductive Indices with Fertility Outcomes, Milk Yield, and Survival in Holstein Cows. J. Dairy Sci. 2020, 103, 6647–6660. [Google Scholar] [CrossRef]
- Bruinjé, T.C.; Morrison, E.I.; Ribeiro, E.S.; Renaud, D.L.; Couto Serrenho, R.; LeBlanc, S.J. Postpartum Health Is Associated with Detection of Estrus by Activity Monitors and Reproductive Performance in Dairy Cows. J. Dairy Sci. 2023, 106, 9451–9473. [Google Scholar] [CrossRef]
- Bruinjé, T.C.; Morrison, E.I.; LeBlanc, S.J. Associations of Early Postpartum Metabolic and Inflammatory Markers with Time to Onset of Cyclicity in Clinically Healthy Dairy Cows (Abstract #1719). In Proceedings of the 2023 American Dairy Science Association (ADSA) Meeting, Ottawa, ON, Canada, 23 June 2023. [Google Scholar]
- Roche, J.R.; Burke, C.R.; Crookenden, M.A.; Heiser, A.; Loor, J.L.; Meier, S.; Mitchell, M.D.; Phyn, C.V.C.; Turner, S.-A. Fertility and the Transition Dairy Cow. Reprod. Fertil. Dev. 2018, 30, 85. [Google Scholar] [CrossRef]
- Cardoso, F.C.; Kalscheur, K.F.; Drackley, J.K. Symposium Review: Nutrition Strategies for Improved Health, Production, and Fertility during the Transition Period. J. Dairy Sci. 2020, 103, 5684–5693. [Google Scholar] [CrossRef] [PubMed]
- Bruinjé, T.C.; LeBlanc, S.J. Graduate Student Literature Review: Implications of Transition Cow Health for Reproductive Function and Targeted Reproductive Management. J. Dairy Sci. 2024, 107, 8234–8246. [Google Scholar] [CrossRef] [PubMed]
- Drackley, J.K. ADSA Foundation Scholar Award: Biology of Dairy Cows during the Transition Period: The Final Frontier? J. Dairy Sci. 1999, 82, 2259–2273. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.W. Regulation of Organic Nutrient Metabolism during Transition from Late Pregnancy to Early Lactation. J. Anim. Sci. 1995, 73, 2804–2819. [Google Scholar] [CrossRef] [PubMed]
- Shenavai, S.; Preissing, S.; Hoffmann, B.; Dilly, M.; Pfarrer, C.; Özalp, G.R.; Caliskan, C.; Seyrek-Intas, K.; Schuler, G. Investigations into the Mechanisms Controlling Parturition in Cattle. Reproduction 2012, 144, 279–292. [Google Scholar] [CrossRef]
- Huzzey, J.M.; Duffield, T.F.; LeBlanc, S.J.; Veira, D.M.; Weary, D.M.; von Keyserlingk, M.A.G. Short Communication: Haptoglobin as an Early Indicator of Metritis. J. Dairy Sci. 2009, 92, 621–625. [Google Scholar] [CrossRef]
- Cheong, S.H.; Filho, O.G.S.; Absalon-Medina, V.A.; Schneider, A.; Butler, W.R.; Gilbert, R.O.; Hon Cheong, S.; Sá Filho, O.G.; Absalon-Medina, V.A.; Schneider, A.; et al. Uterine and Systemic Inflammation Influences Ovarian Follicular Function in Postpartum Dairy Cows. PLoS ONE 2017, 12, e0177356. [Google Scholar] [CrossRef]
- Kehrli, M.E.; Nonnecke, B.J.; Roth, J.A. Alterations in Bovine Neutrophil Function during the Periparturient Period. Am. J. Vet. Res 1989, 50, 207–214. [Google Scholar] [CrossRef]
- Cai, T.Q.; Weston, P.G.; Lund, L.A.; Brodie, B.; McKenna, D.J.; Wagner, W.C. Association between Neutrophil Functions and Periparturient Disorders in Cows. Am. J. Vet. Res. 1994, 55, 934–943. [Google Scholar] [CrossRef]
- Pinedo, P.; Santos, J.E.P.; Chebel, R.C.; Galvão, K.N.; Schuenemann, G.M.; Bicalho, R.C.; Gilbert, R.O.; Rodriguez Zas, S.; Seabury, C.M.; Rosa, G.; et al. Early-Lactation Diseases and Fertility in 2 Seasons of Calving across US Dairy Herds. J. Dairy Sci. 2020, 103, 10560–10576. [Google Scholar] [CrossRef]
- Dubuc, J.; Duffield, T.F.; Leslie, K.E.; Walton, J.S.; LeBlanc, S.J. Risk Factors for Postpartum Uterine Diseases in Dairy Cows. J. Dairy Sci. 2010, 93, 5764–5771. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.E.; Bisinotto, R.S.; Ribeiro, E.S.; Lima, F.S.; Greco, L.F.; Staples, C.R.; Thatcher, W.W. Applying Nutrition and Physiology to Improve Reproduction in Dairy Cattle. Soc. Reprod. Fertil. Suppl. 2010, 67, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Lean, I.J.; Sheedy, D.B.; Santos, J.E.P. Associations of Parity with Health Disorders and Blood Metabolite Concentrations in Holstein Cows in Different Production Systems. J. Dairy Sci. 2023, 106, 500–518. [Google Scholar] [CrossRef] [PubMed]
- Suthar, V.S.; Canelas-Raposo, J.; Deniz, A.; Heuwieser, W. Prevalence of Subclinical Ketosis and Relationships with Postpartum Diseases in European Dairy Cows. J. Dairy Sci. 2013, 96, 2925–2938. [Google Scholar] [CrossRef]
- Reinhardt, T.A.; Lippolis, J.D.; McCluskey, B.J.; Goff, J.P.; Horst, R.L. Prevalence of Subclinical Hypocalcemia in Dairy Herds. Vet. J. 2011, 188, 122–124. [Google Scholar] [CrossRef]
- Venjakob, P.L.; Borchardt, S.; Heuwieser, W. Hypocalcemia—Cow-Level Prevalence and Preventive Strategies in German Dairy Herds. J. Dairy Sci. 2017, 100, 9258–9266. [Google Scholar] [CrossRef]
- Dubuc, J.; Denis-Robichaud, J. A Dairy Herd-Level Study of Postpartum Diseases and Their Association with Reproductive Performance and Culling. J. Dairy Sci. 2017, 100, 3068–3078. [Google Scholar] [CrossRef]
- Van Schyndel, S.J.; Dubuc, J.; Pascottini, O.B.; Carrier, J.; Kelton, D.F.; Duffield, T.F.; LeBlanc, S.J. The Effect of Pegbovigrastim on Early-Lactation Disease, Production, and Reproduction in Dairy Cows. J. Dairy Sci. 2021, 104, 10100–10110. [Google Scholar] [CrossRef]
- McDougall, S.; Burke, C.R. Prevalence of Endometritis Diagnosed by Vaginal Discharge Scoring or Uterine Cytology in Dairy Cows and Herds. J. Dairy Sci. 2020, 103, 6511–6521. [Google Scholar] [CrossRef]
- Carvalho, M.R.; Peñagaricano, F.; Santos, J.E.P.; DeVries, T.J.; McBride, B.W.; Ribeiro, E.S. Long-Term Effects of Postpartum Clinical Disease on Milk Production, Reproduction, and Culling of Dairy Cows. J. Dairy Sci. 2019, 102, 11701–11717. [Google Scholar] [CrossRef]
- Goff, J.P.; Horst, R.L. Physiological Changes at Parturition and Their Relationship to Metabolic Disorders. J. Dairy Sci. 1997, 80, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Bauman, D.E. Regulation of Nutrient Partitioning during Lactation: Homeostasis and Homeorhesis Revisited. Ruminant physiology: Digestion, metabolism, growth and reproduction. In Ruminant Physiology: Digestion, Metabolism, Growth and Reproduction; Cronjé, P.B., Ed.; CABI International: Wallingford, UK, 2000; pp. 311–328. [Google Scholar] [CrossRef]
- De Koster, J.D.; Opsomer, G. Insulin Resistance in Dairy Cows. Vet. Clin. N. Am.—Food Anim. Pract. 2013, 29, 299–322. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.S.; Bradford, B.J.; Oba, M. Board-Invited Review: The Hepatic Oxidation Theory of the Control of Feed Intake and Its Application to Ruminants. J. Anim. Sci. 2009, 87, 3317. [Google Scholar] [CrossRef]
- Kerwin, A.L.; Burhans, W.S.; Mann, S.; Nydam, D.V.; Wall, S.K.; Schoenberg, K.M.; Perfield, K.L.; Overton, T.R. Transition Cow Nutrition and Management Strategies of Dairy Herds in the Northeastern United States: Part II—Associations of Metabolic- and Inflammation-Related Analytes with Health, Milk Yield, and Reproduction. J. Dairy Sci. 2022, 105, 5349–5369. [Google Scholar] [CrossRef]
- Walsh, R.B.; Walton, J.S.; Kelton, D.F.; Leblanc, S.J.; Leslie, K.E.; Duffield, T.F. The Effect of Subclinical Ketosis in Early Lactation on Reproductive Performance of Postpartum Dairy Cows. J. Dairy Sci. 2007, 90, 2788–2796. [Google Scholar] [CrossRef]
- Bruinjé, T.C.; Morrison, E.I.; Ribeiro, E.S.; Renaud, D.L.; LeBlanc, S.J. Associations of Inflammatory and Reproductive Tract Disorders Postpartum with Pregnancy and Early Pregnancy Loss in Dairy Cows. J. Dairy Sci. 2024, 107, 1630–1644. [Google Scholar] [CrossRef]
- LeBlanc, S.J. Review: Relationships between Metabolism and Neutrophil Function in Dairy Cows in the Peripartum Period. Animal 2020, 14, s44–s54. [Google Scholar] [CrossRef]
- Kimura, K.; Reinhardt, T.A.; Goff, J.P. Parturition and Hypocalcemia Blunts Calcium Signals in Immune Cells of Dairy Cattle. J. Dairy Sci. 2006, 89, 2588–2595. [Google Scholar] [CrossRef]
- Martinez, N.; Sinedino, L.D.P.; Bisinotto, R.S.; Ribeiro, E.S.; Gomes, G.C.; Lima, F.S.; Greco, L.F.; Risco, C.A.; Galvão, K.N.; Taylor-Rodriguez, D.; et al. Effect of Induced Subclinical Hypocalcemia on Physiological Responses and Neutrophil Function in Dairy Cows. J. Dairy Sci. 2014, 97, 874–887. [Google Scholar] [CrossRef]
- Goff, J.P.; Hohman, A.; Timms, L.L. Effect of Subclinical and Clinical Hypocalcemia and Dietary Cation-Anion Difference on Rumination Activity in Periparturient Dairy Cows. J. Dairy Sci. 2020, 103, 2591–2601. [Google Scholar] [CrossRef]
- Martinez, N.; Risco, C.A.; Lima, F.S.; Bisinotto, R.S.; Greco, L.F.; Ribeiro, E.S.; Maunsell, F.; Galvão, K.; Santos, J.E.P. Evaluation of Peripartal Calcium Status, Energetic Profile, and Neutrophil Function in Dairy Cows at Low or High Risk of Developing Uterine Disease. J. Dairy Sci. 2012, 95, 7158–7172. [Google Scholar] [CrossRef] [PubMed]
- Venjakob, P.L.; Pieper, L.; Heuwieser, W.; Borchardt, S. Association of Postpartum Hypocalcemia with Early-Lactation Milk Yield, Reproductive Performance, and Culling in Dairy Cows. J. Dairy Sci. 2018, 101, 9396–9405. [Google Scholar] [CrossRef] [PubMed]
- McArt, J.A.A.; Neves, R.C. Association of Transient, Persistent, or Delayed Subclinical Hypocalcemia with Early Lactation Disease, Removal, and Milk Yield in Holstein Cows. J. Dairy Sci. 2020, 103, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Seely, C.R.; McArt, J.A.A. Patterns of Periparturient Rumination and Activity Time in Multiparous Holstein Cows with and without Dyscalcemia in Early Lactation. J. Dairy Sci. 2024, 107, 4871–4880. [Google Scholar] [CrossRef]
- Seely, C.R.; Leno, B.M.; Kerwin, A.L.; Overton, T.R.; McArt, J.A.A. Association of Subclinical Hypocalcemia Dynamics with Dry Matter Intake, Milk Yield, and Blood Minerals during the Periparturient Period. J. Dairy Sci. 2021, 104, 4692–4702. [Google Scholar] [CrossRef]
- Kvidera, S.K.; Horst, E.A.; Abuajamieh, M.; Mayorga, E.J.; Fernandez, M.V.S.; Baumgard, L.H. Glucose Requirements of an Activated Immune System in Lactating Holstein Cows. J. Dairy Sci. 2017, 100, 2360–2374. [Google Scholar] [CrossRef]
- Monks, J.; Geske, F.J.; Lehman, L.; Fadok, V.A. Do Inflammatory Cells Participate in Mammary Gland Involution? J. Mammary Gland. Biol. Neoplasia 2002, 7, 163–176. [Google Scholar] [CrossRef]
- Watson, C.J. Immune Cell Regulators in Mouse Mammary Development and Involution. J. Anim. Sci. 2009, 87, 35–42. [Google Scholar] [CrossRef]
- Kimura, K.; Goff, J.P.; Kehrli, M.E.; Reinhardt, T.A. Decreased Neutrophil Function as a Cause of Retained Placenta in Dairy Cattle. J. Dairy Sci. 2002, 85, 544–550. [Google Scholar] [CrossRef]
- Boro, P.; Kumaresan, A.; Singh, A.K.; Gupta, D.; Kumar, S.; Manimaran, A.; Mohanty, A.K.; Mohanty, T.K.; Pathak, R.; Attupuram, N.M.; et al. Expression of Short Chain Fatty Acid Receptors and Pro-Inflammatory Cytokines in Utero-Placental Tissues Is Altered in Cows Developing Retention of Fetal Membranes. Placenta 2014, 35, 455–460. [Google Scholar] [CrossRef]
- Bruinjé, T.C.; Campora, L.; Van Winters, B.; LeBlanc, S.J. Effects of Systemic or Uterine Endotoxin Challenge in Holstein Cows at 5 or 40 Days Postpartum on Clinical Responses, Uterine and Systemic Inflammation, and Milk Yield. J. Dairy Sci. 2024, 107, 7392–7404. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Mendonça, L.G.D.; Hulbert, L.E.; Mamedova, L.K.; Muckey, M.B.; Shen, Y.; Elrod, C.C.; Bradford, B.J. Yeast Product Supplementation Modulated Humoral and Mucosal Immunity and Uterine Inflammatory Signals in Transition Dairy Cows. J. Dairy Sci. 2015, 98, 3236–3246. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Dixit, V.M. Signaling in Innate Immunity and Inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4, a006049. [Google Scholar] [CrossRef]
- Ceciliani, F.; Ceron, J.J.; Eckersall, P.D.; Sauerwein, H. Acute Phase Proteins in Ruminants. J. Proteom. 2012, 75, 4207–4231. [Google Scholar] [CrossRef]
- Gilbert, R.O. Symposium Review: Mechanisms of Disruption of Fertility by Infectious Diseases of the Reproductive Tract. J. Dairy Sci. 2019, 102, 3754–3765. [Google Scholar] [CrossRef]
- Pascottini, O.B.; Carvalho, M.R.; Van Schyndel, S.J.; Ticiani, E.; Spricigo, J.W.; Mamedova, L.K.; Ribeiro, E.S.; LeBlanc, S.J. Feed Restriction to Induce and Meloxicam to Mitigate Potential Systemic Inflammation in Dairy Cows before Calving. J. Dairy Sci. 2019, 102, 9285–9297. [Google Scholar] [CrossRef]
- Nightingale, C.R.; Sellers, M.D.; Ballou, M.A. Elevated Plasma Haptoglobin Concentrations Following Parturition Are Associated with Elevated Leukocyte Responses and Decreased Subsequent Reproductive Efficiency in Multiparous Holstein Dairy Cows. Vet. Immunol. Immunopathol. 2015, 164, 16–23. [Google Scholar] [CrossRef]
- Huzzey, J.M.; Mann, S.; Nydam, D.V.; Grant, R.J.; Overton, T.R. Associations of Peripartum Markers of Stress and Inflammation with Milk Yield and Reproductive Performance in Holstein Dairy Cows. Prev. Vet. Med. 2015, 120, 291–297. [Google Scholar] [CrossRef]
- Aschenbach, J.R.; Zebeli, Q.; Patra, A.K.; Greco, G.; Amasheh, S.; Penner, G.B. Symposium Review: The Importance of the Ruminal Epithelial Barrier for a Healthy and Productive Cow. J. Dairy Sci. 2019, 102, 1866–1882. [Google Scholar] [CrossRef]
- Emmanuel, D.G.V.; Dunn, S.M.; Ametaj, B.N. Feeding High Proportions of Barley Grain Stimulates an Inflammatory Response in Dairy Cows. J. Dairy Sci. 2008, 91, 606–614. [Google Scholar] [CrossRef]
- Kvidera, S.K.; Dickson, M.J.; Abuajamieh, M.; Snider, D.B.; Fernandez, M.V.S.; Johnson, J.S.; Keating, A.F.; Gorden, P.J.; Green, H.B.; Schoenberg, K.M.; et al. Intentionally Induced Intestinal Barrier Dysfunction Causes Inflammation, Affects Metabolism, and Reduces Productivity in Lactating Holstein Cows. J. Dairy Sci. 2017, 100, 4113–4127. [Google Scholar] [CrossRef] [PubMed]
- Chirivi, M.; Lock, A.L.; Rendon, C.J.; Myers, M.N. Lipopolysaccharide Induces Lipolysis and Insulin Resistance in Adipose Tissue from Dairy Cows. J. Dairy Sci. 2022, 105, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.W.; Miller, A.; Leal Yepes, F.A.; Bitsko, E.; Nydam, D.; Mann, S. The Effect of the Transition Period and Postpartum Body Weight Loss on Macrophage Infiltrates in Bovine Subcutaneous Adipose Tissue. J. Dairy Sci. 2019, 102, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- De Heredia, F.P.; Gómez-Martínez, S.; Marcos, A. Chronic and Degenerative Diseases: Obesity, Inflammation and the Immune System. Proc. Nutr. Soc. 2012, 71, 332–338. [Google Scholar] [CrossRef]
- Osborn, O.; Olefsky, J.M. The Cellular and Signaling Networks Linking the Immune System and Metabolism in Disease. Nat. Med. 2012, 18, 363–374. [Google Scholar] [CrossRef]
- Bogado Pascottini, O.; Koster, J.D.; Nieuwerburgh, F.V.; Poucke, M.V.; Peelman, L.; Fievez, V.; Leroy, J.L.M.R.; Opsomer, G. Effect of Overconditioning on the Hepatic Global Gene Expression Pattern of Dairy Cows at the End of Pregnancy. J. Dairy Sci. 2021, 104, 8152–8163. [Google Scholar] [CrossRef]
- Kuhla, B. Review: Pro-Inflammatory Cytokines and Hypothalamic Inflammation: Implications for Insufficient Feed Intake of Transition Dairy Cows. Animal 2020, 14, S65–S77. [Google Scholar] [CrossRef]
- Yuan, K.; Farney, J.K.; Mamedova, L.K.; Sordillo, L.M.; Bradford, B.J. TNFα Altered Inflammatory Responses, Impaired Health and Productivity, but Did Not Affect Glucose or Lipid Metabolism in Early-Lactation Dairy Cows. PLoS ONE 2013, 8, e80316. [Google Scholar] [CrossRef]
- Schukken, Y.H.; Günther, J.; Fitzpatrick, J.; Fontaine, M.C.; Goetze, L.; Holst, O.; Leigh, J.; Petzl, W.; Schuberth, H.J.; Sipka, A.; et al. Host-Response Patterns of Intramammary Infections in Dairy Cows. Vet. Immunol. Immunopathol. 2011, 144, 270–289. [Google Scholar] [CrossRef]
- Ingvartsen, K.L.; Moyes, K. Nutrition, Immune Function and Health of Dairy Cattle. Animal 2013, 7, 112–122. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Molinari, P.C.C.; Ormsby, T.J.R.; Bromfield, J.J. Preventing Postpartum Uterine Disease in Dairy Cattle Depends on Avoiding, Tolerating and Resisting Pathogenic Bacteria. Theriogenology 2020, 150, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, I.M.; Cronin, J.; Goetze, L.; Donofrio, G.; Schuberth, H.-J. Defining Postpartum Uterine Disease and the Mechanisms of Infection and Immunity in the Female Reproductive Tract in Cattle1. Biol. Reprod. 2009, 81, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, S.J. Reproductive Tract Inflammatory Disease in Postpartum Dairy Cows. Animal 2014, 8, 54–63. [Google Scholar] [CrossRef]
- Galvão, K.N.; Bicalho, R.C.; Jin Jean, S. Symposium Review: The Uterine Microbiome Associated with the Development of Uterine Disease in Dairy Cows. J. Dairy Sci. 2019, 102, 11786–11797. [Google Scholar] [CrossRef]
- Pérez-Báez, J.; Risco, C.A.; Chebel, R.C.; Gomes, G.C.; Greco, L.F.; Tao, S.; Thompson, I.M.; do Amaral, B.C.; Zenobi, M.G.; Martinez, N.; et al. Association of Dry Matter Intake and Energy Balance Prepartum and Postpartum with Health Disorders Postpartum: Part I. Calving Disorders and Metritis. J. Dairy Sci. 2019, 102, 9138–9150. [Google Scholar] [CrossRef]
- Dubuc, J.; Duffield, T.F.; Leslie, K.E.; Walton, J.S.; LeBlanc, S.J. Risk Factors and Effects of Postpartum Anovulation in Dairy Cows. J. Dairy Sci. 2012, 95, 1845–1854. [Google Scholar] [CrossRef]
- Magata, F.; Tsukamura, H.; Matsuda, F. Peptides The Impact of Inflammatory Stress on Hypothalamic Kisspeptin Neurons: Mechanisms Underlying Inflammation-Associated Infertility in Humans and Domestic Animals. Peptides 2023, 162, 170958. [Google Scholar] [CrossRef]
- Monteiro, P.L.J.; Gonzales, B.; Drum, J.N.; Santos, J.E.P.; Wiltbank, M.C.; Sartori, R. Prevalence and Risk Factors Related to Anovular Phenotypes in Dairy Cows. J. Dairy Sci. 2021, 104, 2369–2383. [Google Scholar] [CrossRef]
- Britt, J.H. Impacts of Early Postpartum Metabolism on Follicular Development and Fertility. In American Association of Bovine Practitioners Conference Proceedings; Williams, E.I., Ed.; Frontier Printers, Inc.: St. Paul, MN, USA, 1992; Volume 24, pp. 39–43. [Google Scholar]
- Price, J.C.; Sheldon, I.M. Granulosa Cells from Emerged Antral Follicles of the Bovine Ovary Initiate Inflammation in Response to Bacterial Pathogen-Associated Molecular Patterns via Toll-like Receptor Pathways. Biol. Reprod. 2013, 89, 1–12. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Noakes, D.E.; Rycroft, A.N.; Pfeiffer, D.U.; Dobson, H. Influence of Uterine Bacterial Contamination after Parturition on Ovarian Dominant Follicle Selection and Follicle Growth and Function in Cattle. Reproduction 2002, 123, 837–845. [Google Scholar] [CrossRef]
- Peter, A.T.; Bosu, W.T.; DeDecker, R.J. Suppression of Preovulatory Luteinizing Hormone Surges in Heifers after Intrauterine Infusions of Escherichia coli Endotoxin. Am. J. Vet. Res. 1989, 50, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Herath, S.; Williams, E.J.; Lilly, S.T.; Gilbert, R.O.; Dobson, H.; Bryant, C.E.; Sheldon, I.M. Ovarian Follicular Cells Have Innate Immune Capabilities That Modulate Their Endocrine Function. Reproduction 2007, 134, 683–693. [Google Scholar] [CrossRef]
- Magata, F.; Horiuchi, M.; Echizenya, R.; Miura, R.; Chiba, S.; Matsui, M.; Miyamoto, A.; Kobayashi, Y.; Shimizu, T. Lipopolysaccharide in Ovarian Follicular Fluid Influences the Steroid Production in Large Follicles of Dairy Cows. Anim. Reprod. Sci. 2014, 144, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Wojtulewicz, K.; Krawczyńska, A.; Tomaszewska-Zaremba, D.; Wójcik, M.; Herman, A.P. Effect of Acute and Prolonged Inflammation on the Gene Expression of Proinflammatory Cytokines and Their Receptors in the Anterior Pituitary Gland of Ewes. Int. J. Mol. Sci. 2020, 21, 6939. [Google Scholar] [CrossRef]
- Battaglia, D.F.; Krasa, H.B.; Padmanabhan, V.; Viguié, C.; Karsch, F.J. Endocrine Alterations That Underlie Endotoxin-Induced Disruption of the Follicular Phase in Ewes. Biol. Reprod. 2000, 62, 45–53. [Google Scholar] [CrossRef]
- Lavon, Y.; Leitner, G.; Moallem, U.; Klipper, E.; Voet, H.; Jacoby, S.; Glick, G.; Meidan, R.; Wolfenson, D. Immediate and Carryover Effects of Gram-Negative and Gram-Positive Toxin-Induced Mastitis on Follicular Function in Dairy Cows. Theriogenology 2011, 76, 942–953. [Google Scholar] [CrossRef]
- Marey, M.A.; Liu, J.; Kowsar, R.; Haneda, S.; Matsui, M.; Sasaki, M.; Shimizu, T.; Hayakawa, H.; Wijayagunawardane, M.P.B.; Hussein, F.M.; et al. Bovine Oviduct Epithelial Cells Downregulate Phagocytosis of Sperm by Neutrophils: Prostaglandin E2 as a Major Physiological Regulator. Reproduction 2014, 147, 211–219. [Google Scholar] [CrossRef]
- Pratt, B.R.; Butcher, R.L.; Inskeep, E.K. Antiluteolytic Effect of the Conceptus and of PGE2 in Ewes. J. Anim. Sci. 1977, 45, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Jiemtaweeboon, S.; Shirasuna, K.; Nitta, A.; Kobayashi, A.; Schuberth, H.J.; Shimizu, T.; Miyamoto, A. Evidence That Polymorphonuclear Neutrophils Infiltrate into the Developing Corpus Luteum and Promote Angiogenesis with Interleukin-8 in the Cow. Reprod. Biol. Endocrinol. 2011, 9, 79. [Google Scholar] [CrossRef]
- Pate, J.L.; Keyes, P.L. Immune Cells in the Corpus Luteum: Friends or Foes? Reproduction 2001, 122, 665–676. [Google Scholar] [CrossRef]
- Thatcher, W.W.; Guzeloglu, A.; Mattos, R.; Binelli, M.; Hansen, T.R.; Pru, J.K. Uterine-Conceptus Interactions and Reproductive Failure in Cattle. Theriogenology 2001, 56, 1435–1450. [Google Scholar] [CrossRef] [PubMed]
- Bruinjé, T.C.; Morrison, E.I.; Ribeiro, E.S.; Renaud, D.L.; LeBlanc, S.J. Progesterone Profiles in Postpartum Dairy Cows with Inflammatory Disorders. J. Dairy Sci. 2024, 107, 7153–7164. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, C.; Yoshioka, K.; Iwamura, S.; Hirose, H. Endotoxin Induces Delayed Ovulation Following Endocrine Aberration during the Proestrous Phase in Holstein Heifers. Domest. Anim. Endocrinol. 2001, 20, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Lavon, Y.; Leitner, G.; Goshen, T.; Braw-Tal, R.; Jacoby, S.; Wolfenson, D. Exposure to Endotoxin during Estrus Alters the Timing of Ovulation and Hormonal Concentrations in Cows. Theriogenology 2008, 70, 956–967. [Google Scholar] [CrossRef]
- Herzog, K.; Strüve, K.; Kastelic, J.P.; Piechotta, M.; Ulbrich, S.E.; Pfarrer, C.; Shirasuna, K.; Shimizu, T.; Miyamoto, A.; Bollwein, H. Escherichia coli Lipopolysaccharide Administration Transiently Suppresses Luteal Structure and Function in Diestrous Cows. Reproduction 2012, 144, 467–476. [Google Scholar] [CrossRef]
- Lüttgenau, J.; Wellnitz, O.; Kradolfer, D.; Kalaitzakis, E.; Ulbrich, S.E.; Bruckmaier, R.M. Intramammary Lipopolysaccharide Infusion Alters Gene Expression but Does Not Induce Lysis of the Bovine Corpus Luteum. J. Dairy Sci. 2016, 99, 4018–4031. [Google Scholar] [CrossRef]
- Mateus, L.; Lopes da Costa, L.; Diniz, P.; Ziecik, A.J. Relationship between Endotoxin and Prostaglandin (PGE2 and PGFM) Concentrations and Ovarian Function in Dairy Cows with Puerperal Endometritis. Anim. Reprod. Sci. 2003, 76, 143–154. [Google Scholar] [CrossRef]
- Bruinjé, T.C. Symposium Review: In-Line Milk Progesterone Monitoring as a Tool for Precision Reproductive Management. JDS Commun. 2025, in press. [Google Scholar] [CrossRef]
- Bromfield, J.J.; Sheldon, I.M. Lipopolysaccharide Reduces the Primordial Follicle Pool in the Bovine Ovarian Cortex Ex Vivo and in the Murine Ovary in Vivo. Biol. Reprod. 2013, 88, 1–9. [Google Scholar] [CrossRef]
- Fair, T. Follicular Oocyte Growth and Acquisition of Developmental Competence. Anim. Reprod. Sci. 2003, 78, 203–216. [Google Scholar] [CrossRef]
- Roth, Z.; Dvir, A.; Kalo, D.; Lavon, Y.; Krifucks, O.; Wolfenson, D.; Leitner, G. Naturally Occurring Mastitis Disrupts Developmental Competence of Bovine Oocytes. J. Dairy Sci. 2013, 96, 6499–6505. [Google Scholar] [CrossRef]
- Bromfield, J.J.; Sheldon, I.M. Lipopolysaccharide Initiates Inflammation in Bovine Granulosa Cells via the TLR4 Pathway and Perturbs Oocyte Meiotic Progression in Vitro. Endocrinology 2011, 152, 5029–5040. [Google Scholar] [CrossRef] [PubMed]
- Magata, F.; Shimizu, T. Effect of Lipopolysaccharide on Developmental Competence of Oocytes. Reprod. Toxicol. 2017, 71, 1–7. [Google Scholar] [CrossRef]
- Soto, P.; Natzke, R.P.; Hansen, P.J. Actions of Tumor Necrosis Factor-α on Oocyte Maturation and Embryonic Development in Cattle. Am. J. Reprod. Immunol. 2003, 50, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Piras, C.; Guo, Y.; Soggiu, A.; Chanrot, M.; Greco, V.; Urbani, A.; Charpigny, G.; Bonizzi, L.; Roncada, P.; Humblot, P. Changes in Protein Expression Profiles in Bovine Endometrial Epithelial Cells Exposed to E. Coli LPS Challenge. Mol. BioSystems 2017, 13, 392–405. [Google Scholar] [CrossRef]
- Ribeiro, E.S.; Monteiro, A.P.A.; Bisinotto, R.S.; Lima, F.S.; Greco, L.F.; Ealy, A.D.; Thatcher, W.W.; Santos, J.E.P. Conceptus Development and Transcriptome at Preimplantation Stages in Lactating Dairy Cows of Distinct Genetic Groups and Estrous Cyclic Statuses. J. Dairy Sci. 2016, 99, 4761–4777. [Google Scholar] [CrossRef]
- Ribeiro, E.S.; Carvalho, M.R. Impact and Mechanisms of Inflammatory Diseases on Embryonic Development and Fertility in Cattle. Anim. Reprod. 2017, 14, 589–600. [Google Scholar] [CrossRef]
- Sellmer Ramos, I.; Moraes, J.G.N.; Caldeira, M.O.; Poock, S.E.; Spencer, T.E.; Lucy, M.C. Impact of Postpartum Metritis on the Regeneration of Endometrial Glands in Dairy Cows Impact of Postpartum Metritis on the Regeneration of Endometrial Glands in Dairy Cows. JDSC 2023, 4, 400–405. [Google Scholar] [CrossRef]
- Sellmer Ramos, I.S.; Caldeira, M.O.; Poock, S.E.; Moraes, J.G.N.; Lucy, M.C.; Patterson, A.L. Adenomyosis and Fibrosis Define the Morphological Memory of the Postpartum Uterus of Dairy Cows Previously Exposed to Metritis. JDS Commun. 2024, in press. [Google Scholar] [CrossRef]
| Health Disorder | Definition | Incidence or Prevalence | References |
|---|---|---|---|
| Calving problems | Any of dystocia, twin birth, stillbirth, or retained placenta | 9 to 15% | [10,12,25,26,27] |
| Clinical hypocalcemia | Farm personnel/veterinarian diagnosis or recumbent cow with serum total Ca < 2.0 mM | 1 to 5% | [12,27,28,29,30] |
| Retained placenta | Failure to expel fetal membranes within 24 h after calving | 5 to 12% | [12,25,27,28,31,32] |
| Clinical ketosis | Reduced feed intake or reduced milk yield with hyperketonemia (≥1.2 mM blood BHB) | 3 to 10% | [26,27,28] |
| Displaced abomasum | Veterinary diagnosis of the left- or right-side displacement of abomasum within 30 or 60 DIM | 2 to 4% | [12,27,28,31,32] |
| Metritis | Watery fetid vaginal discharge with or without systemic illness within 15 DIM | 5 to 19% | [12,25,26,27,28,32] |
| PVD | ≥50% pus in vaginal discharge at 35 to 56 DIM | 10 to 25% | [12,25,32,33] |
| Clinical endometritis | ≥5% PMN in endometrial cytology at 35 or ≥4% PMN at 56 DIM with PVD | 4 to 7% | [25,32] |
| Subclinical endometritis | ≥5% PMN in endometrial cytology at 35 DIM, ≥2% at 41 DIM, or ≥4% at 56 DIM without PVD | 10 to 27% | [25,32,33] |
| Mastitis | Abnormal milk or visible inflammation in one or more quarters within 30 DIM, often based on treatment records only | 3 to 15% | [10,12,26,28,32] |
| Lameness | Walking with altered gait or arched back, or defined arbitrarily by farm personnel diagnosis within 30 DIM | 3 to 14% | [10,12,26,28] |
| Respiratory disease | Diagnosis of respiratory disease or pneumonia. Not commonly reported | 2% | [27] |
| At least one clinical disease | Any of dystocia, RP, metritis, PVD, mastitis, lameness, ketosis, DA, or pneumonia | 44 to 48% | [10,12,26,34] |
| Multiple clinical diseases | >1 of dystocia, RP, metritis, PVD, mastitis, lameness, ketosis, DA, or pneumonia | 17 to 26% | [10,12,26] |
| Uterine disease | RP, metritis, endometritis, or PVD | 22 to 45% | [10,12,34] |
| Non-uterine disease | Clinical hypocalcemia, clinical mastitis, lameness, or digestive or respiratory problems | 23 to 32% | [10,12,34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruinjé, T.C.; LeBlanc, S.J. Invited Review: Inflammation and Health in the Transition Period Influence Reproductive Function in Dairy Cows. Animals 2025, 15, 633. https://doi.org/10.3390/ani15050633
Bruinjé TC, LeBlanc SJ. Invited Review: Inflammation and Health in the Transition Period Influence Reproductive Function in Dairy Cows. Animals. 2025; 15(5):633. https://doi.org/10.3390/ani15050633
Chicago/Turabian StyleBruinjé, Tony C., and Stephen J. LeBlanc. 2025. "Invited Review: Inflammation and Health in the Transition Period Influence Reproductive Function in Dairy Cows" Animals 15, no. 5: 633. https://doi.org/10.3390/ani15050633
APA StyleBruinjé, T. C., & LeBlanc, S. J. (2025). Invited Review: Inflammation and Health in the Transition Period Influence Reproductive Function in Dairy Cows. Animals, 15(5), 633. https://doi.org/10.3390/ani15050633






