The Performance, Ingestive Behavior, Nutrient Digestibility, Ruminal Fermentation Profile, Health Status, and Gene Expression of Does Fed a Phytochemical–Lactobacilli Blend in Late Pregnancy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Experimental PEL Feed Additive
2.2. Animals and Management
2.3. Feed Intake
2.4. Digestibility Trial and Chemical Analyses
2.5. Rumen Fermentation Parameters
2.6. Ingestive Behavior
2.7. Blood Sampling
2.8. Total RNA Extraction, Reverse Transcription, and Quantitative Real Time PCR
2.9. Statistical Analysis
3. Results
3.1. Feed Intake, Digestibility, and Ruminal Parameters
3.2. Ingestive Behavior
3.3. Blood Parameters
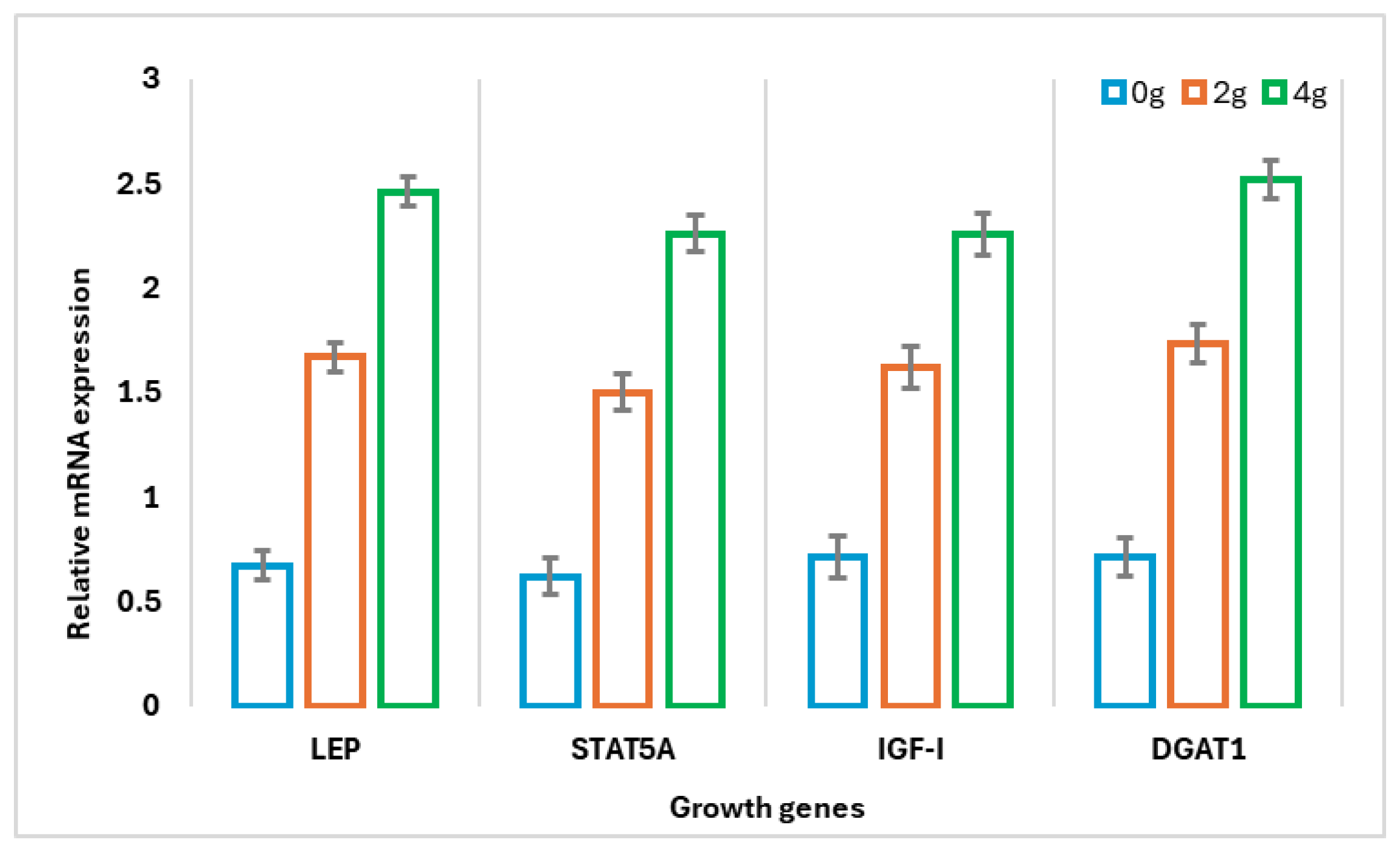
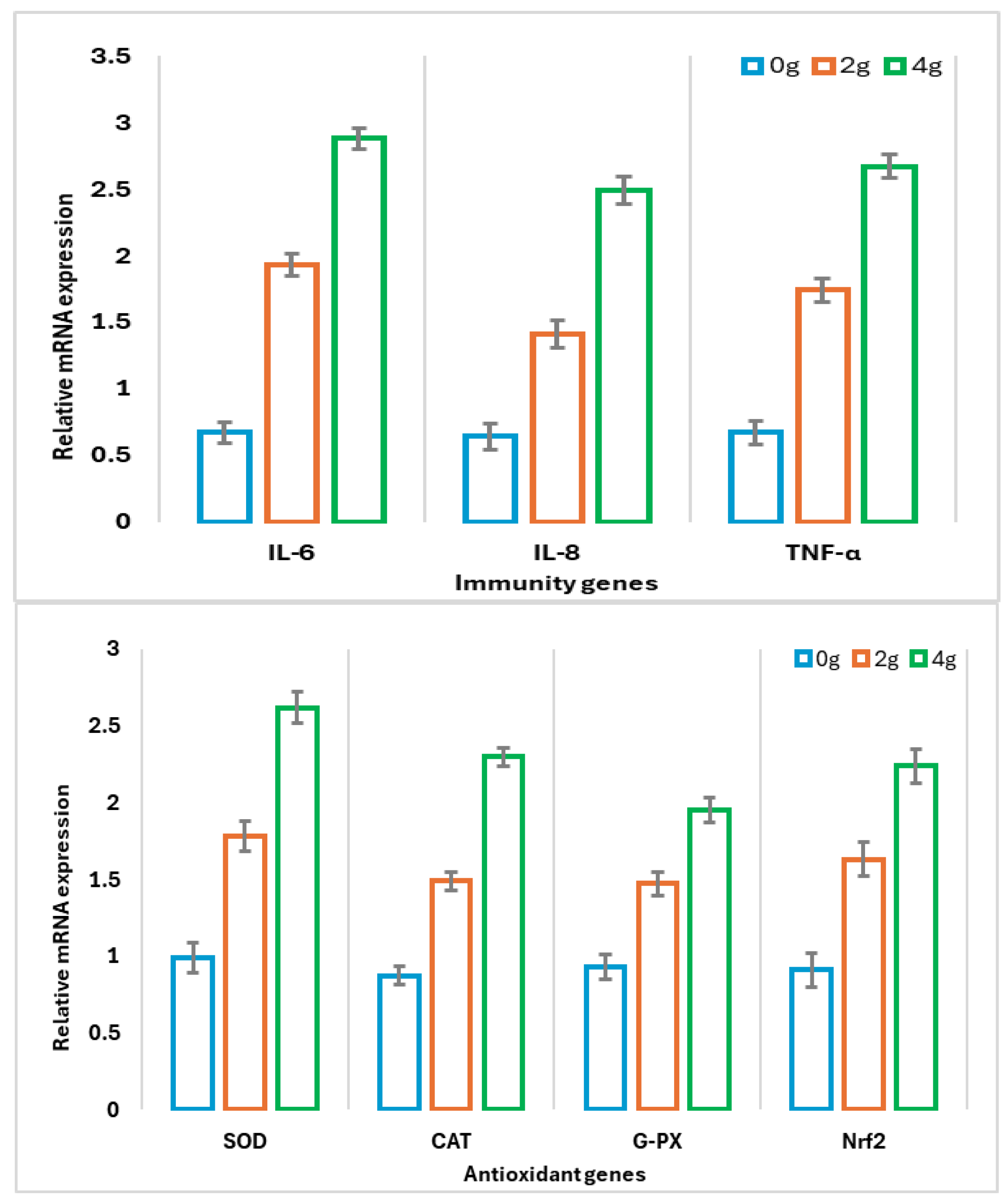
3.4. Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ihejirika, U.D.G.; Jimoh, O.A.; Ojo, O.A.; Kamalu, N.A. Oxidative stress indicators in West African Dwarf goats does (young females) during gestation. J. Anim. Sci. Vet. Med. 2024, 9, 129–138. [Google Scholar]
- Weng, J.; Couture, C.; Girard, S. Innate and Adaptive Immune Systems in Physiological and Pathological Pregnancy. Biology 2023, 12, 402. [Google Scholar] [CrossRef] [PubMed]
- Soltan, Y.A.; Morsy, A.S.; Hashem, N.M.; Sallam, S.M. Boswellia sacra resin as a phytogenic feed supplement to enhance ruminal fermentation, milk yield, and metabolic energy status of early lactating goats. Anim. Feed Sci. Technol. 2021, 277, 114963. [Google Scholar] [CrossRef]
- Morsy, A.S.; Soltan, Y.A.; El-Zaiat, H.M.; Alencar, S.M.; Abdalla, A.L. Role of bee propolis extract on diet digestibility, purine derivatives, mitigating methane formation, and blood metabolites in late pregnant ewes. Anim. Feed Sci. Technol. 2021, 273, 114834. [Google Scholar] [CrossRef]
- Wang, M.; Wu, H.; Lu, L.; Jiang, L.; Yu, Q. Lactobacillus reuteri promotes intestinal development and regulates mucosal immune function in newborn piglets. Front. Vet. Sci. 2020, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Hou, K.; Zhao, J.; Wang, H. The probiotic properties of lactic acid bacteria and their applications in animal husbandry. Curr. Microbiol. 2022, 79, 22. [Google Scholar] [CrossRef]
- Yang, S.; Xu, X.; Peng, Q.; Ma, L.; Qiao, Y.; Shi, B. Exopolysaccharides from lactic acid bacteria, as an alternative to antibiotics, on regulation of intestinal health and the immune system. Anim. Nutr. 2023, 13, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Zhang, C.S.; Liu, G.H.; Chen, Z.M. Research progress of antioxidant activity of lactic acid bacteria in livestock and poultry production. Chin. J. Anim. Nutr. 2023, 35, 3431–3444. [Google Scholar]
- Feng, T.; Wang, J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: A systematic review. Gut Microbes 2020, 12, 1801944. [Google Scholar] [CrossRef]
- Ge, Q.; Yang, B.; Liu, R.; Jiang, D.; Yu, H.; Wu, M.; Zhang, W. Antioxidant activity of Lactobacillus plantarum NJAU-01 in an animal model of aging. BMC Microbiol. 2021, 21, 182. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Deng, L.; Chen, M.; Che, Y.; Li, L.; Zhu, L.; Chen, G.; Feng, T. Phytogenic feed additives as natural antibiotic alternatives in animal health and production: A review of the literature of the last decade. Anim. Nutr. 2024, 17, 244–264. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, S.; Sukhikh, S.; Popov, A.; Shishko, O.; Nikonov, I.; Kapitonova, E.; Krol, O.; Larina, V.; Noskova, S.; Babich, O. Medicinal Plants: A Source of Phytobiotics for the Feed Additives. J. Agric. Food Res. 2024, 16, 101172. [Google Scholar] [CrossRef]
- Ahmed, M.G.; Elwakeel, E.A.; El-Zarkouny, S.Z.; Al-Sagheer, A.A. Environmental impact of phytobiotic additives on greenhouse gas emission reduction, rumen fermentation manipulation, and performance in ruminants: An updated review. Environ. Sci. Pollut. Res. 2024, 31, 37943–37962. [Google Scholar] [CrossRef]
- Soltan, Y.; Morsy, A.; Elazab, M.; El-Nile, A.E.; Hashem, N.; Sultan, M.; Hamad, Y.; Abo El Lail, G.; Abo-Sherif, S.; Dabour, N.; et al. Effects of Pichia manshurica yeast supplementation on ruminal fermentation, nutrient degradability, and greenhouse gas emissions in aflatoxin B1 contaminated diets. Trop. Anim. Health Prod. 2024, 56, 367. [Google Scholar] [CrossRef] [PubMed]
- NRC—National Research Council. Nutrient Requirements of Domestic Animals. In Nutrient Requirements of Domestic Goats; National Research Council: Washington, DC, USA, 1981. [Google Scholar]
- AOAC. Official Methods of Analysis, 21st ed.; AOAC: Rockville, MD, USA, 2019. [Google Scholar]
- NRC—National Research Council. Nutrient Requirement of Small Ruminants, 7th ed.; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- AOAC Official. Methods of Analysis of AOAC International; AOAC: Rockville, MD, USA, 2005. [Google Scholar]
- Palmquist, D.; Conrad, H. Origin of plasma fatty acid in lactating dairy cows fed high fat diets. J. Dairy Sci. 1971, 54, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.R.; Combs, D.K. Effects of prepartum diet, inert rumen bulk, and dietary polythyleneglicol on dry matter intake of lactating dairy cows. J. Dairy Sci. 1991, 74, 933–944. [Google Scholar] [CrossRef]
- Maekawa, M.; Beauchemin, K.A.; Christensen, D.A. Chewing activity, saliva production, and ruminal pH of primiparous and multiparous lactating dairy cows. J. Dairy Sci. 2002, 85, 1176–1182. [Google Scholar] [CrossRef]
- Polli, V.A.; Restle, J.; Senna, D.B.; Almeida, S.D. Aspectos relativos à ruminação de bovinos e bubalinos em regime de confinamento. Rev. Bras. Zootecnia 1996, 25, 987–993. [Google Scholar]
- Carvalho, G.G.P.; Pires, A.J.V.; Silva, F.F.D.; Veloso, C.M.; Silva, R.R.; Silva, H.G.D.O.; Bonomo, P.; Mendonça, S.D.S. Comportamento ingestivo de cabras leiteiras alimentadas com farelo de cacau ou torta de dendê. Pesqui. Agropecuária Brasileira 2004, 39, 919–925. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Basyony, M.; Morsy, A.S.; Soltan, Y.A. Extracts of Apricot (Prunus armeniaca) and Peach (Prunus pérsica) Kernels as Feed Additives: Nutrient Digestibility, Growth Performance, and Immunological Status of Growing Rabbits. Animals 2023, 13, 868. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, W.; Wang, L.; Li, A. Research progress in development and application of feed lactic acid bacteria preparations. Chin. J. Anim. Nutr. 2019, 31, 2012e21. [Google Scholar]
- Yang, C.; Chowdhury, M.A.K.; Hou, Y.; Gong, J. Phytogenic compounds as alternatives to in-feed antibiotics: Potentials and challenges in application. Pathogens 2015, 4, 137–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Huang, H.; Hu, Y.; Liu, Y.; Zeng, X.; Zhuang, Y.; Yang, H.; Wang, L.; Chen, S.; Yin, L.; et al. Effects of dietary supplementation with herbal extract mixture on growth performance, organ weight and intestinal morphology in weaning piglets. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1462–1470. [Google Scholar] [CrossRef]
- El-Essawy, A.M.; Anele, U.; Abdel-Wahed, A.; Abdou, A.R.; Khattab, I. Effects of anise, clove and thyme essential oils supplementation on rumen fermentation, blood metabolites, milk yield and milk composition in lactating goats. Anim. Feed Sci. Tech. 2021, 271, 114760. [Google Scholar] [CrossRef]
- Gabr, A.A.; Ahmed, M.I.; Shaheen, G.F.; Abdel-Gawad, A.G.; Abdelsalam, O.M.; Farag, M.E. Improving productivity and health status of lactating Zaraibi goats with Echinacea Purpurea or/and Chamomile flower supplementation. J. Anim. Poult. Prod. 2023, 14, 157–165. [Google Scholar] [CrossRef]
- Liu, T.; Chen, H.; Bai, Y.; Wu, J.; Cheng, S.; He, B.; Casper, D.P. Calf starter containing a blend of essential oils and prebiotics affects the growth performance of Holstein calves. J. Dairy Sci. 2020, 103, 2315–2323. [Google Scholar] [CrossRef]
- Hashemzadeh, F.; Rafeie, F.; Hadipour, A.; Rezadoust, M.H. Supplementing a phytogenic-rich herbal mixture to heat-stressed lambs: Growth performance, carcass yield, and muscle and liver antioxidant status. Small Rumin. Res. 2022, 206, 106596. [Google Scholar] [CrossRef]
- Dey, A.; Attri, K.; Dahiya, S.S.; Paul, S.S. Influence of dietary phytogenic feed additives on lactation performance, methane emissions and health status of Murrah buffaloes (Bubalus bubalis). J. Sci. Food Agric. 2021, 101, 4390–4397. [Google Scholar] [CrossRef]
- Tian, G.; Zhang, X.; Hao, X.; Zhang, J. Effects of curcumin on growth performance, ruminal fermentation, rumen microbial protein synthesis, and serum antioxidant capacity in housed growing lambs. Animals 2023, 13, 1439. [Google Scholar] [CrossRef]
- Junior, P.C.G.D.; dos Santos, I.J.; da Silva, A.L.; de Assis, R.G.; Vicente, A.C.S.; Carlis, M.S.; Soares, L.C.; Comelli, J.H.; Biava, J.S.; Araujo, R.C.; et al. Essential oil from Arnica montana on feedlot performance, ingestive behavior, carcass characteristics, rumen morphometrics characteristics and meat fatty acids profile of lambs. Small Rumin. Res. 2023, 220, 106920. [Google Scholar] [CrossRef]
- Sun, J.; Cheng, Z.; Zhao, Y.; Wang, Y.; Wang, H.; Ren, Z. Influence of increasing levels of oregano essential oil on intestinal morphology, intestinal flora and performance of Sewa sheep. Ital. J. Anim. Sci. 2022, 21, 463–472. [Google Scholar] [CrossRef]
- Rivera-Chacon, R.; Castillo-Lopez, E.; Ricci, S.; Petri, R.M.; Reisinger, N.; Zebeli, Q. Supplementing a phytogenic feed additive modulates the risk of subacute rumen acidosis, rumen fermentation and systemic inflammation in cattle fed acidogenic diets. Animals 2022, 12, 1201. [Google Scholar] [CrossRef]
- Mickdam, E.; Khiaosa-ard, R.; Metzler-Zebeli, B.U.; Klevenhusen, F.; Chizzola, R.; Zebeli, Q. Rumen microbial abundance and fermentation profile during severe subacute ruminal acidosis and its modulation by plant derived alkaloids in vitro. Anaerobe 2016, 39, 4–13. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, Z.; Wang, D.; Chen, G.; Sun, X.; He, Q.; Luo, J.; Chen, T.; Xi, Q.; Zhang, Y. Effects of fermented herbal tea residues on the intestinal microbiota characteristics of Holstein heifers under heat stress. Front. Microbiol. 2020, 11, 1014. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, X.; Zhang, Y.; Elmhadi, M.; Qin, Y.; Sun, H.; Zhang, H.; Wang, M.; Wang, H. Tannic acid-steeped corn grain modulates in vitro ruminal fermentation pattern and microbial metabolic pathways. Front. Vet. Sci. 2021, 8, 698108. [Google Scholar] [CrossRef]
- Ahmed, M.G.; Al-Sagheer, A.A.; El-Zarkouny, S.Z.; Elwakeel, E.A. Potential of selected plant extracts to control severe subacute ruminal acidosis in vitro as compared with monensin. BMC Vet. Res. 2022, 18, 356. [Google Scholar] [CrossRef]
- Carvalho, G.G.P.; Pires, A.J.V.; Silva, R.R.; Ribeiro, L.S.O.; Chagas, D.M.T. Ingestive behavior of Santa Inês sheep fed diets with cocoa meal. Braz. J. Anim. Sci. 2008, 37, 660–665. [Google Scholar]
- Beauchemin, K.A. Invited review: Current perspectives on eating and rumination activity in dairy cows. J. Dairy Sci. 2018, 101, 4762–4784. [Google Scholar] [CrossRef]
- Dias-Silva, T.P.; Abdalla Filho, A.L. Sheep and goat feeding behavior profile in grazing systems. Acta Sci. Anim. Sci. 2021, 43, e51265. [Google Scholar] [CrossRef]
- Ortencio, M.O.; Araújo, S.A.C.; Rocha, N.S.; Mota, D.A.; Villela, S.D.; Bento, C.B.P.; Araújo, A.M.S.; Domingues, F.N. Ingestive behavior of calves fed diets based on corn grain and supplementary hay. Braz. J. Dev. 2020, 6, 38562–38574. [Google Scholar] [CrossRef]
- Da Silva, C.S.; de Souza, E.J.O.; Pereira, G.F.C.; Cavalcante, E.O.; de Lima, E.I.M.; Torres, T.R.; da Silva, J.R.C.; da Silva, D.C. Plant extracts as phytogenic additives considering intake, digestibility, and feeding behavior of sheep. Trop. Anim. Health Prod. 2017, 49, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Geron, L.J.V.; Veloso, L.E.C.; de Moraes, E.H.B.K.; de Moraes, K.A.K.; Gomes, H.F.B.; Zanin, S.F.P.; Carvalho, G.M.; Brito, T.R.; Silva, P.S.; Almeida, T.A. Phytogenic additive Noni (Morinda citrifolia) in feed of confined lambs. Semin. Ciências Agrárias Londrina 2019, 40, 3679–3690. [Google Scholar] [CrossRef]
- Kalafati, L.; Hatzioannou, A.; Hajishengallis, G.; Chavakis, T. The role of neutrophils in trained immunity. Immunol. Rev. 2023, 314, 142–157. [Google Scholar] [CrossRef]
- Kamelnia, E.; Mohebbati, R.; Kamelnia, R.; El-Seedi, H.R.; Boskabady, M.H. Anti-inflammatory, immunomodulatory and antioxidant effects of Ocimum basilicum L. and its main constituents: A review. Iran. J. Basic Med. Sci. 2023, 26, 617. [Google Scholar]
- Moshfeghinia, R.; Najibi, A.; Moradi, M.; Assadian, K.; Ahmadi, J. The association between hematological markers of inflammation and chronic cannabis use: A systematic review and meta-analysis of observational studies. Front. Psychiatry 2024, 15, 1438002. [Google Scholar] [CrossRef]
- Waqas, M.; Salman, M.; Sharif, M.S. Application of polyphenolic compounds in animal nutrition and their promising effects. J. Anim. Feed Sci. 2023, 32, 233–256. [Google Scholar] [CrossRef]
- Wickramasinghe, H.K.J.P.; Stepanchenko, N.; Oconitrillo, M.J.; Goetz, B.M.; Abeyta, M.A.; Gorden, P.J.; Baumgard, L.H.; Appuhamy, J.A.D.R.N. Effects of a phytogenic feed additive on weaned dairy heifer calves subjected to a diurnal heat stress bout. J. Dairy Sci. 2023, 106, 6114–6127. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Yamazaki, T.; Ohshio, K.; Sugamata, M.; Yoshikawa, M.; Kanauchi, O.; Morita, Y. A specific strain of lactic acid bacteria, lactobacillus paracasei, inhibits inflammasome activation in vitro and prevents inflammation-related disorders. J. Immunol. 2020, 205, 811–821. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, J.; Ou, X.; Han, Y. Anti-cancer substances and safety of lactic acid bacteria in clinical treatment. Front. Microbiol. 2021, 12, 722052. [Google Scholar] [CrossRef]
- Toboła-Wróbel, K.; Pietryga, M.; Dydowicz, P.; Napierała, M.; Brązert, J.; Florek, E. Association of oxidative stress on pregnancy. Oxidative Med. Cell. Longev. 2020, 2020, 6398520. [Google Scholar] [CrossRef] [PubMed]
- Tüfekci, H.; Sejian, V. Stress Factors and Their Effects on Productivity in Sheep. Animals 2023, 13, 2769. [Google Scholar] [CrossRef]
- Nastoh, N.A.; Waqas, M.; Çınar, A.A.; Salman, M. The impact of phytogenic feed additives on ruminant production: A review. J. Anim. Feed Sci. 2024, 33, 431–453. [Google Scholar] [CrossRef]
- Molosse, V.L.; Deolindo, G.L.; Glombosky, P.; Pereira, W.A.; Carvalho, R.A.; Zotti, C.A.; Solivo, G.; Vedovato, M.; Fracasso, M.; Silva, A.D.; et al. Curcumin or microencapsulated phytogenic blend to replace ionophore and non-ionophore antibiotics in weaned calves: Effects on growth performance and health. Livest. Sci. 2022, 263, 105029. [Google Scholar] [CrossRef]
- Bostami, A.R.; Khan, M.R.I.; Rabbi, A.Z.; Siddiqui, M.N.; Islam, M.T. Boosting animal performance, immune index, and antioxidant status in post-weaned bull calves through dietary augmentation of selective traditional medicinal plants. Vet. Anim. Sci. 2021, 14, 100197. [Google Scholar] [CrossRef] [PubMed]
- Shedeed, H.A.; Farrag, B.; Elwakeel, E.A.; Abd El-Hamid, I.S.; El-Rayes, M.A.H. Propolis supplementation improved productivity, oxidative status, and immune response of Barki ewes and lambs. Vet. World 2019, 12, 834–843. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Cheng, L.; Liu, J.; Zhang, S.; Sun, X.; Al-Marashdeh, O. Effects of licorice extract supplementation on feed intake, digestion, rumen function, blood indices and live weight gain of Karakul sheep. Animals 2019, 9, 279. [Google Scholar] [CrossRef] [PubMed]
- Purba, R.A.P.; Paengkoum, P. Exploring the phytochemical profiles and antioxidant, antidiabetic, and antihemolytic properties of Sauropus androgynus dried leaf extracts for ruminant health and production. Molecules 2022, 27, 8580. [Google Scholar] [CrossRef] [PubMed]
- Hrelia, S.; Angeloni, C. New mechanisms of action of natural antioxidants in health and disease. Antioxidants 2020, 9, 344. [Google Scholar] [CrossRef] [PubMed]
- Cetin, N.; Eşki, F.; Mis, L.; Naseer, Z.; Bolacalı, M. Dynamics of oxidants, antioxidants and hormones during different phases of pregnancy in hairy goats. Kafkas Univ. Vet. Fak. Derg. 2021, 27, 117–121. [Google Scholar]
- Piao, M.; Tu, Y.; Zhang, N.; Diao, Q.; Bi, Y. Advances in the application of phytogenic extracts as antioxidants and their potential mechanisms in ruminants. Antioxidants 2023, 12, 879. [Google Scholar] [CrossRef] [PubMed]
- Alipour, F.; Vakili, A.; Mesgaran, M.D.; Ebrahimi, H. The effect of adding ethanolic saffron petal extract and vitamin E on growth performance, blood metabolites, and antioxidant status in Baluchi male lambs. Asian Australas. J. Anim. 2019, 32, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Brunetto, A.L.; Giacomelli, C.M.; Favero, J.F.; Bissacotti, B.F.; Copeti, P.M.; Morsch, V.M.; de Oliveira, F.D.C.; Wagner, R.; Alves, R.; Pereira, W.A.; et al. Phytogenic blend in the diet of growing Holstein steers: Effects on performance, digestibility, rumen volatile fatty acid profile, and immune and antioxidant responses. Anim. Feed Sci. Technol. 2023, 297, 115595. [Google Scholar] [CrossRef]
- Tantawi, A.A.; Imbabi, T.A.; Abdelhakeam, M.A.; Hassan, H.M.; Nasr, M.A.F.; Elgananiny, S. Does phytogenic natural compound improve growth, physiological status, antioxidant parameters, digestibility and nutritive value of Ossimi lambs. Small Rumin. Res. 2023, 229, 107130. [Google Scholar] [CrossRef]
- Basiouni, S.; Tellez-Isaias, G.; Latorre, J.D.; Graham, B.D.; Petrone-Garcia, V.M.; El-Seedi, H.R.; Yalçın, S.; El-Wahab, A.A.; Visscher, C.; May-Simera, H.L.; et al. Anti-Inflammatory and Antioxidative Phytogenic Substances against Secret Killers in Poultry: Current Status and Prospects. Vet. Sci. 2023, 10, 55. [Google Scholar] [CrossRef]
- Kozlov, A.V.; Javadov, S.; Sommer, N. Cellular ROS and Antioxidants: Physiological and Pathological Role. Antioxidants 2024, 13, 602. [Google Scholar] [CrossRef]

| Peaks | Compounds | Retention Time, min | Peak Area (%) | Molecular Weight | Molecular Formula |
|---|---|---|---|---|---|
| 1 | á-d-Glucopyranosiduronic acid, 3-(5-ethylhexahydro-2,4,6-trioxo-5-pyrimidin yl)-1,1-dimethylpropyl 2,3,4-tris-O-(trimethylsilyl)-, methyl ester | 4.22 | 9.99 | 648 | C27H52N2O10Si3 |
| 2 | 4-Hexyl-1-(7-methoxycarbonylheptyl)bicyclo[4.4.0]deca-2,5,7-triene | 4.39 | 2.86 | 372 | C25H40O2 |
| 3 | Decanoic acid, 1,1a,1b,4,4a,5,7a,7b,8,9-decahydro-4a,7b-dihydroxy-3-(hydroxymethyl)-1,1,6,8-tetramet hyl-5-oxo-9aH-cyclopropa[3,4]benz[1,2-e]azulene-9,9a-diyl ester, [1aR-(1aà,1bá,4aá,7aà,7bà,8à,9á,9aà)] | 25.41 | 1.45 | 672 | C40H64O8 |
| 4 | Pentadecanoic acid, 14-methyl-, methyl ester | 25.45 | 1.01 | 270 | C17H34O2 |
| 5 | L-Valine, N-[2-(chloroimino)-3-methyl-1-oxobutyl]- | 25.52 | 1.30 | 248 | C10H17ClN2O3 |
| 6 | l-(+)-Ascorbic acid 2,6-dihexadecanoate | 31.66 | 12.19 | 652 | C38H68O8 |
| 7 | à-d-Galactopyranose, 6-O-(trimethylsilyl)-, cyclic 1,2:3,4-bis(butylboronate) | 33.64 | 4.78 | 384 | C17H34B2O6Si |
| 8 | Linoleic acid ethyl ester | 34.21 | 1.28 | 308 | C20H36O2 |
| 9 | cis-7,cis-11-Hexadecadien-1-yl acetate | 35.39 | 29.13 | 280 | C18H32O2 |
| 10 | Elaidic acid, isopropyl ester | 35.50 | 14.38 | 324 | C21H40O2 |
| 11 | Octadecanal, 2-bromo | 36.16 | 2.36 | 346 | C18H35BrO |
| 12 | 9-Octadecenoic acid, (2-phenyl-1,3-dioxolan-4-yl)methyl ester, cis | 37.08 | 2.13 | 444 | C28H44O4 |
| 13 | Milbemycin B, 6,28-anhydro-15-chloro-25-isopropyl-13-dehydro-5-O-demethyl-4-methyl- | 41.16 | 1.21 | 590 | C33H47ClO7 |
| 14 | à-d-Xylopyranoside, methyl-2,3,4-tris-O-[9 borabicyclo[3.3.1]non-9-yl] | 41.64 | 1.26 | 524 | C30H51B3O5 |
| 15 | Ursa-9(11),12-dien-28-oic acid, 3-(acetyloxy)-, methyl ester, (3á)- | 45.86 | 4.22 | 510 | C33H50O4 |
| 16 | Betulin | 50.64 | 0.84 | 442 | C30H50O2 |
| 17 | Ursodeoxycholic acid | 51.13 | 1.18 | 392 | C24H40O4 |
| 18 | 9,12,15-Octadecatrienoic acid, 2-phenyl-1,3-dioxan-5-yl ester | 51.18 | 0.64 | 440 | C28H40O4 |
| 19 | Milbemycin B,5-demethoxy-5-one-6,28-anhydro-25-ethyl-4-methyl-13-chloro-oxime | 51.22 | 0.39 | 589 | C32H44ClNO7 |
| 20 | á Carotene | 51.26 | 0.80 | 536 | C40H56 |
| 21 | Sulfadiazine | 51.34 | 0.59 | 250 | C10H10N4O2S |
| 22 | á-Sitosterol | 51.51 | 2.21 | 414 | C29H50O |
| 23 | .psi.,.psi.-Carotene | 52.04 | 1.52 | 600 | C42H64O2 |
| 24 | 25-Norisopropyl-9,19-cyclolanostan-22-en-24-one, 3-acetoxy-24-phenyl-4,4,14-trimethyl- | 54.44 | 0.77 | 516 | C35H48O3 |
| 25 | 2,2′-Methylenebis[3,4,6-trichloroanisole] | 54.53 | 1.51 | 432 | C15H10Cl6O2 |
| Item | % (Dry Matter Bases) |
|---|---|
| Ingredients | |
| Egyptain Berssem (Trifolium alexandrinum) clover hay | 25.0 |
| Wheat straw | 25.0 |
| Yellow corn | 22.5 |
| Un-decorticated cottonseed meal | 12.5 |
| Wheat bran | 10.0 |
| Rice bran | 2.0 |
| Molasses | 1.5 |
| Limestone | 1.0 |
| Vitamin and minerals mixture * | 0.5 |
| Chemical composition | |
| Dry matter | 91.0 |
| Organic matter | 89.3 |
| Crude protein | 11.9 |
| Ether extract | 25.0 |
| Nitrogen-free extract | 2.53 |
| Gene | Primer | Product Length (bp) | Annealing Temperature (°C) | Accession Number |
|---|---|---|---|---|
| Leptin | F5′-CAGTCCGTCTCCTCCAAACA-3′ R5′-CGGAGGTTCTCCAGGTCATT-3′ | 170 | 60 | EU158187.1 |
| STAT5A | F5′-TGGGGCCTTCCTGTAGTAAC-3′ R5′-CGGGGATATTCCAGCCCAAA-3′ | 194 | 58 | JN688205.1 |
| IGF-I | F5′-ATCAGCAGTCTTCCAACCCA-3′ R5′-AGAGCATCCACCAACTCAGC-3′ | 179 | 58 | NM_001285697.1 |
| DGAT1 | F5′-ACTACTACGTGCTCAACCGC-3′ R5′-AGACTGCAATCGCGTGTCG-3′ | 126 | 60 | MT221183.1 |
| IL-6 | F5′-TTCAGTCCACTCGCTGTCTC-3′ R5′-TGCTTGGGGTGGTGTCATTC-3′ | 106 | 58 | NM_001285640.1 |
| IL-8 | F5′-CTGGCCAGGATTCACGAGTT-3′ R5′-TGCTTCCACATGTCCTCACA-3′ | 117 | 60 | XM_005681749.3 |
| TNF-α | F5′-GCATGAGCACCAAAAGCATGA-3′ R5′-CTGGGGACTGCTCTTCCCTCT-3′ | 198 | 60 | NM_001286442.1 |
| SOD1 | F5′-ATCCACTTCGAGGCAAAGGG-3′ R5′-CTGCACTGGTACAGCCTTGT-3′ | 124 | 60 | NM_001285550.1 |
| CAT | F5′-ACACAGGCACATGAACGGAT-3′ R5′-CCGTAGTCAGGGTCTTCGTG-3′ | 159 | 58 | GQ204786.1 |
| GPx1 | F5′-AAGTTCATCACGTGGTCCCC-3′ R5′-CTGGGACAGCAGGGTTTCAA-3′ | 153 | 58 | XM_005695962.3 |
| Nrf2 | F5′-CTACGGGCAAAAGCTCTCCA-3′ R5′-TCTGCAATTCTGAGCAGCCA-3′ | 171 | 60 | KM576769.1 |
| β. actin | F5′-CGTGCTGCTGACGGAGGCCCC-3′ R5′-GCACAGCCTGGATGGCCACATAC-3′ | 113 | 60 | AF481159.1 |
| Control | PEL | SEM | p-Values | |||
|---|---|---|---|---|---|---|
| 0 g/Day | 2 g/Day | 4 g/Day | Linear | Quadratic | ||
| Feed intake, g/day | ||||||
| Dry matter | 1089 | 1095 | 1104 | 1.02 | <0.001 | 0.322 |
| Total digestible nutrient | 700 | 722 | 755 | 0.62 | <0.001 | 0.145 |
| Digestible crude protein | 11.3 | 11.7 | 12.2 | 0.01 | <0.001 | 0.258 |
| Nutrient digestibility, % | ||||||
| Dry matter | 63.8 | 66.6 | 68.7 | 0.19 | <0.001 | 0.006 |
| Organic matter | 64.2 | 67.0 | 69.1 | 0.18 | <0.001 | <0.001 |
| Crude protein | 66.1 | 68.3 | 70.6 | 0.22 | <0.001 | <0.001 |
| Crude fiber | 53.7 | 55.2 | 57.5 | 0.17 | <0.001 | 0.827 |
| Ether extract | 60.2 | 63.5 | 68.4 | 0.19 | <0.001 | 0.026 |
| Nitrogen-free extract | 67.9 | 69.1 | 71.3 | 0.19 | <0.001 | 0.557 |
| Feeding value, % | ||||||
| Total digestible nutrients | 58.6 | 60.0 | 62.2 | 0.12 | <0.001 | 0.338 |
| Digestible crude protein | 7.89 | 8.15 | 8.43 | 0.03 | <0.001 | <0.001 |
| Ruminal parameters | ||||||
| pH values | 6.43 | 6.35 | 6.31 | 0.030 | 0.003 | 0.699 |
| Ammoina, mg/100 mL | 21.5 | 20.5 | 19.7 | 0.10 | <0.001 | 0.235 |
| Volatial fatty acids (mmol/L) | ||||||
| Acetic | 46.8 | 49.8 | 52.1 | 0.12 | <0.001 | <0.001 |
| Propionic | 24.8 | 26.5 | 28.6 | 0.16 | <0.001 | 0.388 |
| Butyric | 22.4 | 19.3 | 18.5 | 0.15 | <0.001 | 0.001 |
| Control | PEL | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| 0 g/Day | 2 g/Day | 4 g/Day | Linear | Quadratic | ||
| Hemoglobin, g/dL | 7.30 | 8.86 | 9.67 | 0.110 | <0.001 | 0.970 |
| Red blood cells, ×106/µL | 6.35 | 6.88 | 7.11 | 0.042 | <0.001 | 0.005 |
| Hematocrit, % | 32.1 | 35.5 | 38.3 | 0.453 | <0.001 | 0.185 |
| Mean corpuscular hemoglobin, pg/cell | 27.8 | 24.8 | 23.9 | 0.431 | <0.001 | 0.661 |
| Mean corpuscular volume, µm3 | 19.2 | 18.8 | 18.2 | 0.224 | <0.001 | 0.044 |
| Mean corpuscular hemoglobin concentration, % | 58.9 | 56.2 | 53.1 | 0.254 | <0.001 | 0.492 |
| Platelet count | 465 | 475 | 485 | 1.120 | <0.001 | 0.362 |
| White blood cells, ×103/µL | 11.3 | 8.84 | 7.86 | 0.310 | <0.001 | <0.001 |
| Neutrophils, % | 42.2 | 50.6 | 55.7 | 0.540 | <0.001 | 0.648 |
| Lymphocytes, % | 48.6 | 43.0 | 39.1 | 0.411 | <0.001 | 0.696 |
| Monocytes, % | 4.71 | 3.86 | 2.86 | 0.262 | <0.001 | 0.919 |
| Eosinophils, % | 2.73 | 1.63 | 1.31 | 0.181 | <0.001 | 0.616 |
| Control | PEL | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| 0 g/Day | 2 g/Day | 4 g/Day | Linear | Quadratic | ||
| Serum protein, g/dL | ||||||
| Total protein | 5.39 | 5.96 | 6.97 | 0.030 | <0.001 | 0.018 |
| Albumin | 3.16 | 3.37 | 3.79 | 0.021 | <0.001 | 0.003 |
| Globulin | 2.23 | 2.60 | 3.21 | 0.032 | <0.001 | 0.167 |
| Albumin/Globulin ratio | 1.42 | 1.30 | 1.18 | 0.021 | <0.001 | 0.385 |
| Liver function, IU/L | ||||||
| Aspartate aminotransferase | 43.9 | 41.4 | 40.2 | 0.110 | <0.001 | 0.027 |
| Alanine aminotransferase | 30.2 | 28.1 | 25.9 | 0.201 | <0.001 | <0.001 |
| Kidney function, mg/dL | ||||||
| Urea | 37.8 | 33.7 | 24.2 | 0.550 | <0.001 | 0.009 |
| Creatinine | 1.21 | 0.95 | 0.82 | 0.011 | <0.001 | 0.004 |
| Serum lipids, mg/dL | ||||||
| Total Cholesterol | 130 | 122 | 116 | 0.911 | <0.001 | 0.020 |
| Triglyceride | 92.2 | 86.2 | 77.3 | 0.468 | <0.001 | 0.032 |
| High-density lipoprotein | 41.7 | 48.4 | 63.6 | 0.780 | <0.001 | <0.001 |
| Low-density lipoprotein | 61.9 | 54.5 | 46.4 | 0.851 | <0.001 | 0.089 |
| Very low-density lipoprotein | 18.4 | 17.2 | 15.5 | 0.090 | <0.001 | 0.032 |
| Glucose | 113.1 | 98.1 | 88.4 | 0.900 | <0.001 | <0.001 |
| Lipase, U/L | 46.7 | 108 | 129 | 0.983 | <0.001 | <0.001 |
| Amylase, U/L | 78.7 | 83.4 | 87.9 | 0.621 | <0.001 | 0.049 |
| Cortisol, ug/dL | 1.57 | 0.84 | 0.47 | 0.040 | <0.001 | 0.014 |
| Thyroid hormones, ng/ml | ||||||
| Triiodothyronine (T3) | 0.52 | 0.48 | 0.33 | 0.010 | <0.001 | 0.187 |
| Thyroxine (T4) | 29.4 | 30.3 | 38.4 | 0.440 | <0.001 | 0.002 |
| Control | PEL | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| 0 g/Day | 2 g/Day | 4 g/Day | Linear | Quadratic | ||
| Immune indicators | ||||||
| Immunoglobulin G, mg/dL | 31.4 | 45.8 | 50.3 | 0.430 | <0.001 | <0.001 |
| Immunoglobulin M, mg/dL | 50.7 | 61.3 | 65.3 | 0.333 | <0.001 | <0.001 |
| Antioxidant indicators | ||||||
| Total antioxidant capacity (TAC), mmol/L | 0.87 | 1.18 | 1.62 | 0.030 | <0.001 | <0.001 |
| Malondialdehyde (MDA), nmol/mL | 14.5 | 12.4 | 9.61 | 0.171 | <0.001 | 0.041 |
| Catalase (CAT), U/g | 464.1 | 538.9 | 616.3 | 4.560 | <0.001 | 0.920 |
| Glutathione peroxidase (GPx), U/I | 1.13 | 1.89 | 2.20 | 0.021 | <0.001 | <0.001 |
| Inflammation indicators, pg/mg | ||||||
| Interleukin B (ILB) | 881.1 | 673.6 | 408.3 | 14.72 | <0.001 | 0.095 |
| Interleukin6 (IL6) | 386.3 | 234.1 | 175.1 | 4.501 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabr, A.A.; Farrag, F.; Ahmed, M.; Soltan, Y.A.; Ateya, A.; Mafindi, U. The Performance, Ingestive Behavior, Nutrient Digestibility, Ruminal Fermentation Profile, Health Status, and Gene Expression of Does Fed a Phytochemical–Lactobacilli Blend in Late Pregnancy. Animals 2025, 15, 598. https://doi.org/10.3390/ani15040598
Gabr AA, Farrag F, Ahmed M, Soltan YA, Ateya A, Mafindi U. The Performance, Ingestive Behavior, Nutrient Digestibility, Ruminal Fermentation Profile, Health Status, and Gene Expression of Does Fed a Phytochemical–Lactobacilli Blend in Late Pregnancy. Animals. 2025; 15(4):598. https://doi.org/10.3390/ani15040598
Chicago/Turabian StyleGabr, Amr A., Fayek Farrag, Mohamed Ahmed, Yosra A. Soltan, Ahmed Ateya, and Umar Mafindi. 2025. "The Performance, Ingestive Behavior, Nutrient Digestibility, Ruminal Fermentation Profile, Health Status, and Gene Expression of Does Fed a Phytochemical–Lactobacilli Blend in Late Pregnancy" Animals 15, no. 4: 598. https://doi.org/10.3390/ani15040598
APA StyleGabr, A. A., Farrag, F., Ahmed, M., Soltan, Y. A., Ateya, A., & Mafindi, U. (2025). The Performance, Ingestive Behavior, Nutrient Digestibility, Ruminal Fermentation Profile, Health Status, and Gene Expression of Does Fed a Phytochemical–Lactobacilli Blend in Late Pregnancy. Animals, 15(4), 598. https://doi.org/10.3390/ani15040598








