Epidemiology and Molecular Characterization of Feline Calicivirus in Beijing, China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Information and Treatment
2.2. Statistical Analysis
2.3. Virus Isolation
2.4. Genome Amplification and Sequencing
2.5. FCV Sequences Analysis
3. Results
3.1. Epidemiological Investigation
3.2. Virus Isolation, Amplification and Sequencing
3.3. Phylogenetic and Homology Analysis of FCV Isolates
3.4. Amino Acid Analysis of the VP1 Protein
3.5. Detection of Recombinant of FCV Isolates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Isolate | Amino-Acid Residues and Physico-Chemical Properties Associated with VSD Pathotype | ||||||
|---|---|---|---|---|---|---|---|
| 438 | 440 | 448 | 452 | 455 | 465 | 492 | |
| VSD-FCV | V | Q | K/R | E | T | S | V |
| URTD-FCV | T | G | A | D | D | G | V |
| FCV1-BJ 2023 | T | S | G | D | D | S | I |
| FCV2-BJ 2023 | A | S | A | D | G | S | V |
| FCV3-BJ 2023 | V | S | P | D | D | G | V |
| FCV4-BJ 2023 | T | S | R | E | D | G | I |
| FCV5-BJ 2023 | T | S | R | E | D | G | V |
| FCV6-BJ 2023 | T | G | A | D | T | S | V |
| FCV7-BJ 2023 | T | G | A | D | D | S | V |
| FCV8-BJ 2023 | T | G | A | D | D | S | I |
| FCV9-BJ 2023 | T | S | A | D | D | G | V |
| FCV12-BJ2023 | T | G | A | D | S | S | V |
| FCV13-BJ2023 | T | S | A | D | S | S | V |
| FCV14-BJ2023 | T | E | K | E | T | S | L |
| FCV15-BJ2023 | T | S | A | D | S | S | V |
| FCV20-BJ 2023 | T | G | S | D | D | S | V |
| FCV22-BJ 2023 | T | Q | A | E | T | S | L |
| FCV23-BJ 2023 | T | S | K | E | D | G | I |
| FCV24-BJ 2023 | T | E | P | D | N | S | V |
| FCV26-BJ 2023 | T | G | A | D | D | S | I |
| FCV30-BJ 2023 | T | E | P | D | V | S | V |
| FCV32-BJ 2023 | T | E | A | D | T | S | V |
| FCV34-BJ 2023 | T | G | A | D | D | S | V |
| FCV36-BJ 2023 | T | G | A | D | D | G | L |
| FCV37-BJ 2023 | T | E | P | D | V | S | I |
| FCV40-BJ 2023 | T | G | A | D | D | S | V |
| FCV41-BJ 2023 | T | G | K | E | D | S | I |
| FCV42-BJ 2023 | T | G | K | E | D | S | I |
| FCV43-BJ 2023 | T | T | A | D | D | S | V |
| FCV45-BJ 2023 | T | S | R | E | D | G | I |
| FCV46-BJ 2023 | V | Q | K | E | I | S | L |
| FCV47-BJ 2023 | T | G | A | D | D | G | V |
| FCV49-BJ 2023 | T | S | S | D | D | G | L |
| FCV50-BJ 2023 | T | G | A | D | D | S | V |
| FCV51-BJ 2023 | T | G | A | D | D | S | V |
| FCV52-BJ 2023 | T | N | A | D | D | S | V |
| FCV53-BJ 2023 | T | S | R | E | D | G | I |
| FCV54-BJ 2023 | T | L | R | E | N | S | I |
| FCV55-BJ 2023 | T | A | P | D | D | G | I |
| FCV56-BJ 2023 | T | G | P | E | D | S | V |
| FCV57-BJ 2023 | V | E | A | D | V | S | I |
| FCV58-BJ 2023 | T | G | S | D | S | S | V |
| FCV63-BJ 2023 | T | A | A | D | D | S | V |
| FCV64-BJ 2023 | T | S | P | D | D | S | V |
| FCV66-BJ 2023 | T | S | L | E | D | G | V |
| FCV67-BJ 2023 | T | Q | K | D | L | S | L |
| FCV69-BJ 2023 | T | G | P | D | D | S | V |
| FCV70-BJ 2023 | V | L | K | E | Q | S | L |
| FCV71-BJ 2023 | T | G | A | D | D | G | I |
| FCV72-BJ 2023 | T | S | K | E | E | G | I |
| FCV73-BJ 2023 | V | E | R | E | N | S | V |
| FCV74-BJ 2023 | T | E | A | D | G | S | V |
| FCV75-BJ 2023 | T | L | R | E | D | G | I |
| FCV76-BJ 2023 | L | L | A | D | T | S | I |
| FCV78-BJ 2023 | T | G | R | E | D | S | V |
| FCV80-BJ 2023 | T | K | A | D | D | G | L |
| FCV81-BJ 2023 | T | S | A | D | D | G | L |
| FCV82-BJ 2023 | T | A | A | D | G | G | I |
| FCV84-BJ 2023 | T | G | A | D | N | S | V |
| FCV85-BJ 2023 | V | L | K | E | Q | S | L |
| FCV86-BJ 2023 | T | G | P | D | Q | G | L |
| FCV87-BJ 2023 | T | G | A | D | G | G | L |
References
- Fastier, L.B. A New Feline Virus Isolated in Tissue Culture. Am. J. Vet. Res. 1957, 18, 382–389. [Google Scholar] [PubMed]
- Hou, J.; Sánchez-Vizcaíno, F.; McGahie, D.; Lesbros, C.; Almeras, T.; Howarth, D.; O’Hara, V.; Dawson, S.; Radford, A.D. European Molecular Epidemiology and Strain Diversity of Feline Calicivirus. Vet. Rec. 2016, 178, 114–115. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Ohe, K.; Murakami, M.; Fukuyama, M.; Furuhata, K.; Kishikawa, S.; Suzuki, Y.; Kiuchi, A.; Hara, M.; Ishikawa, Y.; et al. Phylogenetic Analysis of Field Isolates of Feline Calcivirus (FCV) in Japan by Sequencing Part of Its Capsid Gene. Vet. Res. Commun. 2002, 26, 205–219. [Google Scholar] [CrossRef]
- Mao, J.; Ye, S.; Li, Q.; Bai, Y.; Wu, J.; Xu, L.; Wang, Z.; Wang, J.; Zhou, P.; Li, S. Molecular Characterization and Phylogenetic Analysis of Feline Calicivirus Isolated in Guangdong Province, China from 2018 to 2022. Viruses 2022, 14, 2421. [Google Scholar] [CrossRef]
- Hoover, E.A.; Kahn, D.E. Experimentally Induced Feline Calicivirus Infection: Clinical Signs and Lesions. J. Am. Vet. Med. Assoc. 1975, 166, 463–468. [Google Scholar]
- Reubel, G.H.; Hoffmann, D.E.; Pedersen, N.C. Acute and Chronic Faucitis of Domestic Cats. A Feline Calicivirus-Induced Disease. Vet. Clin. N. Am. Small Anim. Pract. 1992, 22, 1347–1360. [Google Scholar] [CrossRef]
- Cooper, L.M.; Sabine, M. Paw and Mouth Disease in a Cat. Aust. Vet. J. 1972, 48, 644. [Google Scholar] [CrossRef]
- Dawson, S.; Bennett, D.; Carter, S.D.; Bennett, M.; Meanger, J.; Turner, P.C.; Carter, M.J.; Milton, I.; Gaskell, R.M. Acute Arthritis of Cats Associated with Feline Calicivirus Infection. Res. Vet. Sci. 1994, 56, 133–143. [Google Scholar] [CrossRef]
- Battilani, M.; Vaccari, F.; Carelle, M.S.; Morandi, F.; Benazzi, C.; Kipar, A.; Dondi, F.; Scagliarini, A. Virulent Feline Calicivirus Disease in a Shelter in Italy: A Case Description. Res. Vet. Sci. 2013, 95, 283–290. [Google Scholar] [CrossRef]
- Bordicchia, M.; Fumian, T.M.; Van Brussel, K.; Russo, A.G.; Carrai, M.; Le, S.-J.; Pesavento, P.A.; Holmes, E.C.; Martella, V.; White, P.; et al. Feline Calicivirus Virulent Systemic Disease: Clinical Epidemiology, Analysis of Viral Isolates and In Vitro Efficacy of Novel Antivirals in Australian Outbreaks. Viruses 2021, 13, 2040. [Google Scholar] [CrossRef]
- Wilhelm, S.; Truyen, U. Real-Time Reverse Transcription Polymerase Chain Reaction Assay to Detect a Broad Range of Feline Calicivirus Isolates. J. Virol. Methods 2006, 133, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Pesavento, P.A.; Chang, K.-O.; Parker, J.S.L. Molecular Virology of Feline Calicivirus. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Green, K.Y.; Ando, T.; Balayan, M.S.; Berke, T.; Clarke, I.N.; Estes, M.K.; Matson, D.O.; Nakata, S.; Neill, J.D.; Studdert, M.J.; et al. Taxonomy of the Caliciviruses. J. Infect. Dis. 2000, 181, S322–S330. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Huang, J.; Liu, Y.; Pan, Y.; Li, Y.; Miao, Q.; Qu, L.; Tian, J. Feline Calicivirus Proteinase-Polymerase Protein Degrades mRNAs To Inhibit Host Gene Expression. J. Virol. 2021, 95, e0033621. [Google Scholar] [CrossRef]
- Mao, J.; Ye, S.; Deng, J.; Song, J.; Wang, Z.; Chen, A.; Zhou, P.; Li, S. Feline Calicivirus P39 Inhibits Innate Immune Responses by Autophagic Degradation of Retinoic Acid Inducible Gene I. Int. J. Mol. Sci. 2023, 24, 5254. [Google Scholar] [CrossRef]
- Cubillos-Zapata, C.; Angulo, I.; Almanza, H.; Borrego, B.; Zamora-Ceballos, M.; Castón, J.R.; Mena, I.; Blanco, E.; Bárcena, J. Precise Location of Linear Epitopes on the Capsid Surface of Feline Calicivirus Recognized by Neutralizing and Non-Neutralizing Monoclonal Antibodies. Vet. Res. 2020, 51, 59. [Google Scholar] [CrossRef]
- Brunet, S.; Sigoillot-Claude, C.; Pialot, D.; Poulet, H. Multiple Correspondence Analysis on Amino Acid Properties within the Variable Region of the Capsid Protein Shows Differences between Classical and Virulent Systemic Feline Calicivirus Strains. Viruses 2019, 11, 1090. [Google Scholar] [CrossRef]
- Geissler, K.; Schneider, K.; Truyen, U. Mapping Neutralizing and Non-Neutralizing Epitopes on the Capsid Protein of Feline Calicivirus. J. Vet. Med. B Infect. Dis. Vet. Public Health 2002, 49, 55–60. [Google Scholar] [CrossRef]
- Radford, A.D.; Willoughby, K.; Dawson, S.; McCracken, C.; Gaskell, R.M. The Capsid Gene of Feline Calicivirus Contains Linear B-Cell Epitopes in Both Variable and Conserved Regions. J. Virol. 1999, 73, 8496–8502. [Google Scholar] [CrossRef]
- Fujita, S.; Koba, R.; Tohya, Y. Identification of Amino Acid Substitutions Escaping from a Broadly Neutralizing Monoclonal Antibody of Feline Calicivirus. Virus Res. 2022, 318, 198848. [Google Scholar] [CrossRef]
- Chowdhury, M.Z.I.; Turin, T.C. Variable Selection Strategies and Its Importance in Clinical Prediction Modelling. Fam. Med. Community Health 2020, 8, e000262. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Miao, Q.; Zhu, J.; Yang, Z.; Liu, G. Isolation and Molecular Characterization of a Virulent Systemic Feline Calicivirus Isolated in China. Infect. Genet. Evol. 2018, 65, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Kim, Y.-K.; Kim, Y.-S.; Na, E.-J.; Kim, Y.-J.; Oem, J.-K. Intergenic Recombination in Feline Calicivirus Associated with a Hemorrhagic-like Disease in the Republic of Korea. Acta Virol. 2021, 65, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Ludwig-Begall, L.F.; Mauroy, A.; Thiry, E. Norovirus Recombinants: Recurrent in the Field, Recalcitrant in the Lab—A Scoping Review of Recombination and Recombinant Types of Noroviruses. J. Gen. Virol. 2018, 99, 970–988. [Google Scholar] [CrossRef]
- Kim, S.; Cheng, Y.; Fang, Z.; Liu, X.; Zhongqi, Q.; Weidong, Y.; Yilmaz, A.; Yilmaz, H.; Umar, S. Molecular Epidemiology and Phylogenetic Analysis of Feline Calicivirus in Kunshan, China. Virol. J. 2024, 21, 50. [Google Scholar] [CrossRef]
- Komina, A.; Krasnikov, N.; Kucheruk, O.; Zhukova, E.; Yuzhakov, A.; Gulyukin, A. Distribution and Genetic Diversity of Feline Calicivirus in Moscow Metropolitan Area. J. Vet. Sci. 2022, 23, e92. [Google Scholar] [CrossRef]
- Zheng, M.; Li, Z.; Fu, X.; Lv, Q.; Yang, Y.; Shi, F. Prevalence of Feline Calicivirus and the Distribution of Serum Neutralizing Antibody against Isolate Strains in Cats of Hangzhou, China. J. Vet. Sci. 2021, 22, e73. [Google Scholar] [CrossRef]
- Berger, A.; Willi, B.; Meli, M.L.; Boretti, F.S.; Hartnack, S.; Dreyfus, A.; Lutz, H.; Hofmann-Lehmann, R. Feline Calicivirus and Other Respiratory Pathogens in Cats with Feline Calicivirus-Related Symptoms and in Clinically Healthy Cats in Switzerland. BMC Vet. Res. 2015, 11, 282. [Google Scholar] [CrossRef]
- Wang, Z.; Xin, T.; Wei, J.; Jiang, Y.; Liu, X.; Song, W.; Guo, X.; Yuan, W.; Cui, Y.; Zhu, H.; et al. Isolation and Phylogenetic Analysis of Strains of Feline Calicivirus in Beijing, China. Arch. Virol. 2021, 166, 2521–2527. [Google Scholar] [CrossRef]
- Coyne, K.P.; Gaskell, R.M.; Dawson, S.; Porter, C.J.; Radford, A.D. Evolutionary Mechanisms of Persistence and Diversification of a Calicivirus within Endemically Infected Natural Host Populations. J. Virol. 2007, 81, 1961–1971. [Google Scholar] [CrossRef]
- Wardley, R.C. Feline Calicivirus Carrier State a Study of the Host/Virus Relationship. Arch. Virol. 1976, 52, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Radford, A.D.; Dawson, S.; Ryvar, R.; Coyne, K.; Johnson, D.R.; Cox, M.B.; Acke, E.F.J.; Addie, D.D.; Gaskell, R.M. High Genetic Diversity of the Immunodominant Region of the Feline Calicivirus Capsid Gene in Endemically Infected Cat Colonies. Virus Genes. 2003, 27, 145–155. [Google Scholar] [CrossRef]
- Johnson, R.P.; Povey, R.C. Transfer and Decline of Maternal Antibody to Feline Calicivirus. Can. Vet. J. 1983, 24, 6–9. [Google Scholar] [PubMed]
- Digangi, B.A.; Levy, J.K.; Griffin, B.; Reese, M.J.; Dingman, P.A.; Tucker, S.J.; Dubovi, E.J. Effects of Maternally-Derived Antibodies on Serologic Responses to Vaccination in Kittens. J. Feline Med. Surg. 2012, 14, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Pesavento, P.A.; Stokol, T.; Liu, H.; van der List, D.A.; Gaffney, P.M.; Parker, J.S. Distribution of the Feline Calicivirus Receptor Junctional Adhesion Molecule a in Feline Tissues. Vet. Pathol. 2011, 48, 361–368. [Google Scholar] [CrossRef]
- Povey, C.; Ingersoll, J. Cross-Protection among Feline Caliciviruses. Infect. Immun. 1975, 11, 877–885. [Google Scholar] [CrossRef]
- Spiri, A.M.; Novacco, M.; Meli, M.L.; Stirn, M.; Riond, B.; Fogle, J.E.; Boretti, F.S.; Herbert, I.; Hosie, M.J.; Hofmann-Lehmann, R. Modified-Live Feline Calicivirus Vaccination Elicits Cellular Immunity against a Current Feline Calicivirus Field Strain in an Experimental Feline Challenge Study. Viruses 2021, 13, 1736. [Google Scholar] [CrossRef]
- Porter, C.J.; Radford, A.D.; Gaskell, R.M.; Ryvar, R.; Coyne, K.P.; Pinchbeck, G.L.; Dawson, S. Comparison of the Ability of Feline Calicivirus (FCV) Vaccines to Neutralise a Panel of Current UK FCV Isolates. J. Feline Med. Surg. 2008, 10, 32–40. [Google Scholar] [CrossRef]
- Zhou, L.; Fu, N.; Ding, L.; Li, Y.; Huang, J.; Sha, X.; Zhou, Q.; Song, X.; Zhang, B. Molecular Characterization and Cross-Reactivity of Feline Calicivirus Circulating in Southwestern China. Viruses 2021, 13, 1812. [Google Scholar] [CrossRef]
- Afonso, M.M.; Pinchbeck, G.L.; Smith, S.L.; Daly, J.M.; Gaskell, R.M.; Dawson, S.; Radford, A.D. A Multi-National European Cross-Sectional Study of Feline Calicivirus Epidemiology, Diversity and Vaccine Cross-Reactivity. Vaccine 2017, 35, 2753–2760. [Google Scholar] [CrossRef]
- Lu, Z.; Ledgerwood, E.D.; Hinchman, M.M.; Dick, R.; Parker, J.S.L. Conserved Surface Residues on the Feline Calicivirus Capsid Are Essential for Interaction with Its Receptor Feline Junctional Adhesion Molecule A (fJAM-A). J. Virol. 2018, 92, e00035-18. [Google Scholar] [CrossRef] [PubMed]
- Mathijs, E.; Muylkens, B.; Mauroy, A.; Ziant, D.; Delwiche, T.; Thiry, E. Experimental Evidence of Recombination in Murine Noroviruses. J. Gen. Virol. 2010, 91, 2723–2733. [Google Scholar] [CrossRef] [PubMed]
- Symes, S.J.; Job, N.; Ficorilli, N.; Hartley, C.A.; Browning, G.F.; Gilkerson, J.R. Novel Assay to Quantify Recombination in a Calicivirus. Vet. Microbiol. 2015, 177, 25–31. [Google Scholar] [CrossRef]
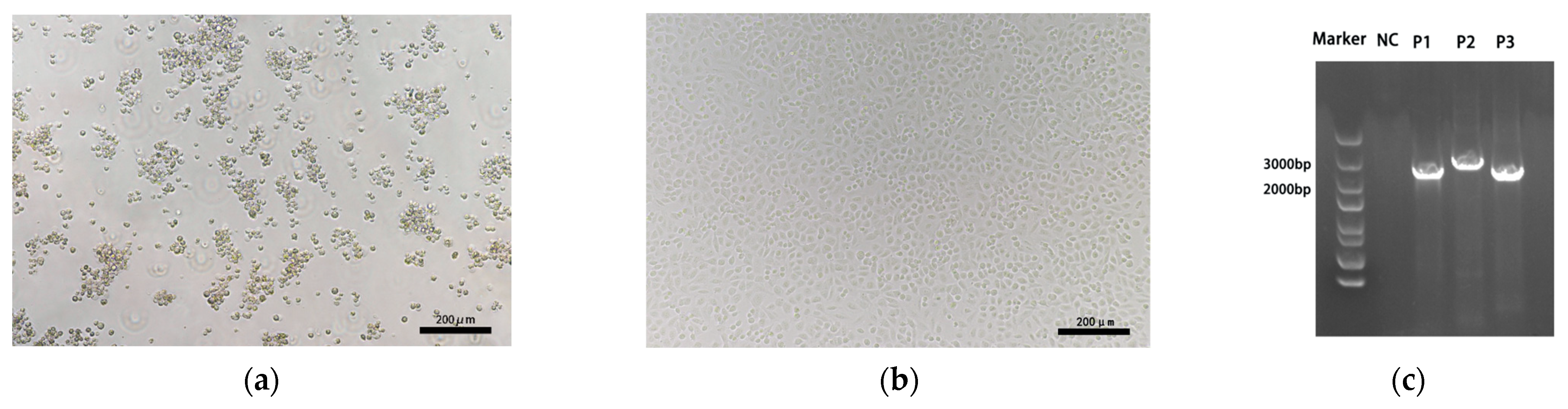
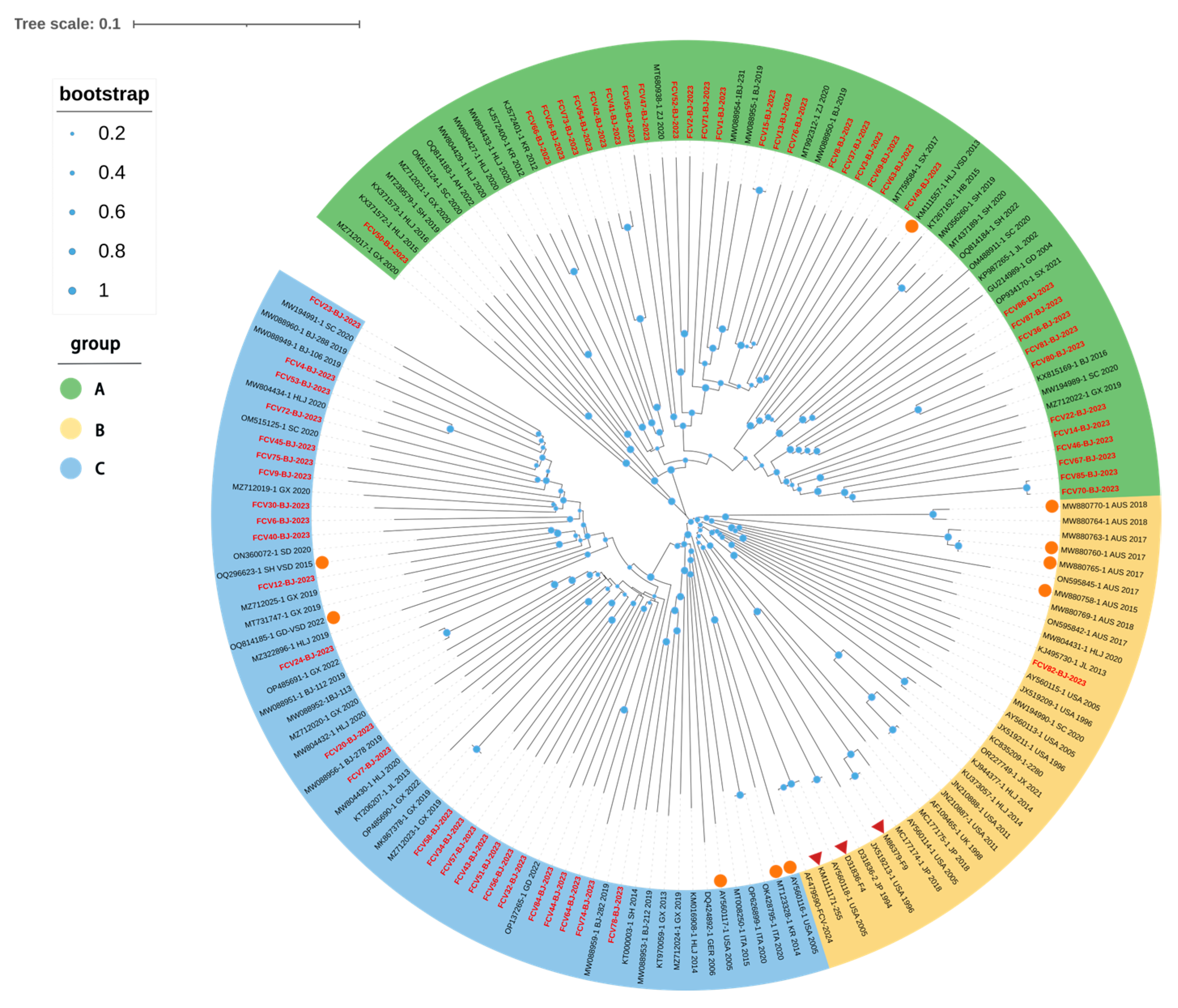

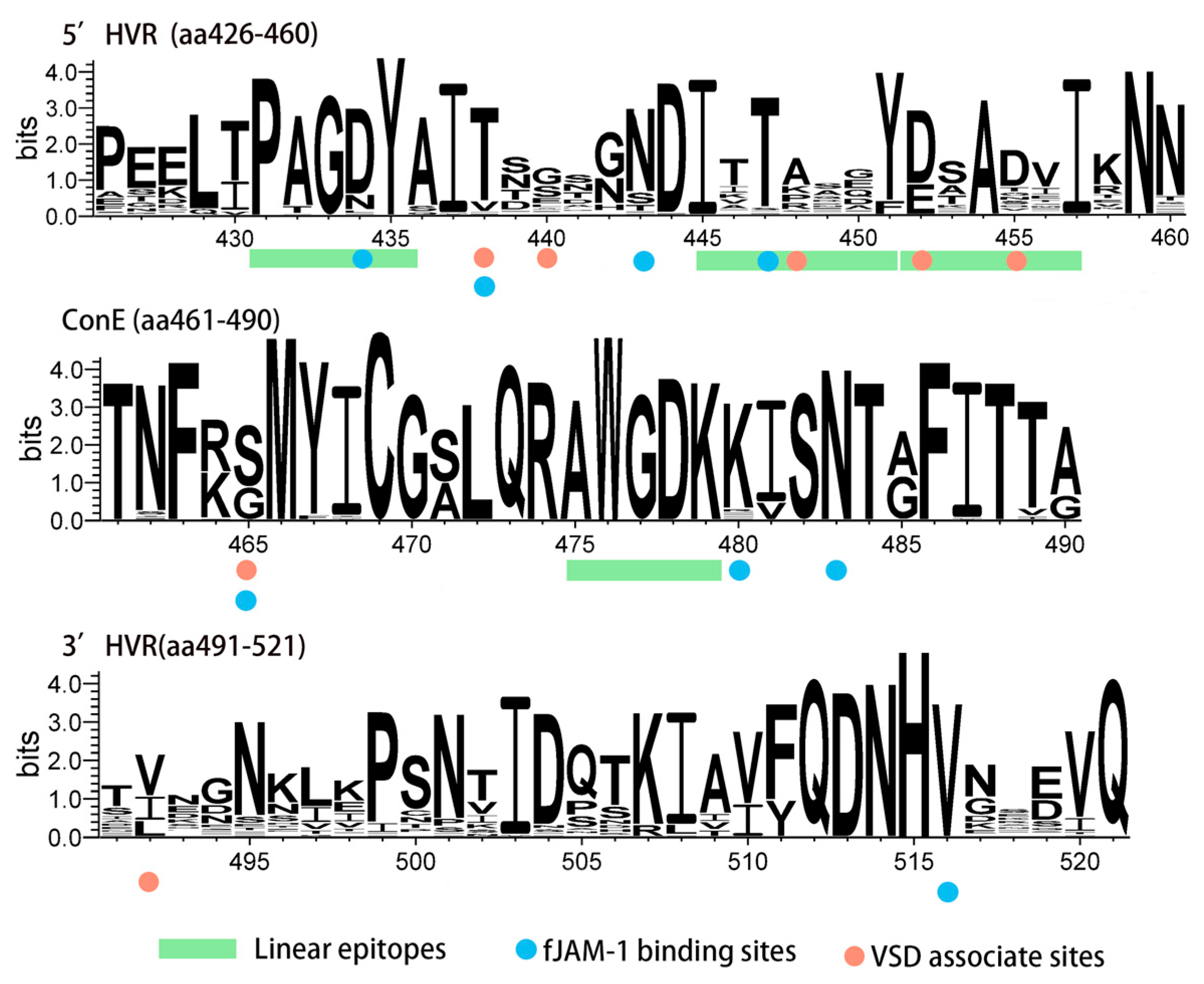

| Primer | Sequence (5′-3′) | Position | Amplicon Size (bp) |
|---|---|---|---|
| P1-F | GTAAAAGAAATTTGAGACAATGTCTCAAAC | 1–30 | 2556 |
| P1-R | TTGTCAGGGGCAGTAAGCACATC | 2534–2556 | |
| P2-F | GTGGTAACCGTTAATTCGGTGTTT | 2441–2464 | 2897 |
| P2-R | CACGTTAGCGCAGGTTGAGCAC | 5316–5337 | |
| P3-F | CAACAGCCAGTTTAATGGTGTG | 5221–5242 | 2463 |
| P3-R | CCCTGGGGTTAGGCGC | 7668–7683 |
| Variable | Category | Proportion | Frequency (%) | χ2 | p Value |
|---|---|---|---|---|---|
| Overall | 126/402 | 31.3 | |||
| Age | 0–3 months | 32/109 | 29.4 | 3.3932 | 0.1833 |
| 4–12 months | 49/131 | 37.4 | |||
| >1 years | 45/162 | 27.8 | |||
| Gender | Male | 83/247 | 33.6 | 1.2602 | 0.2616 |
| Female | 43/155 | 27.7 | |||
| Vaccination | Unvaccinated | 55/148 | 37.3 | 11.581 | 0.003056 |
| Not proper | 47/131 | 35.9 | |||
| Proper | 24/123 | 18.2 | |||
| Residential density | ≥3 cats | 30/58 | 51.7 | 16.395 | 0.0002754 |
| 2 cats | 38/110 | 34.5 | |||
| 1 cat | 58/234 | 24.8 | |||
| Breed | Chinese domestic cats | 53/136 | 39.0 | 12.18 | 0.0324 |
| British shorthair | 23/94 | 24.5 | |||
| Ragdoll | 15/40 | 37.5 | |||
| American shorthair | 4/33 | 12.1 | |||
| Doven | 7/24 | 29.2 | |||
| Others | 24/75 | 32.0 |
| Variable | OR | 95%CI | p Value |
|---|---|---|---|
| Age | |||
| 0–3 months | 0.99 | 0.51–1.94 | 0.985 |
| 4–12 months | 2.20 | 1.25–3.94 | 0.007 |
| >1 years | 1.0 * | ||
| Gender | |||
| Male | 1.41 | 0.88–2.28 | 0.160 |
| Female | 1.0 * | ||
| Vaccination | |||
| Unvaccinated | 2.97 | 1.49–6.03 | 0.002 |
| Not proper | 2.79 | 1.52–5.29 | 0.001 |
| Proper | 1.0 * | ||
| Residential density | |||
| ≥3 cats | 3.47 | 1.80–6.76 | <0.001 |
| 2 cats | 1.85 | 1.09–3.13 | 0.021 |
| 1 cat | 1.0 * | ||
| Breed | |||
| Chinese domestic cats | 1.16 | 0.60–2.28 | 0.664 |
| British shorthair | 0.85 | 0.41–1.75 | 0.662 |
| Ragdoll | 1.52 | 0.64–3.60 | 0.339 |
| American shorthair | 0.33 | 0.08–1.03 | 0.074 |
| Doven Rex | 0.98 | 0.33–2.75 | 0.972 |
| Others | 1.0 * | ||
| Recombinant | Major (Similarity) | Minor (Similarity) | p-Value of 7 Detection Methods | ||||||
|---|---|---|---|---|---|---|---|---|---|
| RDP | GENECONV | BootScan | MaxChi | Chimaera | SiScan | Phylpro | |||
| FCV-26 | MW088950.1 | MW088952.1 | 1.661 × 10−54 | 1.152 × 10−23 | 2.789 × 10−48 | 3.192 × 10−25 | 4.049 × 10−29 | 8.496 × 10−33 | 1.248 × 10−10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Zhu, J.; Yang, H.; Lyu, Y. Epidemiology and Molecular Characterization of Feline Calicivirus in Beijing, China. Animals 2025, 15, 494. https://doi.org/10.3390/ani15040494
Wang D, Zhu J, Yang H, Lyu Y. Epidemiology and Molecular Characterization of Feline Calicivirus in Beijing, China. Animals. 2025; 15(4):494. https://doi.org/10.3390/ani15040494
Chicago/Turabian StyleWang, Daoqi, Jingru Zhu, Hanyu Yang, and Yanli Lyu. 2025. "Epidemiology and Molecular Characterization of Feline Calicivirus in Beijing, China" Animals 15, no. 4: 494. https://doi.org/10.3390/ani15040494
APA StyleWang, D., Zhu, J., Yang, H., & Lyu, Y. (2025). Epidemiology and Molecular Characterization of Feline Calicivirus in Beijing, China. Animals, 15(4), 494. https://doi.org/10.3390/ani15040494




