African Swine Fever Vaccine Candidate ASFV-G-ΔI177L/ΔLVR Protects Against Homologous Virulent Challenge and Exhibits Long-Term Maintenance of Antibodies
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Virus
2.2. Animal Experiments
2.3. Quantitative Real Time PCR (qPCR) for Detection of the ASFV Genome
2.4. Detection of Anti-ASFV Antibodies
3. Results
3.1. Efficacy of ASFV-G-ΔI177L/ΔLVR Against ASFV FieldStrain Hwacheon/2020
3.2. Evaluation of ASF Vaccine DNA (ASFV p72 Gene) in Challenged Pigs After ASFV-G-ΔI177L/ΔLVR Vaccination
3.3. Evaluation of ASF Vaccine Antibodies in Challenged Pigs After ASFV-G-ΔI177L/ΔLVR Vaccination
3.4. Evaluation of ASF Vaccine DNA in Rectal and Oral Swabs from the Post-Vaccine Challenge Group and Vaccination-Only (Long-Term) Group
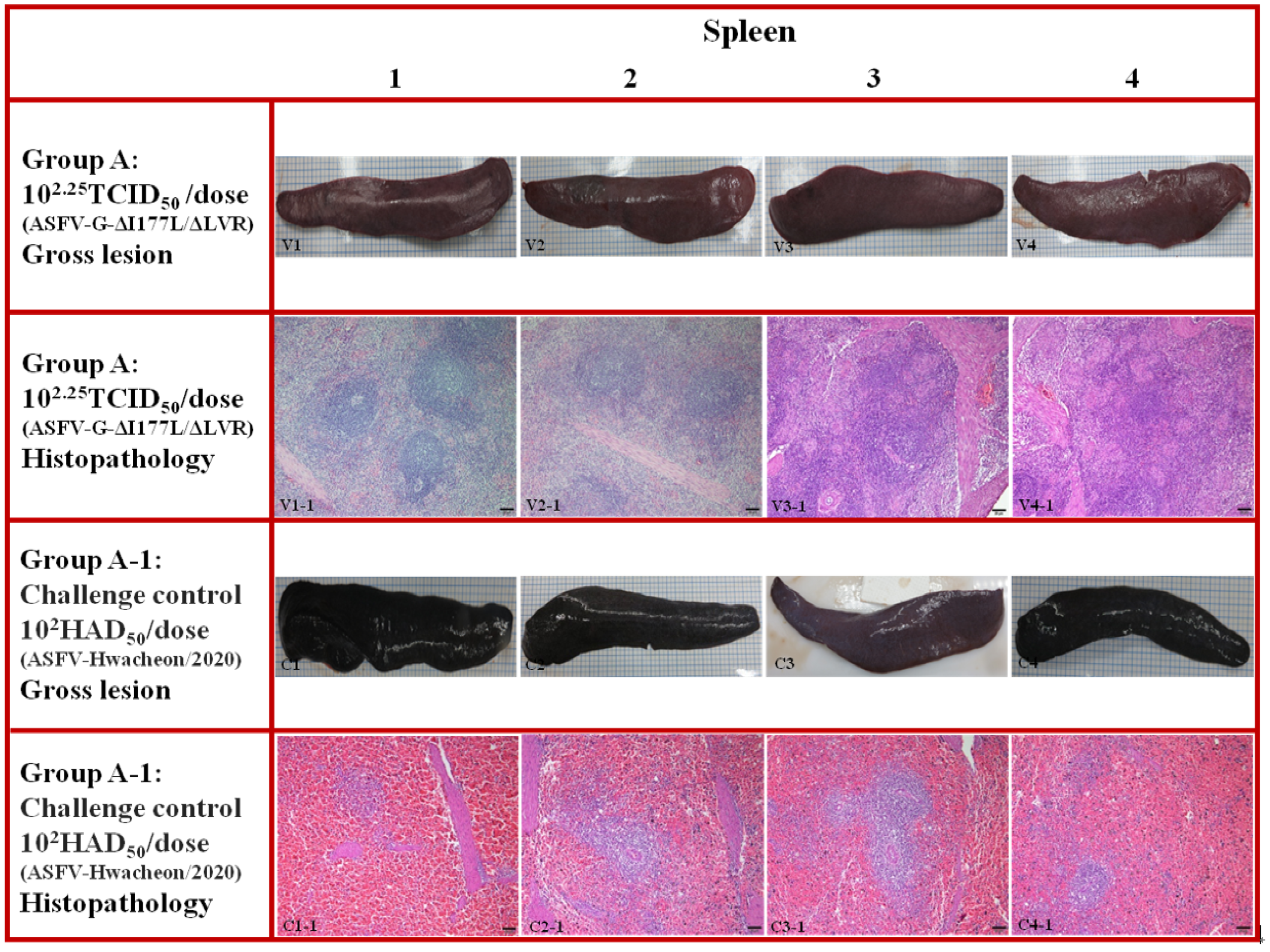
3.5. Pathological Findings in Pigs Challenged After ASFV-G-ΔI177L/ΔLVR Vaccination
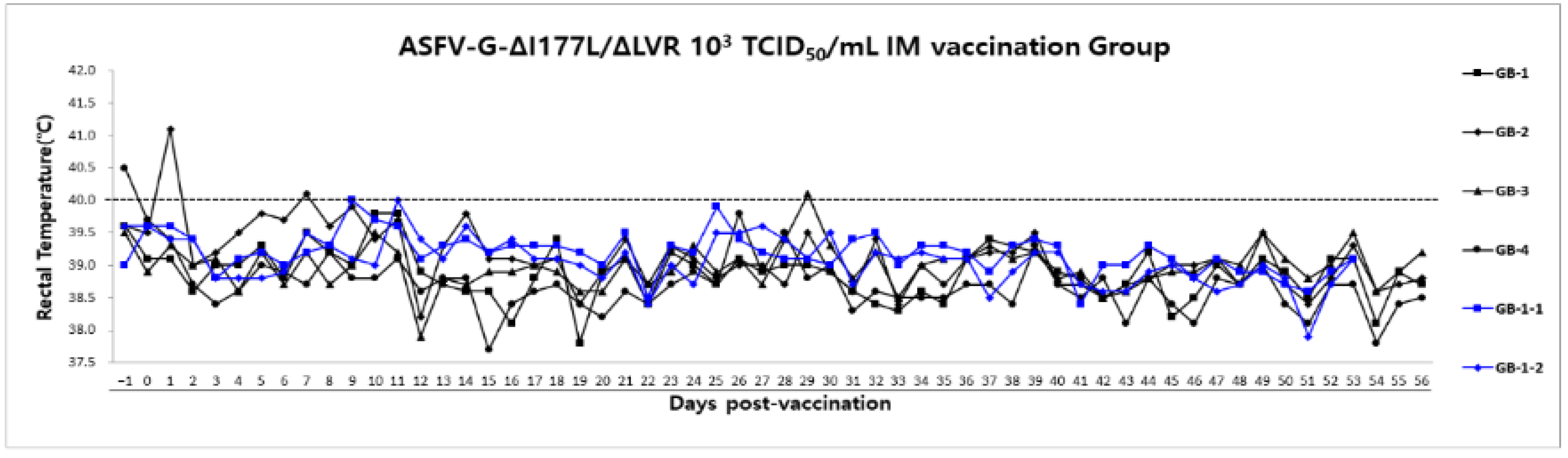
3.6. Long-Term Safety Evaluation After ASFV-G-ΔI177L/ΔLVR Vaccination in Growing Pigs
3.7. Long-Term Evaluation of ASF Vaccine DNA (ASFV p72 Gene After ASFV-G-ΔI177L/ΔLVR Vaccination
3.8. Long-Term Evaluation of ASFV-G-ΔI177L/ΔLVR Vaccine Antibodies After Immunization
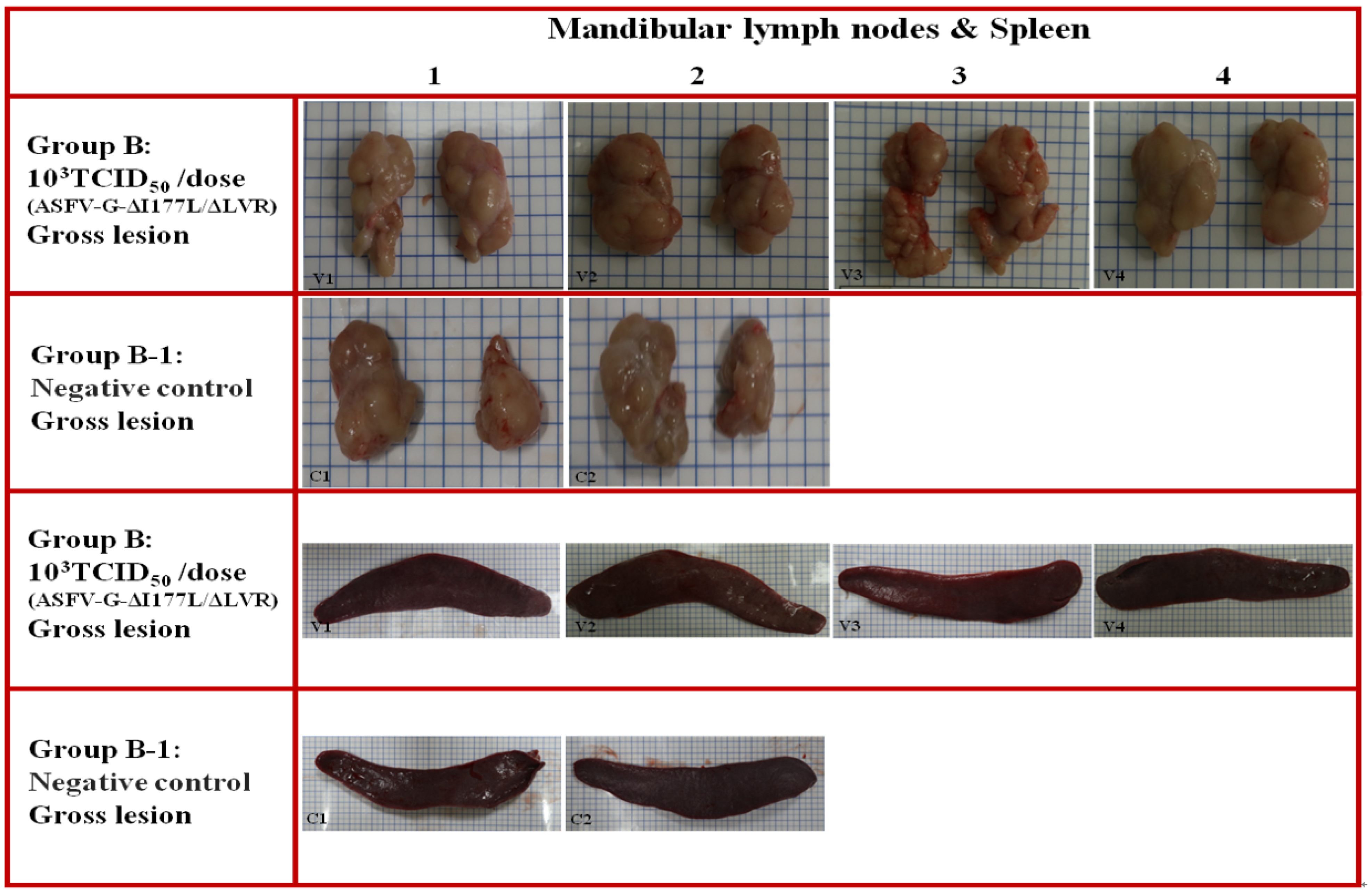
3.9. Long-Term Pathological Evaluation After ASFV-G-ΔI177L/ΔLVR Vaccination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DPV | Days post-vaccination |
| DPC | Days post-challenge |
| ASFV | African swine fever virus |
| LAVs | Live attenuated vaccines |
| PIPEC | Plum Island porcine epithelial cells |
| WOAH | World Organization for Animal Health |
References
- Cwynar, P.; Stojkov, J.; Wlazlak, K. African swine fever status in Europe. Viruses 2019, 11, 310. [Google Scholar] [CrossRef]
- Ma, J.; Chen, H.; Gao, X.; Xiao, J.; Wang, H. African swine fever emerging in China: Distribution characteristics and high-risk areas. Prev. Vet. Med. 2020, 175, 104861. [Google Scholar] [CrossRef] [PubMed]
- Tao, D.; Sun, D.; Liu, Y.; Yang, Z.; An, T.; Shan, F.; Chen, Z.; Liu, J. One year of African swine fever outbreak in China. Acta Tropica. 2020, 211, 105602. [Google Scholar] [CrossRef] [PubMed]
- World Organization for Animal Health. African Swine Fever (ASF)—Situation Report 46. 2024. Available online: https://www.woah.org/app/uploads/2024/02/asf-report46.pdf (accessed on 29 January 2024).
- Li, Z.; Chen, W.; Qiu, Z.; Li, Y.; Fan, J.; Wu, K.; Li, X.; Zhao, M.; Ding, H.; Fan, S.; et al. African Swine Fever Virus: A Review. Life 2022, 12, 1255. [Google Scholar] [CrossRef]
- Dixon, L.K.; Chapman, D.A.; Netherton, C.L.; Upton, C. African swine fever virus replication and genomics. Virus Res. 2013, 173, 3–14. [Google Scholar] [CrossRef]
- Galindo, I.; Alonso, C. African swine fever virus: A review. Viruses 2017, 9, 103. [Google Scholar] [CrossRef]
- Zsak, L.; Borca, M.V.; Risatti, G.R.; Zsak, A.; French, R.A.; Lu, Z.; Kutish, G.F.; Neilan, J.G.; Callahan, J.D.; Nelson, W.M.; et al. Preclinical diagnosis of African swine fever virus in contact-exposed swine by a real-time PCR assay. J. Clin. Microbiol. 2005, 43, 112–119. [Google Scholar] [CrossRef]
- Gallardo, C.; Fernandez-Pinero, J.; Arias, M. African swine fever (ASF) diagnosis, an essential tool in the epidemiological investigation. Virus Res. 2019, 271, 197676. [Google Scholar] [CrossRef]
- Chen, S.; Wang, T.; Luo, R.; Lu, Z.; Lan, J.; Fu, Q.; Qiu, H.-J. Genetic variations of African swine fever virus: Major challenges and prospects. Viruses 2024, 16, 913. [Google Scholar] [CrossRef]
- Sánchez-Cordón, P.J.; Montoya, M.; Reis, A.L.; Dixon, L.K. African swine fever: A re-emerging viral disease threatening the global pig industry. Vet. J. 2018, 233, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Rogoll, L.; Güttner, A.K.; Schulz, K.; Bergmann, H.; Staubach, C.; Conraths, F.J.; Sauter-Louis, C. Seasonal occurrence of African swine fever in wild boar and domestic pigs in EU member states. Viruses 2023, 15, 1955. [Google Scholar] [CrossRef] [PubMed]
- Adesehinwa, A.O.K.; Boladuro, B.A.; Dunmade, A.S.; Idowu, A.B.; Moreki, J.C.; Wachira, A.M. Pig production in Africa: Current status, challenges, prospects and opportunities. Anim. Biosci. 2024, 37, 730–741. [Google Scholar] [CrossRef]
- Brake, D.A. African swine fever modified live vaccine candidates: Transitioning from discovery to product development through harmonized standards and guidelines. Viruses 2022, 14, 2619. [Google Scholar] [CrossRef] [PubMed]
- Gladue, D.P.; Borca, M.V. Recombinant ASF live attenuated virus strains as experimental vaccine candidates. Viruses 2022, 14, 878. [Google Scholar] [CrossRef]
- Muñoz-Pérez, C.; Jurado, C.; Sánchez-Vizcaíno, J.M. African swine fever vaccines: Turning a dream into reality. Transbound Emerg. Dis. 2021, 68, 2657–2668. [Google Scholar] [CrossRef]
- Sang, H.; Miller, G.; Lokhandwala, S.; Sangewar, N.; Waghela, S.D.; Bishop, R.P.; Mwangi, W. Progress toward development of effective and safe African swine fever virus vaccines. Front. Vet. Sci. 2020, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Rock, D.L. Thoughts on African swine fever vaccines. Viruses 2021, 13, 943. [Google Scholar] [CrossRef] [PubMed]
- Borca, M.V.; Ramirez-Medina, E.; Silva, E.; Vuono, E.; Rai, A.; Pruitt, S.; Holinka, L.G.; Velazquez-Salinas, L.; Zhu, J.; Gladue, D.P. Development of a highly effective African swine fever virus vaccine by deletion of the I177L gene results in sterile immunity against the current epidemic Eurasia strain. J. Virol. 2020, 94, e02017-19. [Google Scholar] [CrossRef]
- Borca, M.V.; Rai, A.; Ramirez-Medina, E.; Silva, E.; Velazquez-Salinas, L.; Vuono, E.; Pruitt, S.; Espinoza, N.; Gladue, D.P. A cell culture-adapted vaccine virus against the current African swine fever virus pandemic strain. J. Virol. 2021, 95, e00123-21. [Google Scholar] [CrossRef] [PubMed]
- Teklue, T.; Wang, T.; Luo, Y.; Hu, R.; Qin, H.-J. Generation and evaluation of an African swine fever virus mutant with deletion of the CD2v and UK genes. Vaccines 2020, 8, 763. [Google Scholar] [CrossRef]
- Tran, X.H.; Le, T.T.P.; Nguyen, Q.H.; Do, T.T.; Nguyen, V.D.; Gay, C.G.; Borca, M.V.; Gladue, D.P. African swine fever virus vaccine candidate ASFV-G-∆I177L efficiently protects European and native pig breeds against circulating Vietnamese field strain. Transbound. Emerg. Dis. 2021, 69, e497–e504. [Google Scholar] [CrossRef] [PubMed]
- Tran, X.H.; Phuong, L.T.T.; Huy, N.Q.; Thuy, D.T.; Nguyen, V.D.; Quang, P.H.; Ngôn, Q.V.; Rai, A.; Gay, C.G.; Gladue, D.P.; et al. Evaluation of the safety profile of the ASFV vaccine candidate ASFV-G-∆I177L. Viruses 2022, 14, 896. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Liu, Y.; Di, D.; Liu, J.; Gong, L.; Chen, Z.; Li, Y.; Yu, W.; Lv, L.; Zhong, Q.; et al. Protection evaluation of a five-gene-deleted African swine fever virus vaccine candidate against homologous challenge. Front. Microbiol. 2022, 13, 902932. [Google Scholar] [CrossRef]
- World Organization for Animal Health. Report of the Meeting of the WOAH Biological Standards Commission. Annex 16. Item 5.1.—Chapter 3.9.1. African Swine Fever (Infectious with African Swine Fever Virus). Available online: https:www.woah.org/app/uploads/2023/10/a-bsc-report-sept-2023-6.pdf (accessed on 4 September 2023).
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Montgomery, E.R. On a form of swine fever occurring in British East Africa (Kenya Colony). J. Comp. Pathol. Ther. 1921, 34, 159–191. [Google Scholar] [CrossRef]
- O’Donnell, V.; Holinka, L.G.; Krug, P.W.; Gladue, D.P.; Carlson, J.; Sanford, B.; Alfano, M.; Kramer, E.; Lu, Z.; Arzt, J.; et al. African swine fever virus Georgia 2007 with a deletion of virulence-associated gene 9GL (B119L), when administrated at low doses, leads to virus attenuation in swine and induces an effective protection against homologous challenge. J. Virol. 2015, 89, 8556–8566. [Google Scholar] [CrossRef]
- O’Donnell, V.; Risatti, G.R.; Holinka, L.G.; Krug, P.W.; Carlson, J.; Velazquez-Salinas, L.; Azzinaro, P.A.; Gladue, D.P.; Borca, M.V. Simultaneous deletion of the 9GL and UK genes from the African swine fever virus Georgia 2007 isolate offers increased safety and protection against homologous challenge. J. Virol. 2017, 91, e01760-16. [Google Scholar] [CrossRef]
- Monteagudo, P.L.; Lacasta, A.; López, E.; Bosch, L.; Collado, J.; Pina-Pedrero, S.; Correa-Fiz, F.; Accensi, F.; Navas, M.J.; Vidal, E.; et al. BA71ΔCD2: A new recombinant live attenuated African swine fever virus with cross-protective capabilities. J. Virol. 2017, 91, e01058-17. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, D.; He, X.; Liu, R.; Wang, Z.; Zhang, X.; Li, F.; Shan, D.; Chen, H.; Zhang, J.; et al. A seven-gene-deleted African swine fever virus is safe and effective as a live attenuated vaccine in pigs. Sci. China Life Sci. 2020, 63, 623–634. [Google Scholar] [CrossRef]
- O’Donnell, V.; Holinka, L.G.; Gladue, D.P.; Sanford, B.; Krug, P.W.; Lu, X.; Arzt, J.; Reese, B.; Carrillo, C.; Risatti, G.R.; et al. African swine fever virus Georgia isolate harboring deletions of MGF360 and MGF505 genes is attenuated in swine and confers protection against challenge with virulent parental virus. J. Virol. 2015, 89, 6048–6056. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture. Notice of Withdrawal of Select Agent Regulatory Exclusion for African Swine Fever Virus-G-ΔI177L. Published Document: 2024-10770 (89FR 42834). Federal Register: Notice of Withdrawal of Select Agent Regulatory Exclusion for African Swine Fever Virus-G-ΔI177L. Available online: https://www.govinfo.gov/content/pkg/FR-2024-05-16/pdf/2024-10770.pdf (accessed on 29 January 2024).
- United States Department of Agriculture. Notice of Withdrawal of Select Agent Regulatory Exclusions for Two Strains of African Swine Fever Virus. Published Document: 2022-23446 (87 FR 65023). Federal Register: Notice of Withdrawal of Select Agent Regulatory Exclusions for Two Strains of African Swine Fever Virus. Available online: https://www.govinfo.gov/content/pkg/FR-2022-10-27/pdf/2022-23446.pdf (accessed on 29 January 2024).
- Zhao, D.; Sun, E.; Huang, L.; Zhu, Y.; Zhang, J.; Shen, D.; Zhang, X.; Zhang, Z.; Ren, T.; Wang, W.; et al. Highly lethal genotype I and II recombinant African swine fever viruses detected in pigs. Nat. Commun. 2023, 14, 3096. [Google Scholar] [CrossRef]


| Trial Group | Animal No. (No. of Pigs) | Vaccine/Dose | Challenge/Dose | Route |
|---|---|---|---|---|
| A | 4 (1–4) | ASFV-G-ΔI177L/ΔLVR/102.25 TCID50 | ASFV-Hwacheon/2020(Genotype-II)/102.0 HAD50 | IM |
| A-1 | 4 (1–4) | Not applicable | ASFV-Hwacheon/2020(Genotype-II)/102.0 HAD50 | IM |
| A-2 | 4 (1–4) | Not applicable | Not applicable | - |
| B | 4 (1–4) | ASFV-G-ΔI177L/ΔLVR/103.0 TCID50 | Not applicable | IM |
| B-1 | 2 (1–2) | Not applicable | Not applicable | - |
| Group | Ct * (qPCR, Whole Blood) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DPV (Days Post-Vaccination) | DPC (Days Post-Challenge) | ||||||||||||||
| 0 | 4 | 7 | 10 | 14 | 18 | 21 | 25 | 28 | 4 | 7 | 11 | 14 | |||
| 102.25 TCID50 /dose (IM) | GA1 | 45.00 | 45.00 | 37.6 | 36.6 | 35.7 | 33.6 | 39.0 | 36.3 | 30.4 | 30.5 | 33.1 | 34.9 | 36.7 | |
| GA2 | 45.00 | 45.00 | 45.00 | 45.00 | 33.6 | 25.1 | 25.8 | 26.9 | 30.8 | 36.9 | 18.5 | 19.8 | 21.2 | ||
| GA3 | 45.00 | 36.9 | 20.8 | 19.5 | 21.5 | 21.2 | 21.6 | 21.9 | 24.3 | 23.4 | 23.7 | 21.1 | 22.0 | ||
| GA4 | 45.00 | 37.5 | 26.4 | 19.7 | 22.8 | 23.0 | 22.2 | 23.2 | 27.7 | 25.8 | 26.9 | 25.9 | 22.2 | ||
| Challenge Control | GA-1-1 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 16.6 | 13.5 | D ** | D | |
| GA-1-2 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 16.6 | 15.3 | D | D | ||
| GA-1-3 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 17.1 | 14.1 | D | D | ||
| GA-1-4 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 16.4 | 14.1 | D | D | ||
| Negative Control | GA-2-1 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | |
| GA-2-2 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | ||
| GA-2-3 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | ||
| GA-2-4 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | ||
| Group | S/N% * (cELISA) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DPV (Days Post-Vaccination) | DPC (Days Post-Challenge) | ||||||||||||||
| 0 | 4 | 7 | 10 | 14 | 18 | 21 | 25 | 28 | 4 | 7 | 11 | 14 | |||
| 102.25 TCID50 /dose | GA1 | 74.2 | 79.1 | 69.4 | 48.9 | 38.2 | 36.5 | 33.0 | 24.0 | 20.0 | 14.3 | 12.2 | 11.9 | 8.8 | |
| GA2 | 77.5 | 82.3 | 58.0 | 39.6 | 39.0 | 39.3 | 38.2 | 36.6 | 26.4 | 31.5 | 13.5 | 8.4 | 9.3 | ||
| GA3 | 93.0 | 89.9 | 84.5 | 45.1 | 28.2 | 32.1 | 24.2 | 16.6 | 11.4 | 9.9 | 8.1 | 9.5 | 7.2 | ||
| GA4 | 78.2 | 79.4 | 76.6 | 64.3 | 30.0 | 33.1 | 30.0 | 31.1 | 29.2 | 21.7 | 17.5 | 20.6 | 14.8 | ||
| Challenge Control | GA-1-1 | 77.8 | 86.5 | 86.8 | 80.7 | 82.3 | 76.6 | 78.2 | 68.6 | 76.3 | 81.5 | 49.9 | D ** | D | |
| GA-1-2 | 84.2 | 80.9 | 76.1 | 72.0 | 75.5 | 79.5 | 85.7 | 85.2 | 87.3 | 86.9 | 50.4 | D | D | ||
| GA-1-3 | 89.9 | 94.5 | 86.8 | 87.1 | 90.0 | 86.0 | 91.7 | 88.1 | 76.5 | 68.8 | 49.7 | D | D | ||
| GA-1-4 | 85.0 | 79.2 | 78.5 | 76.7 | 82.0 | 72.5 | 73.2 | 72.0 | 72.4 | 79.6 | 44.7 | D | D | ||
| Negative Control | GA-2-1 | 83.8 | 87.4 | 87.5 | 94.2 | 89.5 | 71.1 | 68.7 | 76.2 | 77.4 | 85.8 | 75.6 | 91.0 | 94.5 | |
| GA-2-2 | 89.2 | 92.5 | 76.3 | 92.4 | 85.6 | 85.2 | 76.3 | 87.4 | 83.0 | 92.8 | 82.6 | 99.9 | 95.4 | ||
| GA-2-3 | 87.4 | 83.4 | 67.2 | 86.1 | 85.4 | 84.4 | 80.8 | 79.3 | 82.2 | 86.2 | 79.4 | 102.4 | 91.3 | ||
| GA-2-4 | 66.0 | 70.9 | 68.1 | 85.9 | 83.3 | 81.1 | 78.5 | 68.4 | 73.6 | 75.4 | 72.0 | 99.2 | 91.8 | ||
| Group | Ct * (qPCR, Whole Blood) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DPV (Days Post-Vaccination) | ||||||||||||||||||
| 0 | 4 | 7 | 11 | 14 | 18 | 21 | 24 | 28 | 32 | 35 | 39 | 42 | 46 | 49 | 53 | 56 | ||
| 103 TCID50 /dose(IM) | GB1 | 45.00 | 29.92 | 19.50 | 21.58 | 21.85 | 22.36 | 23.14 | 26.61 | 26.81 | 27.58 | 29.22 | 31.28 | 31.47 | 30.56 | 30.31 | 32.73 | 31.63 |
| GB2 | 45.00 | 29.32 | 20.15 | 21.29 | 21.92 | 21.93 | 22.21 | 23.82 | 24.60 | 25.27 | 26.23 | 27.62 | 29.09 | 28.65 | 28.94 | 27.50 | 28.97 | |
| GB3 | 45.00 | 45.00 | 38.30 | 27.68 | 28.94 | 32.34 | 34.06 | 36.54 | 26.38 | 23.53 | 25.18 | 28.54 | 30.64 | 30.48 | 30.67 | 32.89 | 34.15 | |
| GB4 | 45.00 | 39.49 | 41.06 | 45.00 | 45.00 | 45.00 | 45.00 | 24.55 | 20.93 | 22.07 | 23.04 | 24.55 | 26.00 | 26.79 | 27.79 | 30.35 | 29.96 | |
| Negative Control | GB-1-1 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 |
| GB-1-2 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | 45.00 | |
| Group | S/N% * (cELISA) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DPV (Days Post-Vaccination) | ||||||||||||||||||
| 0 | 4 | 7 | 11 | 14 | 18 | 21 | 24 | 28 | 32 | 35 | 39 | 42 | 46 | 49 | 53 | 56 | ||
| 103 TCID50 /dose (IM) | GB1 | 86.0 | 82.0 | 71.2 | 21.2 | 24.2 | 23.0 | 21.8 | 19.7 | 18.4 | 16.0 | 13.9 | 12.6 | 9.7 | 10.8 | 9.6 | 10.1 | 9.2 |
| GB2 | 85.2 | 77.8 | 57.8 | 12.1 | 13.4 | 14.5 | 15.1 | 11.1 | 9.3 | 10.2 | 9.9 | 9.1 | 8.8 | 7.2 | 6.3 | 4.7 | 4.4 | |
| GB3 | 76.2 | 78.4 | 56.9 | 40.1 | 45.3 | 44.4 | 33.0 | 22.3 | 16.2 | 10.9 | 8.3 | 7.8 | 6.5 | 6.0 | 4.8 | 4.7 | 4.4 | |
| GB4 | 88.4 | 87.1 | 73.7 | 56.4 | 59.7 | 59.4 | 45.8 | 23.1 | 5.4 | 3.8 | 3.6 | 3.9 | 3.9 | 3.5 | 2.9 | 3.6 | 3.5 | |
| Negative Control | GB-1-1 | 86.6 | 83.0 | 87.1 | 83.4 | 84.9 | 87.7 | 87.1 | 83.5 | 69.2 | 70.0 | 78.2 | 76.7 | 73.7 | 75.4 | 74.9 | - | - |
| GB-1-2 | 79.6 | 80.8 | 83.0 | 87.5 | 89.3 | 88.6 | 90.7 | 86.6 | 78.7 | 83.9 | 82.8 | 80.3 | 77.7 | 81.0 | 85.1 | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.A.; Kim, Y.; Lee, S.J.; Moon, S.C.; Ahn, K.S.; Zheng, X.; Kim, D.S.; Lee, S.Y.; Shin, S.P.; Tark, D.; et al. African Swine Fever Vaccine Candidate ASFV-G-ΔI177L/ΔLVR Protects Against Homologous Virulent Challenge and Exhibits Long-Term Maintenance of Antibodies. Animals 2025, 15, 473. https://doi.org/10.3390/ani15040473
Choi SA, Kim Y, Lee SJ, Moon SC, Ahn KS, Zheng X, Kim DS, Lee SY, Shin SP, Tark D, et al. African Swine Fever Vaccine Candidate ASFV-G-ΔI177L/ΔLVR Protects Against Homologous Virulent Challenge and Exhibits Long-Term Maintenance of Antibodies. Animals. 2025; 15(4):473. https://doi.org/10.3390/ani15040473
Chicago/Turabian StyleChoi, Sun A, Yeonji Kim, Su Jin Lee, Seong Cheol Moon, Keun Seung Ahn, Xinghua Zheng, Do Soon Kim, Se Young Lee, Seung Pyo Shin, Dongseob Tark, and et al. 2025. "African Swine Fever Vaccine Candidate ASFV-G-ΔI177L/ΔLVR Protects Against Homologous Virulent Challenge and Exhibits Long-Term Maintenance of Antibodies" Animals 15, no. 4: 473. https://doi.org/10.3390/ani15040473
APA StyleChoi, S. A., Kim, Y., Lee, S. J., Moon, S. C., Ahn, K. S., Zheng, X., Kim, D. S., Lee, S. Y., Shin, S. P., Tark, D., Kim, W., Shin, Y., Jheong, W., & Sur, J. H. (2025). African Swine Fever Vaccine Candidate ASFV-G-ΔI177L/ΔLVR Protects Against Homologous Virulent Challenge and Exhibits Long-Term Maintenance of Antibodies. Animals, 15(4), 473. https://doi.org/10.3390/ani15040473






