Morphological Variation in the Striped Field Mouse Across Three Countries
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
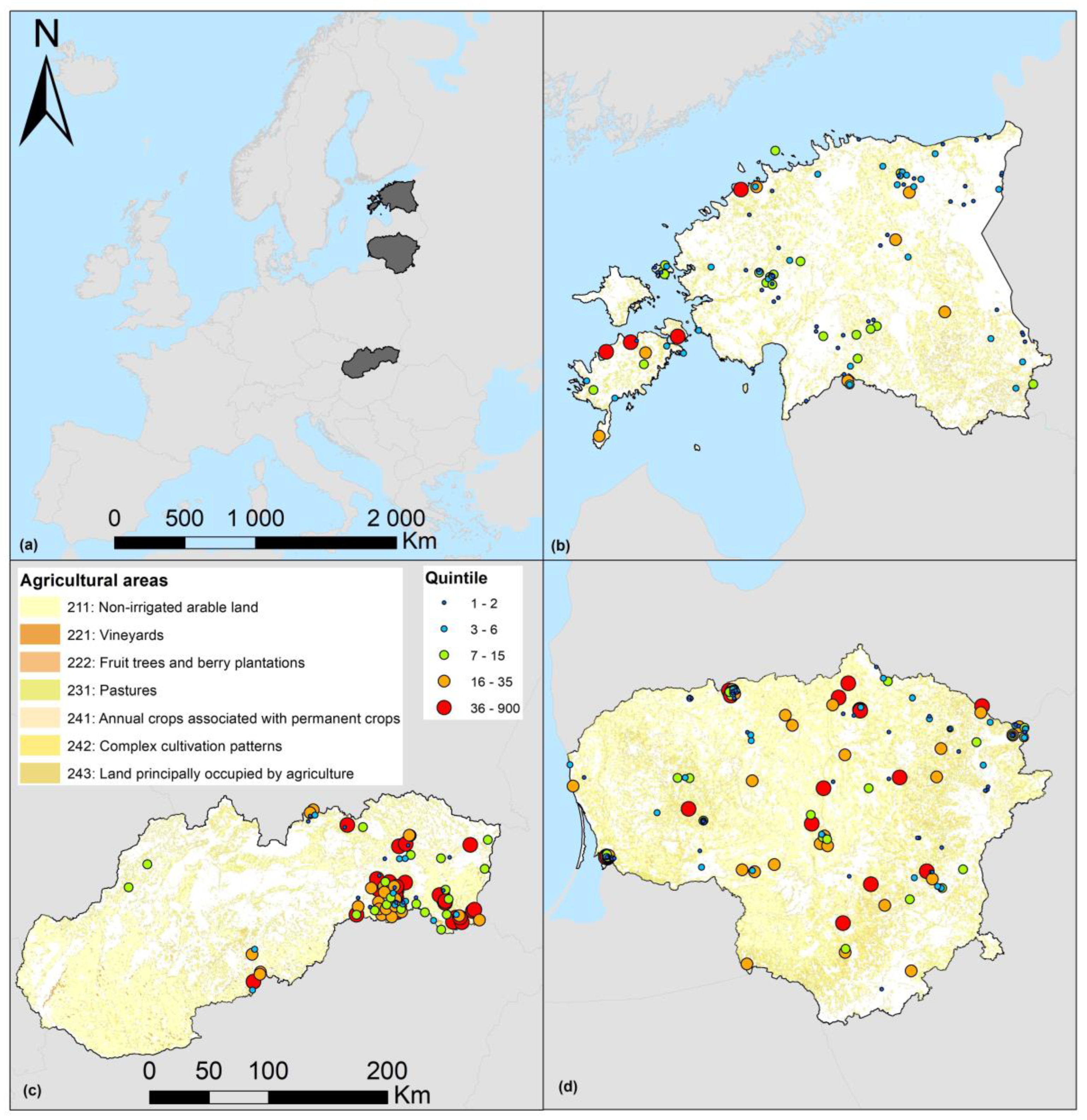
2.1. Countries and Gradients
2.2. Trapping and Sample Size
2.3. Statistical Analysis
3. Results
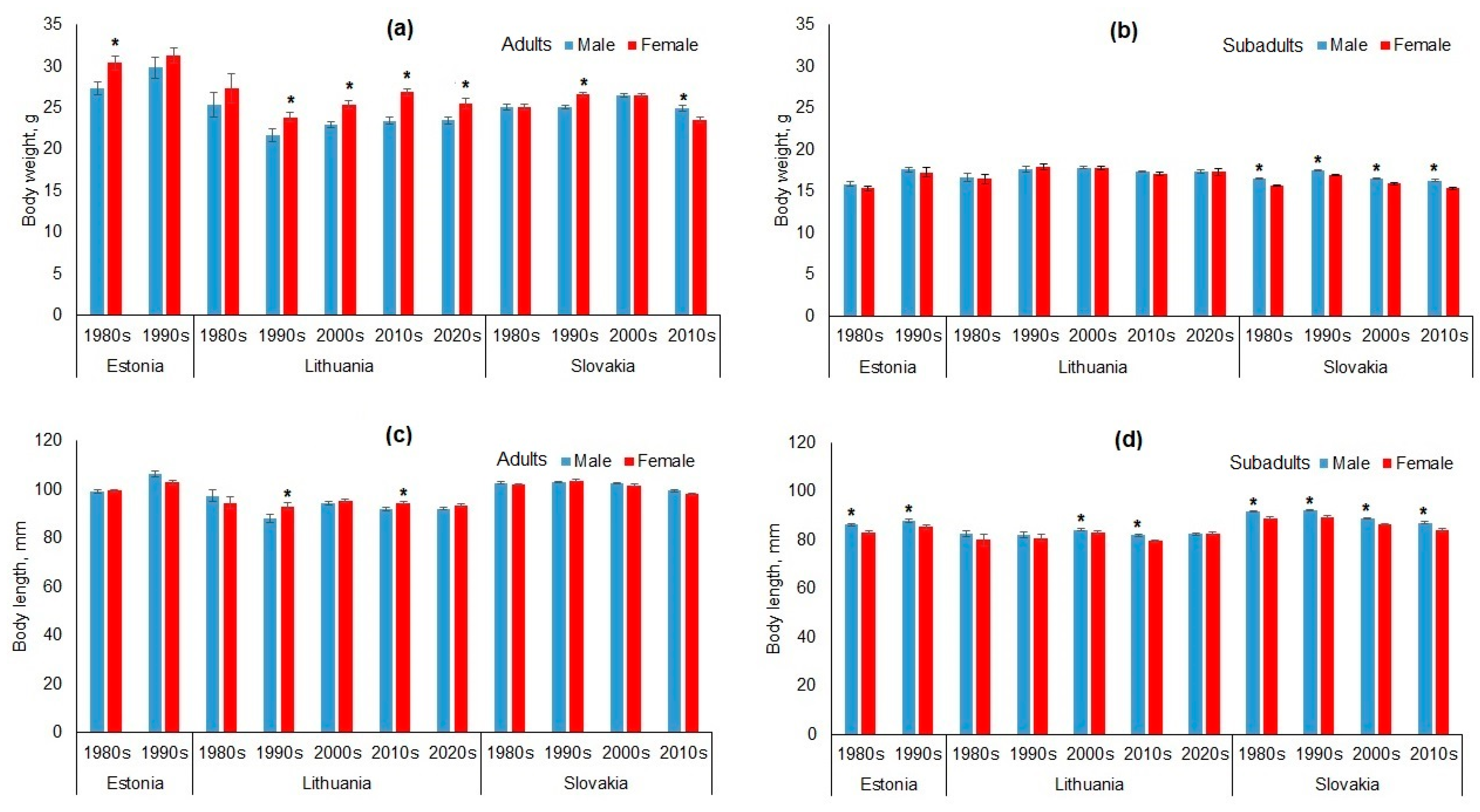
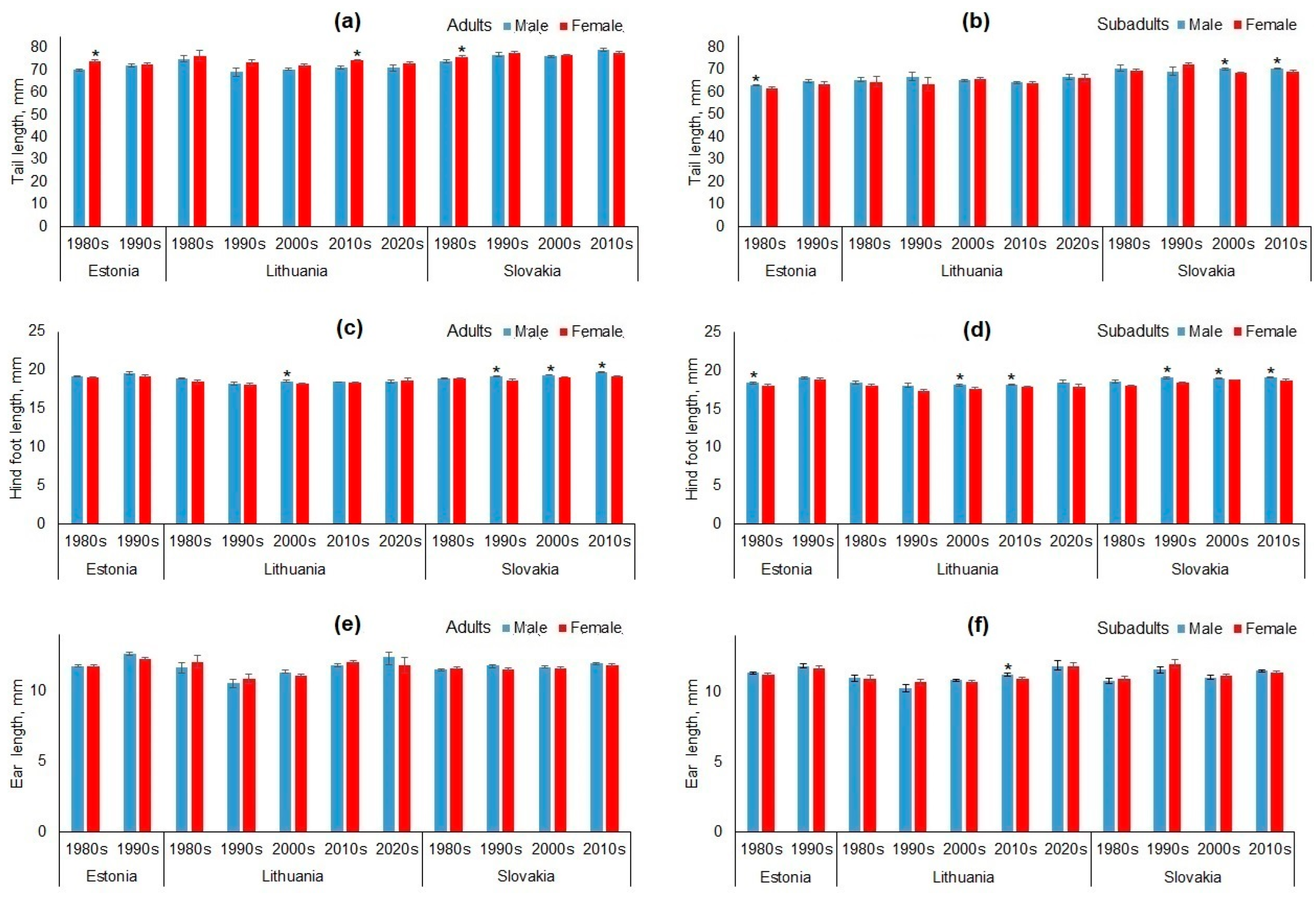
3.1. Sex-Based Dimorphism in Apodemus agrarius
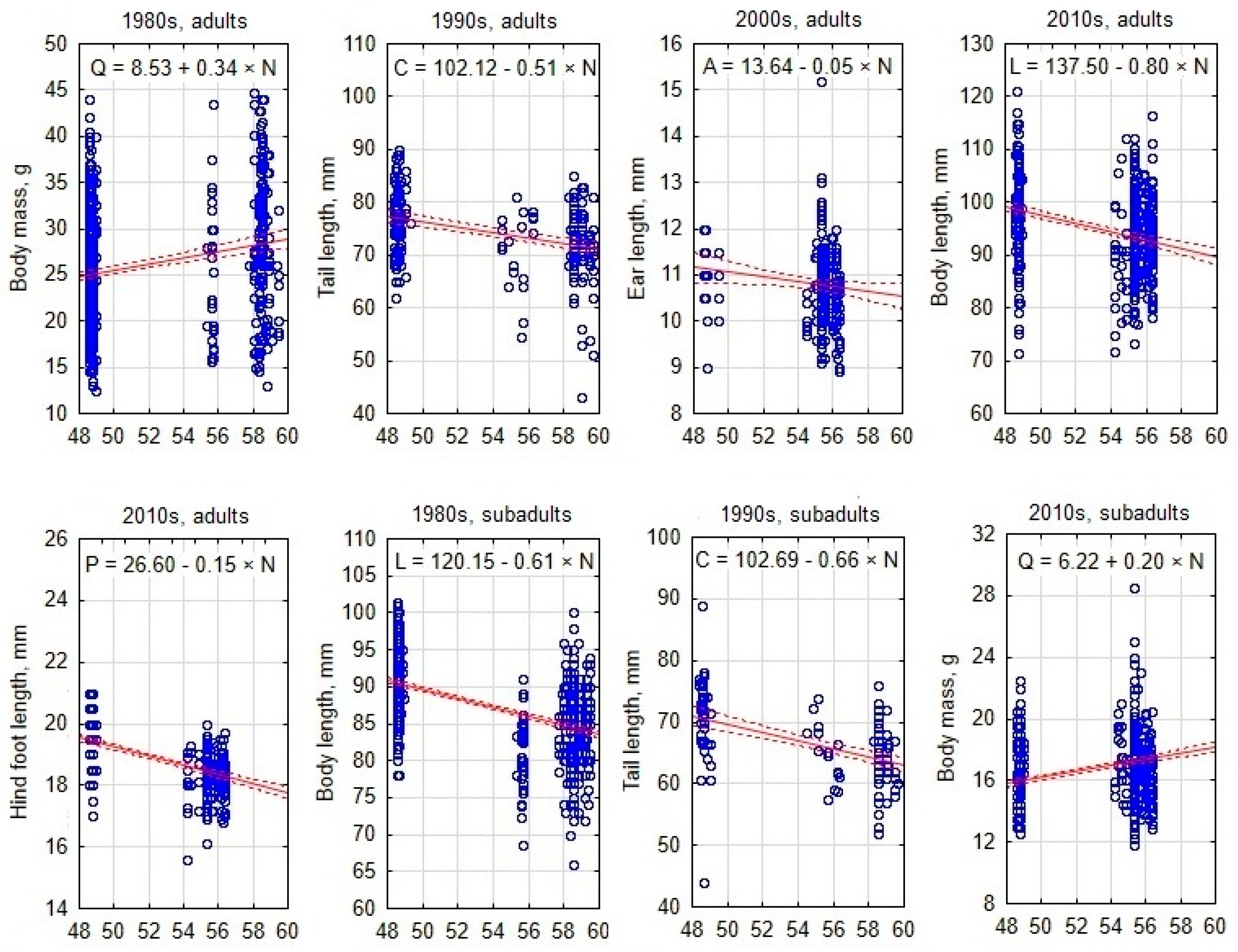
3.2. South–North Gradient of Body Size in Apodemus agrarius
3.3. West–East Gradient of Body Size in Apodemus agrarius
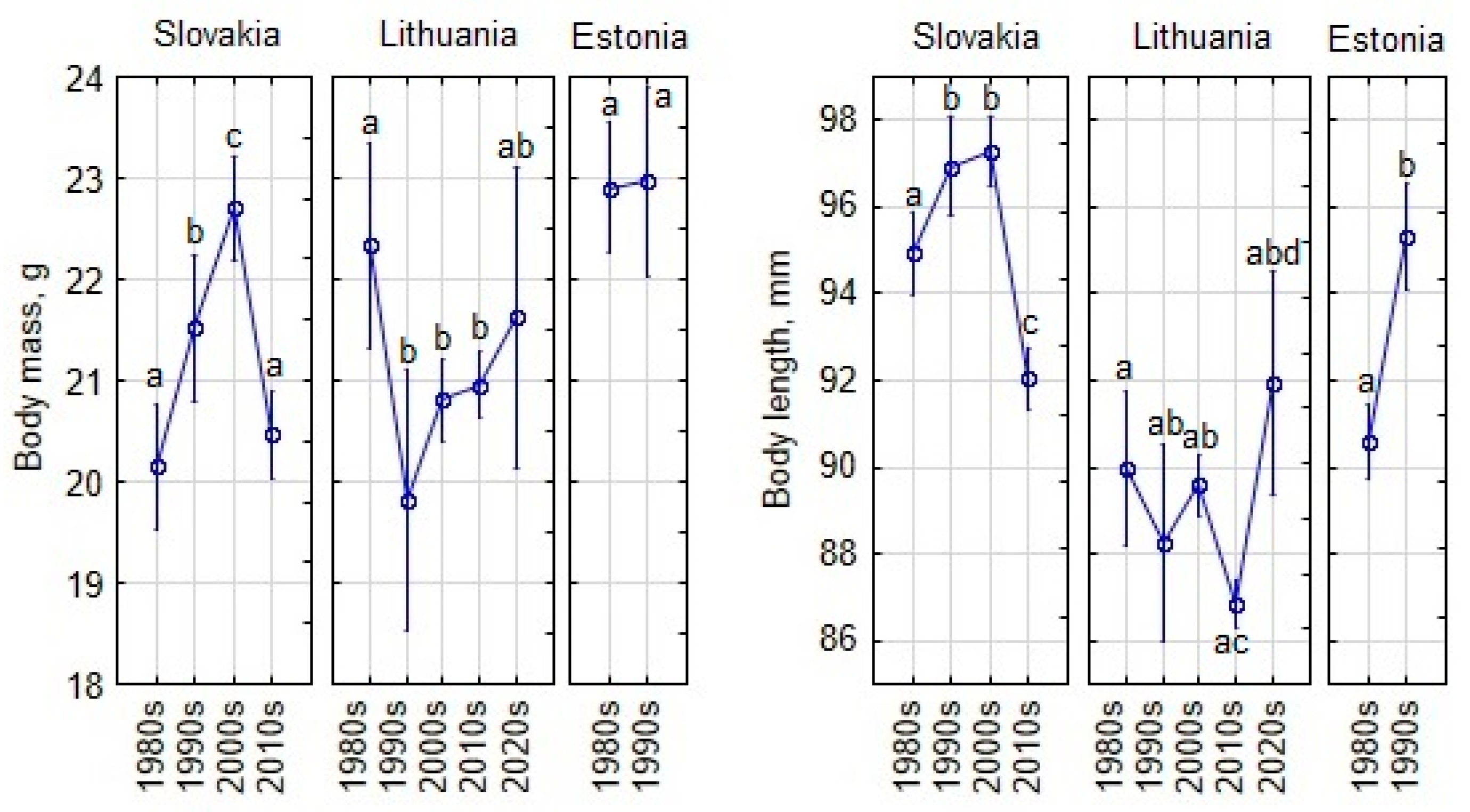
3.4. Temporal Changes in Body Size of Adult and Subadult Apodemus agrarius
4. Discussion
4.1. Overview of Sex-Based Dimorphism and Temporal Trends in Apodemus agrarius
4.2. Overview of Geographic Gradients of Body Size in Apodemus agrarius
4.3. Connecting Climate Trends to Morphological Variability in Apodemus agrarius
5. Conclusions
- Our study highlights the interplay between climatic and geographic factors in shaping the size of A. agrarius. Results confirm significant morphological variation across geographic gradients: body size increases northward (Bergmann’s rule), while appendage size (tail, ears, and hind feet) decreases (Allen’s rule).
- Temporal changes in body size and appendages vary by region, with increases in Slovakia and mixed trends in Lithuania and Estonia. In Slovakia, however, local differences are also confirmed by previous studies.
- Sex-based dimorphism in A. agrarius varies by region and time period, with adult females generally heavier than males, while subadult males are often heavier. Dimorphism in appendage length is less pronounced.
- The species descriptions should be updated due to the changes in body and appendage size of A. agrarius, which have been particularly pronounced in the last few decades.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Q | Body mass |
| L | Body length |
| C | Tail length |
| P | Hind foot length |
| A | Ear length |
References
- Kozłowski, J.; Konarzewski, M.; Czarnoleski, M. Coevolution of body size and metabolic rate in vertebrates: A life-history perspective. Biol. Rev. 2020, 95, 1393–1417. [Google Scholar] [CrossRef] [PubMed]
- Barbault, R. Body Size, Ecological Constraints, and the Evolution of Life-History Strategies. In Evolutionary Biology; Hecht, M.K., Wallace, B., Prance, G.T., Eds.; Springer: Boston, MA, USA, 1988; Volume 22. [Google Scholar] [CrossRef]
- Cui, J.; Lei, B.; Newman, C.; Zhou, Y.; Wang, Z. Food resources and competition rather than eco-geographic rules explain trait variations in two contrasting rat species: Implications for future climate change. Glob. Ecol. Conserv. 2022, 40, e02339. [Google Scholar] [CrossRef]
- Brown, J.H.; Lee, A.K. Bergmann’s rule and climatic adaptation in woodrats (Neotoma). Evolution 1969, 23, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Alhajeri, B.H.; Steppan, S.J. Association between climate and body size in rodents: A phylogenetic test of Bergmann’s rule. Mamm. Biol. 2016, 81, 219–225. [Google Scholar] [CrossRef]
- Ochocińska, D.; Taylor, J.R. Bergmann’s rule in shrews: Geographical variation of body size in Palearctic Sorex species. Biol. J. Linn. Soc. 2003, 78, 365–381. [Google Scholar] [CrossRef]
- Alhajeri, B.H.; Fourcade, Y.; Upham, N.S.; Alhaddad, H. A Global Test of Allen’s rule in rodents. Glob. Ecol. Biogeogr. 2020, 29, 2248–2260. [Google Scholar] [CrossRef]
- Hantak, M.M.; McLean, B.S.; Li, D.; Guralnick, R.P. Mammalian body size is determined by interactions between climate, urbanization, and ecological traits. Commun. Biol. 2021, 4, 972. [Google Scholar] [CrossRef]
- Haight, J.D.; Hall, S.J.; Fidino, M.; Adalsteinsson, S.A.; Ahlers, A.A.; Mowry, C.; Murray, M.; Nininger, K.; Pendergast, M.E.; Remine, K.R.; et al. Urbanization, climate, and species traits shape mammal communities from local to continental scales. Nat. Ecol. Evol. 2023, 7, 1654–1666. [Google Scholar] [CrossRef]
- Boratyński, Z. Energetic constraints on mammalian distribution areas. J. Anim. Ecol. 2021, 90, 1854–1863. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.E.; Bielby, J.; Cardillo, M.; Fritz, S.A.; O’Dell, J.; Orme, C.D.L.; Safi, K.; Sechrest, W.; Boakes, E.H.; Carbone, C.; et al. PanTHERIA: A species-level database of life-history, ecology, and geography of extant and recently extinct mammals. Ecology 2009, 90, 2648. [Google Scholar] [CrossRef]
- Smith, F.A.; Lyons, S.K. How Big Should a Mammal Be? A Macroecological look at mammalian body size over space and time. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2364–2378. [Google Scholar] [CrossRef] [PubMed]
- Stanchak, K.E.; Santana, S.E. Do Ecogeographical rules explain morphological variation in a diverse, Holarctic genus of small mammals? J. Biogeogr. 2019, 46, 110–122. [Google Scholar] [CrossRef]
- Cooper, N.; Purvis, A. Body size evolution in mammals: Complexity in tempo and mode. Am. Nat. 2010, 175, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Yom-Tov, Y.; Geffen, E. Recent spatial and temporal changes in body size of terrestrial vertebrates: Probable causes and pitfalls. Biol. Rev. 2011, 86, 531–541. [Google Scholar] [CrossRef]
- Huang, S.; Saarinen, J.J.; Eyres, A.; Eronen, J.T.; Fritz, S.A. Mammalian body size evolution was shaped by habitat transitions as an indirect effect of climate change. Glob. Ecol. Biogeogr. 2022, 31, 2463–2474. [Google Scholar] [CrossRef]
- Juman, M.M.; Millien, V.; Olson, L.E.; Sargis, E.J. Recent and rapid ecogeographical rule reversals in northern treeshrews. Sci. Rep. 2022, 12, 19689. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Kryštufek, B.; Zagarondnyuk, I.; Vohralík, V.; Batsaikhan, N.; Avirmed, D.; Sukhchuluun, G. Apodemus agrarius (errata version published in 2017). In The IUCN Red List of Threatened Species 2016; IUCN: Gland, Switzerland, 2016; p. e.T1888A115057408. [Google Scholar] [CrossRef]
- Latinne, A.; Navascués, M.; Pavlenko, M.; Kartavtseva, I.; Ulrich, R.G.; Tiouchichine, M.-L.; Catteau, G.; Sakka, H.; Quéré, J.-P.; Chelomina, G.; et al. Phylogeography of the striped field mouse, Apodemus agrarius (Rodentia: Muridae), throughout its distribution range in the Palaearctic region. Mamm. Biol. 2020, 100, 19–31. [Google Scholar] [CrossRef]
- Tulis, F.; Ambros, M.; Baláž, I.; Žiak, D.; Sládkovičová, V.H.; Miklós, P.; Dudich, A.; Stollmann, A.; Klimant, P.; Somogyi, B.; et al. Expansion of the striped field mouse (Apodemus agrarius) in the South-Western Slovakia during 2010–2015. Folia Oecologica 2016, 43, 64–73. [Google Scholar]
- Balčiauskas, L.; Balčiauskienė, L. Striped Field Mouse Invading Human-Modified Environments of Lithuania during Last Five Decades. Land 2024, 13, 1555. [Google Scholar] [CrossRef]
- Programmi Üldandmed. Available online: https://kese.envir.ee/kese/viewProgramNew.action?uid=789267 (accessed on 17 January 2025).
- Balčiauskas, L.; Balčiauskienė, L. Small Mammal Diversity Changes in a Baltic Country, 1975–2021: A Review. Life 2022, 12, 1887. [Google Scholar] [CrossRef] [PubMed]
- Tulis, F.; Ševčík, M.; Jánošíková, R.; Baláž, I.; Ambros, M.; Zvaríková, L.; Horváth, G. The impact of the striped field mouse’s range expansion on communities of native small mammals. Sci. Rep. 2023, 13, 753. [Google Scholar] [CrossRef] [PubMed]
- Ondríková, J.; Miklisová, D.; Ribas, A.; Stanko, M. The helminth parasites of two sympatric species of the genus Apodemus (Rodentia, Muridae) from south-eastern Slovakia. Acta Parasitologica 2010, 55, 369–378. [Google Scholar] [CrossRef]
- Andrzejewski, R.; Babińska-Werka, J.; Gliwicz, J.; Goszczyński, J. Synurbization processes in population of Apodemus agrarius. I. Characteristics of populations in an urbanization gradient. Acta Theriol. 1978, 23, 341–358. [Google Scholar] [CrossRef]
- Dimitrov, H.; Chassovnikarova, T.; Markov, G. Craniometrical description of striped mouse (Apodemus agrarius Pallas, 1771) in Bulgaria as concerns of discovering of its European population patterns of similarity. J. BioSci. Biotechnol. 2015, 4, 131–138. [Google Scholar]
- Liro, A. Variation in weights of body and internal organs of the field mouse in a gradient of urban habitats. Acta Theriol. 1985, 30, 359–377. [Google Scholar] [CrossRef]
- Velickovic, M. Measures of the developmental stability, body size, and body condition in the black-striped mouse (Apodemus agrarius) as indicators of a disturbed environment in Northern Serbia. Belg. J. Zool. 2007, 137, 147. [Google Scholar]
- Balčiauskas, L.; Balčiauskienė, L. Insight into Body Condition Variability in Small Mammals. Animals 2024, 14, 1686. [Google Scholar] [CrossRef] [PubMed]
- Baláž, I.; Ambros, M.; Tulis, F. Biology and Distribution of the Species of the Family Muridae (Rodentia) in Slovakia. 2nd Part: Apodemus flavicollis, Apodemus sylvaticus, Apodemus uralensis, Apodemus agrarius; Constantine the Philosopher University in Nitra, Faculty of Natural Sciences: Nitra, Slovakia, 2012; pp. 87–88. [Google Scholar]
- Li, K.; Sommer, S.; Guo, Y.; Ozgul, A.; Wang, D. Appendage sizes in three rodent species are affected indirectly by climate factors via their effects on body size. Integr. Zool. 2024, 0, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.S.; Tikhonova, G. Morphometric analyses with 15 subspecies of striped field mouse, Apodemus agrarius Pallas (Mammalia, Rodentia) from Eurasia. Anim. Syst. Evol. Divers. 1998, 14, 341–355. [Google Scholar]
- Slovak Hydrometeorological Institute. Available online: http://www.shmu.sk (accessed on 29 December 2024).
- Ulrych, L.; Guttová, A.; Urban, P.; Hindáková, A.; Kučera, J.; Kučera, V.; Šibík, J.; Mútňanová, M. Biodiversity in Slovakia. In Global Biodiversity: Volume 2: Selected Countries in Europe; Pullaiah, T., Ed.; Apple Academic Press: Oakville, ON, Canada, 2018; pp. 325–374. ISBN 978-1-77188-717-5. [Google Scholar]
- Černecký, J.; Gajdoš, P.; Špulerová, J.; Halada, Ľ.; Mederly, P.; Ulrych, L.; Rybanič, R. Ecosystems in Slovakia. J. Maps 2020, 16, 28–35. [Google Scholar] [CrossRef]
- Lietuvos Hidrometeorologijos Tarnyba. Available online: https://www.meteo.lt/klimatas/lietuvos-klimatas/klimato-duomenys/ (accessed on 20 December 2024).
- Land Resource Monitoring Information System. Available online: https://zisis.lt/en/ (accessed on 20 December 2024).
- Official Statistics Portal. Available online: https://osp.stat.gov.lt/geografiniai-duomenys1 (accessed on 20 December 2024).
- Raukas, A. Briefly about Estonia. Dyn. Environnementales J. Int. Géosciences L’environnement 2018, 42, 284–291. [Google Scholar] [CrossRef]
- Estonian Environment Agency. Available online: https://www.ilmateenistus.ee/kliima/kliimanormid/ohutemperatuur/?lang=en (accessed on 17 January 2025).
- Surface Area Distribution by Land Use in Climate Report. Available online: https://andmed.stat.ee/en/stat/keskkond__loodusvarad-ja-nende-kasutamine__maakasutuse-muutumine/KK07 (accessed on 17 January 2025).
- Population Figure. Available online: https://www.stat.ee/en/find-statistics/statistics-theme/population/population-figure (accessed on 17 January 2025).
- Stanko, M. Striped Field Mouse (Apodemus agrarius, Rodentia) in Slovakia; Institute of Parasitology, SAS: Košice, Slovakia, 2014; p. 182. [Google Scholar]
- Blackwell, G.L.; Bassett, S.M.; Dickman, C.R. Measurement error associated with external measurements commonly used in small-mammal studies. J. Mammal. 2006, 87, 216–223. [Google Scholar] [CrossRef]
- Pelikán, J. Reproduction, population structure and elimination of males in Apodemus agrarius (Pall.). Zool. Listy 1965, 14, 317–332. [Google Scholar]
- Adamczewska-Andrzejewska, K.A. Growth, variations, and age criteria in Apodemus agrarius (Pallas, 1771). Acta Theriol. 1973, 18, 353–394. [Google Scholar] [CrossRef]
- Theriot, M.K.; Olson, L.E.; Lanier, H.C. Accounting for age: Uncovering the nuanced drivers of mammal body-size responses to climate change. J. Mammal. 2024, 105, 512–523. [Google Scholar] [CrossRef]
- Razali, N.M.; Wah, Y.B. Power comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, Lilliefors, and Anderson-Darling tests. J. Stat. Model. Anal. 2011, 2, 21–33. [Google Scholar]
- Smith, R.J. Statistics of sexual size dimorphism. J. Hum. Evol. 1999, 36, 423–458. [Google Scholar] [CrossRef] [PubMed]
- Past 4—The Past of the Future. Available online: https://www.nhm.uio.no/english/research/resources/past/ (accessed on 1 January 2024).
- TIBCO Software Inc. Data Science Textbook. Available online: https://docs.tibco.com/data-science/textbook (accessed on 15 January 2024).
- Millien, V.; Lyons, S.K.; Olson, L.; Smith, F.A.; Wilson, A.B.; Yom-Tov, Y. Ecotypic variation in the context of global climate change: Revisiting the rules. Ecol. Lett. 2006, 9, 853–869. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, J.; Bisschop, K.; D’Alba, L.; Shawkey, M.D. The link between body size, colouration and thermoregulation and their integration into ecogeographical rules: A critical appraisal in light of climate change. Oikos 2022, 2022, e09152. [Google Scholar] [CrossRef]
- Searing, K.B.; Lomolino, M.V.; Rozzi, R. Melting climates shrink North American small mammals. Proc. Natl. Acad. Sci. USA 2023, 120, e2310855120. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Amshokova, A.; Balčiauskienė, L.; Benedek, A.M.; Cichocki, J.; Csanady, A.; De Mendonça, P.G.; Nistreanu, V. Geographical clines in the size of the herb field mouse (Apodemus uralensis). Integr. Zool. 2020, 15, 55–68. [Google Scholar] [CrossRef]
- Balčiauskienė, L.; Balčiauskas, L.; Timm, U. Changes in size of Baltic field voles over the last 50 years: Are they really shrinking? Biologia 2018, 73, 247–257. [Google Scholar] [CrossRef]
- Kratochvíl, J.; Rosický, B. K bionomii a taxonomii myší rodu Apodemus žijících v ČSR I. Zool. A Entomol. Listy 1952, 1, 57–70. [Google Scholar]
- Mošanský, A. Príspevok k poznaniu rozšírenia a taxonometrie niektorých druhov drobných cicavcov na východnom Slovensku. Zborník Slovenského národného múzea, Prírodné vedy 1957, 3, 1–42. [Google Scholar]
- Mošanský, L. Geografická variabilnosť populácií mikromammálií suburbaných ekosystémov Košickej aglomerácie. Lynx (Praha) 1988, 24, 47–62. [Google Scholar]
- Dudich, A.; Štollmann, A. Drobné zemné cicavce a ich ektoparazity (Siphonaptera) reliktného luhu v Liptove. Liptov 1979, 5, 91–107. [Google Scholar]
- Štollmann, A.; Dudich, A.; Kováčik, J. Drobné zemné cicavce Chránenej krajinnej oblasti Vihorlat. Ochr. Prírody 1982, 3, 267–282. [Google Scholar]
- Mačionis, A. Micromammalia Wylenszczyzny: Study (Manuscript); Vilnius University: Vilnius, Lithuania, 1936; no. F282-189. [Google Scholar]
- Prūsaitė, J. (Comp.). Fauna of Lithuania. Mammals; Mokslas: Vilnius, Lithuania, 1988; p. 131. [Google Scholar]
- Li, K.; Sommer, S.; Yang, Z.; Guo, Y.; Yue, Y.; Ozgul, A.; Wang, D. Distinct body size responses to warming climate in three rodent species. Proc. R. Soc. B 2022, 289, 20220015. [Google Scholar] [CrossRef]
- Cunningham, S.J.; Gardner, J.L.; Martin, R.O. Opportunity costs and the response of birds and mammals to climate warming. Front. Ecol. Environ. 2021, 19, 300–307. [Google Scholar] [CrossRef]
- Kaneko, Y. Latitudinal geographical variation of external and cranial measurements in Apodemus peninsulae, A. draco, and A. latronum. Mammal Study 2015, 40, 143–165. [Google Scholar] [CrossRef]
- Cui, J.; Wang, B.; Ji, S.; Su, H.; Zhou, Y. Revisiting classic ecogeographical rules, using a widely distributed mouse species (Apodemus draco). Anim. Biol. 2020, 70, 359–372. [Google Scholar] [CrossRef]
- Petrosyan, V.; Dinets, V.; Osipov, F.; Dergunova, N.; Khlyap, L. Range dynamics of striped field mouse (Apodemus agrarius) in Northern Eurasia under global climate change based on ensemble species distribution models. Biology 2023, 12, 1034. [Google Scholar] [CrossRef]
- World Bank. Climate Change Knowledge Portal. Available online: https://climateknowledgeportal.worldbank.org/ (accessed on 12 January 2025).
- Gera, M.; Damborská, I.; Lapin, M.; Melo, M. Climate changes in Slovakia: Analysis of past and present observations and scenarios of future developments. In Water Resources in Slovakia: Part II.; Negm, A., Zeleňáková, M., Eds.; The Handbook of Environmental Chemistry; Springer: Cham, Switzerland, 2017; Volume 70. [Google Scholar] [CrossRef]
- Bernáth, S.; Paulen, O.; Šiška, B.; Kusá, Z.; Tóth, F. Influence of Climate Warming on Grapevine (Vitis vinifera L.) Phenology in Conditions of Central Europe (Slovakia). Plants 2021, 10, 1020. [Google Scholar] [CrossRef] [PubMed]
- Montibeller, B.; Jaagus, J.; Mander, Ü.; Uuemaa, E. Evapotranspiration intensification over unchanged temperate vegetation in the Baltic Countries is being driven by climate shifts. Front. For. Glob. Change 2021, 4, 663327. [Google Scholar] [CrossRef]
- Meier, H.M.; Kniebusch, M.; Dieterich, C.; Gröger, M.; Zorita, E.; Elmgren, R.; Zhang, W. Climate change in the Baltic Sea region: A summary. Earth Syst. Dyn. 2022, 13, 457–593. [Google Scholar] [CrossRef]
- Jaagus, J. Climatic changes in Estonia during the second half of the 20th Century in relationship with changes in large-scale atmospheric circulation. Theor. Appl. Climatol. 2006, 83, 77–88. [Google Scholar] [CrossRef]
- Kotta, J.; Herkül, K.; Jaagus, J.; Kaasik, A.; Raudsepp, U.; Alari, V.; Arula, T.; Haberman, J.; Järvet, A.; Kangur, K.; et al. Linking atmospheric, terrestrial and aquatic environments: Regime shifts in the Estonian climate over the past 50 years. PLoS ONE 2018, 13, e0209568. [Google Scholar] [CrossRef]
- Henry, E.; Santini, L.; Huijbregts, M.A.; Benítez-López, A. Unveiling the Environmental Drivers of Intraspecific Body Size Variation in Terrestrial Vertebrates. Glob. Ecol. Biogeogr. 2023, 32, 267–280. [Google Scholar] [CrossRef]


| Country | N | M | F | Ad | Sub | Juv | 1980s | 1990s | 2000s | 2010s | 2020s |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Slovakia | 7004 | 3581 | 3291 | 2900 | 3392 | 319 | 1824 | 1677 | 2857 | 646 | 780 |
| Lithuania | 3823 | 2179 | 1595 | 788 | 1087 | 1906 | 95 | 274 | 879 | 1795 | |
| Estonia | 1101 | 613 | 460 | 415 | 443 | 154 | 836 | 265 |
| Trait | Cases in Males | Cases in Females | ||
|---|---|---|---|---|
| Normal | Non-Normal | Normal | Non-Normal | |
| Body mass, Q | 30 | 68 | 43 | 55 |
| Body length, L | 58 | 40 | 55 | 43 |
| Tail length, C | 67 | 31 | 54 | 44 |
| Hind foot length, P | 45 | 52 | 39 | 59 |
| Ear length, A | 42 | 55 | 30 | 68 |
| Factor | Df | Body Mass, Q | Body Length, L | Tail Length, C | Hind Foot Length, P | Ear Length, A |
|---|---|---|---|---|---|---|
| Latitude | 1 | F = 70.2, p < 0.001 | F = 184.5, p < 0.001 | F = 229.3, p < 0.001 | F = 6.1, p < 0.05 | F = 4.8, p < 0.05 |
| Longitude | 1 | F = 35.4, p < 0.001 | F = 0.1, NS | F = 3.8, p = 0.05 | F = 205.9, p < 0.001 | F = 25.1, p < 0.001 |
| Decade | 4 | F = 16.8, p < 0.001 | F = 97.2, p < 0.001 | F = 3.8, p < 0.005 | F = 24.8, p < 0.001 | F = 28.3, p < 0.001 |
| Age | 2 | F = 2374.1, p < 0.001 | F = 2113.1, p < 0.001 | F = 1115.4, p < 0.001 | F = 300.3, p < 0.001 | F = 305.7, p < 0.001 |
| Sex | 1 | F = 1.30, NS | F = 29.1, p < 0.001 | F = 1.3, NS | F = 74.3, p < 0.001 | F = 4.7, p < 0.05 |
| Decade | Age | Body Mass, Q | Body Length, L | Tail Length, C | Hind Foot Length, P | Ear Length, A |
|---|---|---|---|---|---|---|
| 1980s | ad | r = 0.21, p < 0.001 | r = −0.20, p < 0.001 | r = −0.21, p < 0.001 | r = 0.08, NS | r = 0.10, p < 0.05 |
| sub | r = −0.08, p < 0.01 | r = −0.55, p < 0.001 | r = −0.40, p < 0.001 | r = 0.02, NS | r = 0.17, p < 0.001 | |
| 1990s | ad | r = 0.19, p < 0.001 | r = −0.05, NS | r = −0.36, p < 0.001 | r = 0.14, p < 0.05 | r = 0.31, p < 0.001 |
| sub | r = 0.05, NS | r = −0.39, p < 0.001 | r = −0.50, p < 0.001 | r = 0.11, NS | r = −0.05, NS | |
| 2000s | ad | r = −0.17, p < 0.001 | r = −0.34, p < 0.001 | r = −0.23, p < 0.001 | r = −0.33, p < 0.001 | r = −0.22, p < 0.001 |
| sub | r = 0.28, p < 0.001 | r = −0.33, p < 0.001 | r = −0.37, p < 0.001 | r = −0.44, p < 0.001 | r = −0.14, p < 0.001 | |
| 2010s | ad | r = 0.10, p < 0.05 | r = −0.34, p < 0.001 | r = −0.41, p < 0.001 | r = −0.52, p < 0.001 | r = 0.01, NS |
| sub | r = 0.32, p < 0.001 | r = −0.43, p < 0.001 | r = −0.50, p < 0.001 | r = −0.53, p < 0.001 | r = −0.16, p < 0.001 |
| Decade | Age | Body Mass, Q | Body Length, L | Tail Length, C | Hind Foot Length, P | Ear Length, A |
|---|---|---|---|---|---|---|
| 1980s | ad | r = 0.09, p < 0.02 | r = −0.24, p < 0.001 | r = −0.25, p < 0.001 | r = −0.27, p < 0.001 | r = 0.00, NS |
| sub | r = −0.09, p < 0.005 | r = −0.54, p < 0.001 | r = −0.37, p < 0.001 | r = −0.41, p < 0.001 | r = −0.12, p < 0.02 | |
| 1990s | ad | r = 0.05, NS | r = −0.19, p < 0.001 | r = −0.43, p < 0.001 | r = −0.17, p < 0.001 | r = 0.05, NS |
| sub | r = 0.08, p < 0.001 | r = −0.37, p < 0.001 | r = −0.43, p < 0.001 | r = −0.20, p < 0.001 | r = −0.34, p < 0.001 | |
| 2000s | ad | r = 0.00, NS | r = −0.09, p < 0.005 | r = −0.20, p < 0.001 | r = −0.31, p < 0.001 | r = −0.31, p < 0.001 |
| sub | r = 0.23, p < 0.001 | r = −0.16, p < 0.001 | r = −0.26, p < 0.001 | r = −0.37, p < 0.001 | r = −0.24, p < 0.001 | |
| 2010s | ad | r = 0.03, NS | r = −0.23, p < 0.001 | r = −0.30, p < 0.001 | r = −0.37, p < 0.001 | r = −0.03, NS |
| sub | r = 0.07, NS | r = −0.19, p < 0.001 | r = −0.21, p < 0.001 | r = −0.34, p < 0.001 | r = −0.09, p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balčiauskas, L.; Csanády, A.; Stanko, M.; Timm, U.; Balčiauskienė, L. Morphological Variation in the Striped Field Mouse Across Three Countries. Animals 2025, 15, 452. https://doi.org/10.3390/ani15030452
Balčiauskas L, Csanády A, Stanko M, Timm U, Balčiauskienė L. Morphological Variation in the Striped Field Mouse Across Three Countries. Animals. 2025; 15(3):452. https://doi.org/10.3390/ani15030452
Chicago/Turabian StyleBalčiauskas, Linas, Alexander Csanády, Michal Stanko, Uudo Timm, and Laima Balčiauskienė. 2025. "Morphological Variation in the Striped Field Mouse Across Three Countries" Animals 15, no. 3: 452. https://doi.org/10.3390/ani15030452
APA StyleBalčiauskas, L., Csanády, A., Stanko, M., Timm, U., & Balčiauskienė, L. (2025). Morphological Variation in the Striped Field Mouse Across Three Countries. Animals, 15(3), 452. https://doi.org/10.3390/ani15030452









